Reliability, Validity and Temporal Stability of the Serbian Version of the Boston Carpal Tunnel Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Electrophysiological Grading
2.3. Boston Carpal Tunnel Questionnaire
2.4. Adaptation Process
2.5. Statistical Analysis
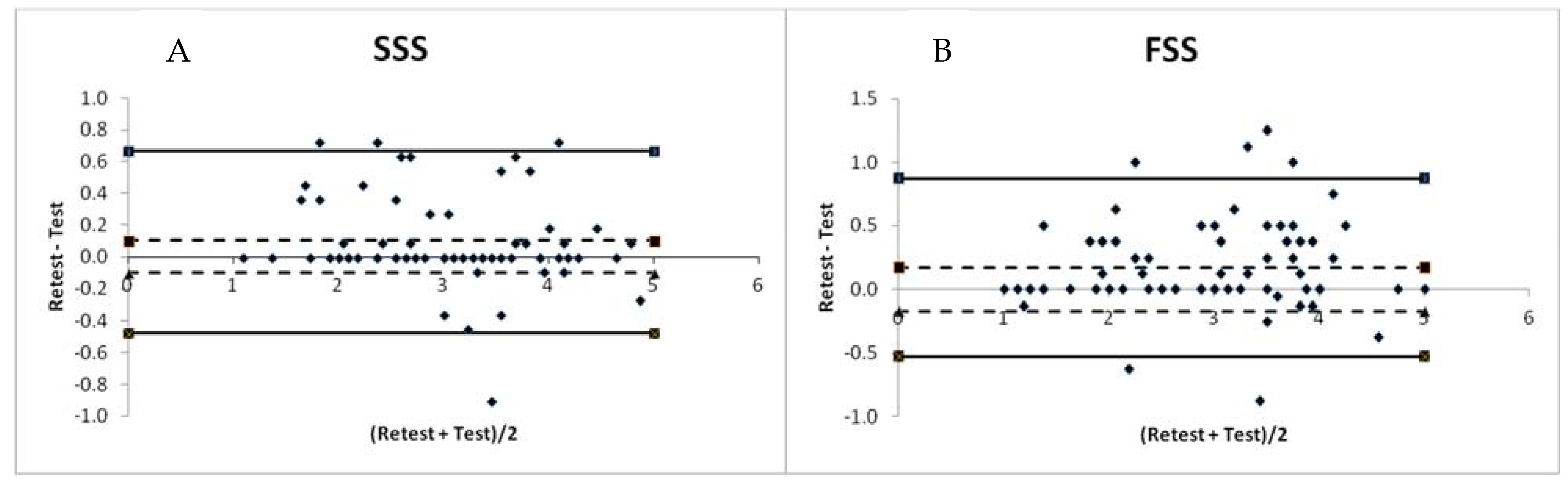
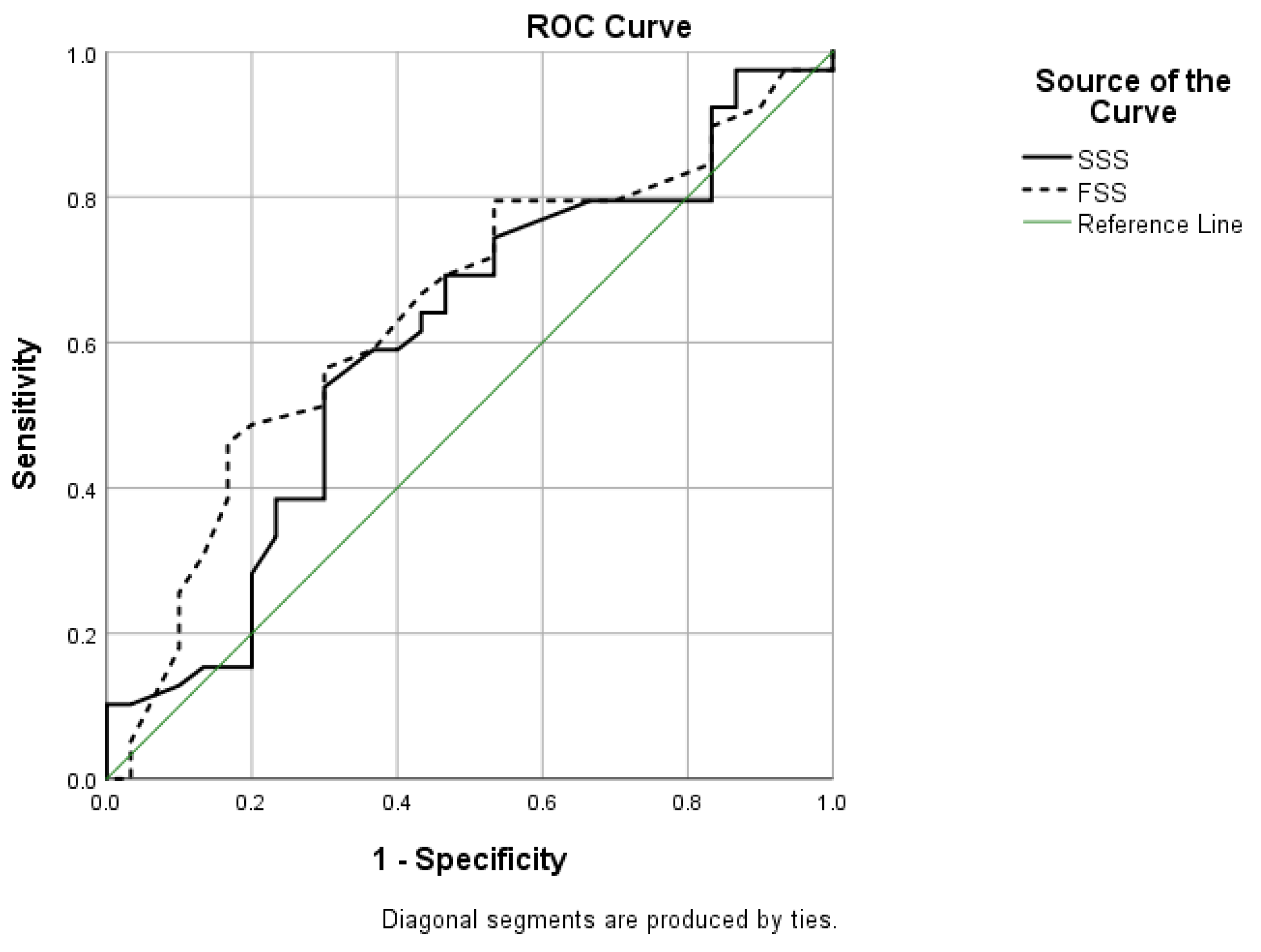
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, A.R.; Atkinson, R.E. Carpal Tunnel Syndrome: An Update for the Primary Care Physician. Hawai’i J. Health Soc. Welf. 2019, 78 (Suppl. S2), 6–10. [Google Scholar]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Scalise, V.; Brindisino, F.; Pellicciari, L.; Minnucci, S.; Bonetti, F. Carpal Tunnel Syndrome: A National Survey to Monitor Knowledge and Operating Methods. Int. J. Environ. Res. Public Health 2021, 18, 1995. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S.D.; Anakwe, R.E. Carpal tunnel syndrome. BMJ 2014, 349, g6437. [Google Scholar] [CrossRef] [PubMed]
- Al Shahrani, E.; Al Shahrani, A.; Al-Maflehi, N. Personal factors associated with carpal tunnel syndrome (CTS): A case-control study. BMC Musculoskelet. Disord. 2021, 22, 1050. [Google Scholar] [CrossRef]
- Lampainen, K.; Shiri, R.; Auvinen, J.; Karppinen, J.; Ryhänen, J.; Hulkkonen, S. Weight-Related and Personal Risk Factors of Carpal Tunnel Syndrome in the Northern Finland Birth Cohort 1966. J. Clin. Med. 2022, 11, 1510. [Google Scholar] [CrossRef]
- Scanlon, A.; Maffei, J. Carpal tunnel syndrome. J. Neurosci. Nurs. 2009, 41, 140–147. [Google Scholar] [CrossRef]
- Farioli, A.; Curti, S.; Bonfiglioli, R.; Baldasseroni, A.; Spatari, G.; Mattioli, S.; Violante, F.S. Observed Differences between Males and Females in Surgically Treated Carpal Tunnel Syndrome Among Non-manual Workers: A Sensitivity Analysis of Findings from a Large Population Study. Ann. Work Expo. Health 2018, 62, 505–515. [Google Scholar] [CrossRef]
- Calandruccio, J.H.; Thompson, N.B. Carpal Tunnel Syndrome: Making Evidence-Based Treatment Decisions. Orthop. Clin. N. Am. 2018, 49, 223–229. [Google Scholar] [CrossRef]
- Alanazy, M.H. Clinical and electrophysiological evaluation of carpal tunnel syndrome: Approach and pitfalls. Neurosciences 2017, 22, 169–180. [Google Scholar] [CrossRef]
- Wipperman, J.; Goerl, K. Carpal tunnel syndrome: Diagnosis and management. Am. Fam. Physician 2016, 94, 993–999. [Google Scholar] [PubMed]
- Bougea, A.; Zambelis, T.; Voskou, P.; Katsika, P.Z.; Tzavara, C.; Kokotis, P.; Karandreas, N. Reliability and Validation of the Greek Version of the Boston Carpal Tunnel Questionnaire. HAND 2018, 13, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Shetty, K.D.; Robbins, M.; Aragaki, D.; Basu, A.; Conlon, C.; Dworsky, M.; Benner, D.; Seelam, R.; Nuckols, T.K. The quality of electrodiagnostic tests for carpal tunnel syndrome: Implications for surgery, outcomes, and expenditures. Muscle Nerve 2020, 62, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Karabinov, V.; Slavchev, S.A.; Georgiev, G.P. Translation and Validation of the Bulgarian Version of the Boston Carpal Tunnel Questionnaire. Cureus 2020, 12, e10901. [Google Scholar] [CrossRef]
- Leite, J.C.D.C.; Jerosch-Herold, C.; Song, F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet. Disord. 2006, 7, 78–79. [Google Scholar] [CrossRef]
- De Kleermaeker, F.G.C.M.; Levels, M.; Verhagen, W.I.M.; Meulstee, J. Validation of the Dutch Version of the Boston Carpal Tunnel Questionnaire. Front. Neurol. 2019, 10, 1154. [Google Scholar] [CrossRef]
- Lue, Y.-J.; Lu, Y.-M.; Lin, G.-T.; Liu, Y.-F. Validation of the Chinese Version of the Boston Carpal Tunnel Questionnaire. J. Occup. Rehabil. 2014, 24, 139–145. [Google Scholar] [CrossRef]
- de Campos, C.C.; Manzano, G.M.; de Andrade, L.B.; Castelo Filho, A.; Nóbrega, J.A. Translation and validation of an instrument for evaluation of severity of symptoms and the functional status in carpal tunnel syndrome. Arq. Neuro-Psiquiatr. 2003, 61, 51–55. [Google Scholar]
- Sezgin, M.; Incel, N.A.; Sevim, S.; Çamdeviren, H.; As, I.; ErdoĞan, C. Assessment of symptom severity and functional status in patients with carpal tunnel syndrome: Reliability and validity of the Turkish version of the Boston questionnaire. Disabil. Rehabil. 2006, 28, 1281–1286. [Google Scholar] [CrossRef]
- Park, D.-J.; Kang, J.-H.; Lee, J.-W.; Lee, K.-E.; Wen, L.; Kim, T.-J.; Park, Y.-W.; Nam, T.-S.; Kim, M.-S.; Lee, S.-S. Cross-Cultural Adaptation of the Korean Version of the Boston Carpal Tunnel Questionnaire: Its Clinical Evaluation in Patients with Carpal Tunnel Syndrome Following Local Corticosteroid Injection. J. Korean Med. Sci. 2013, 28, 1095–1099. [Google Scholar] [CrossRef]
- Oteo-Álvaro, Á.; Marín, M.T.; Matas, J.A.; Vaquero, J. Validación al castellano de la escala Boston Carpal Tunnel Questionnaire. Med. Clin. 2016, 146, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Multanen, J.; Ylinen, J.; Karjalainen, T.; Kautiainen, H.; Repo, J.; Häkkinen, A. Reliability and Validity of The Finnish Version of The Boston Carpal Tunnel Questionnaire among Surgically Treated Carpal Tunnel Syndrome Patients. Scand. J. Surg. 2020, 109, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ulbrichtová, R.; Jakušová, V.; Švihrová, V.; Dvorštiaková, B.; Hudečková, H. Validation of the Slovakian version of Boston Carpal Tunnel Syndrome Questionnaire (BCTSQ). Acta Med. 2019, 62, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, H.H.; Alworikat, N.A. Cross cultural adaptation, reliability and construct validity of the Boston Carpal Tunnel Questionnaire in standard Arabic language. Disabil. Rehabil. 2021, 43, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jerosch-Herold, C.J.; Bland, J.D.P.; Horton, M. Is it time to revisit the Boston Carpal Tunnel Questionnaire? New insights from a Rasch model analysis. Muscle Nerve 2021, 63, 484–489. [Google Scholar] [CrossRef]
- Bland, J.D. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve 2000, 23, 1280–1283. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, J.; Jin, H. Reliability and Concurrent Validity of a Chinese Version of the Alberta Infant Motor Scale Administered to High-Risk Infants in China. BioMed Res. Int. 2018, 2018, 2197163. [Google Scholar] [CrossRef]
- Mendoza-Pulido, C.; Ortiz-Corredor, F. Measurement properties of the Boston Carpal Tunnel Questionnaire in subjects with neurophysiological confirmation of carpal tunnel syndrome: A Rasch analysis perspective. Qual. Life Res. 2021, 30, 2697–2710. [Google Scholar] [CrossRef]
- Mondelli, M.; Aprile, I.; Ballerini, M.; Ginanneschi, F.; Reale, F.; Romano, C.; Rossi, S.; Padua, L. Sex differences in carpal tunnel syndrome: Comparison of surgical and non-surgical populations. Eur. J. Neurol. 2005, 12, 976–983. [Google Scholar] [CrossRef]
- Atroshi, I.; Lyrén, P.-E.; Gummesson, C. The 6-item CTS symptoms scale: A brief outcomes measure for carpal tunnel syndrome. Qual. Life Res. 2009, 18, 347–358. [Google Scholar] [CrossRef] [PubMed]

| Gender (n = 69) | |
| Male, n (%) | 10 (14.49) |
| Female, n (%) | 59 (85.51) |
| Age (MV ± SD) | 55.67 ± 10.77 |
| Occupation (n = 69) | |
| Physical job, n (%) | 20 (28.98) |
| Office job, n (%) | 32 (46.38) |
| Unemployed, n (%) | 6 (8.70) |
| Retired, n (%) | 11 (15.94) |
| Dominant Hand (n = 69) | |
| Right, n (%) | 66 (95.65) |
| Left, n (%) | 1 (1.45) |
| Ambidextrous | 2 (2.90) |
| Lateralization of Symptoms (n = 69) | |
| Both sides, symmetrically, n (%) | 35 (50.72) |
| Both sides, more right, n (%) | 17 (24.64) |
| Both sides, more left, n (%) | 6 (8.70) |
| Only right side, n (%) | 10 (14.49) |
| Only left side, n (%) | 1 (1.45) |
| Electrophysiological Grading: Right Hand (n = 66) | |
| Grade 0, n (%) | 1 (1.52) |
| Grade 1, n (%) | 1 (1.52) |
| Grade 2, n (%) | 30 (45.45) |
| Grade 3, n (%) | 29 (43.93) |
| Grade 4, n (%) | 2 (3.03) |
| Grade 5, n (%) | 1 (1.52) |
| Grade 6, n (%) | 2 (3.03) |
| Electrophysiological Grading: Left Hand (n = 69) | |
| Grade 0, n (%) | 10 (14.49) |
| Grade 1, n (%) | 9 (13.04) |
| Grade 2, n (%) | 26 (37.68) |
| Grade 3, n (%) | 21 (30.44) |
| Grade 4, n (%) | 2 (2.90) |
| Grade 5, n (%) | 1 (1.45) |
| Grade 6, n(%) | 0 |
| BCTQSR Items | MV ± SD | Cronbach’s α if Item Deleted | Total Cronbach’s α |
|---|---|---|---|
| SSS | |||
| 1 | 3.01 ± 1.37 | 0.89 | - |
| 2 | 2.94 ± 1.43 | 0.90 | - |
| 3 | 2.74 ± 1.18 | 0.90 | - |
| 4 | 3.07 ± 1.36 | 0.90 | - |
| 5 | 2.83 ± 1.39 | 0.91 | - |
| 6 | 3.28 ± 1.23 | 0.91 | - |
| 7 | 2.90 ± 1.20 | 0.90 | - |
| 8 | 3.13 ± 1.25 | 0.91 | - |
| 9 | 3.45 ± 1.27 | 0.90 | - |
| 10 | 3.17 ± 1.33 | 0.90 | - |
| 11 | 2.59 ± 1.28 | 0.90 | - |
| Total | 3.01 ± 0.94 | - | 0.91 |
| FSS | |||
| 1 | 2.43 ± 1.24 | 0.93 | - |
| 2 | 2.61 ± 1.20 | 0.91 | - |
| 3 | 2.81 ± 1.29 | 0.92 | - |
| 4 | 2.86 ± 1.25 | 0.92 | - |
| 5 | 3.22 ± 1.29 | 0.92 | - |
| 6 | 3.19 ± 1.09 | 0.91 | - |
| 7 | 3.28 ± 1.22 | 0.92 | - |
| 8 | 2.39 ± 1.20 | 0.92 | - |
| Total | 2.85 ± 1.00 | - | 0.93 |
| Tested Variables | SSS | FSS | ||
|---|---|---|---|---|
| p-Value | p-Value | |||
| Gender | ||||
| Male (MV ± SD) | 2.88 ± 0.99 | 0.643 * | 2.75 ± 1.18 | 0.741 * |
| Female (MV ± SD) | 3.03 ± 0.94 | 2.86 ± 0.98 | ||
| Age | ||||
| r ** | −0.044 | 0.719 ** | 0.131 | 0.284 ** |
| EG | ||||
| 1–2 (MV ± SD) | 2.80 ± 0.91 | 0.103 * | 2.58 ± 0.97 | 0.053 * |
| ≥3 (MV ± SD) | 3.17 ± 0.94 | 3.05 ± 0.99 | ||
| Correlations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCTQSR SSS | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | |
| Item 1 | r | 1 | ||||||||||
| p | ||||||||||||
| Item 2 | r | 0.811 | 1 | |||||||||
| p | <0.001 | |||||||||||
| Item 3 | r | 0.639 | 0.606 | 1 | ||||||||
| p | <0.001 | <0.001 | ||||||||||
| Item 4 | r | 0.630 | 0.483 | 0.731 | 1 | |||||||
| p | <0.001 | <0.001 | <0.001 | |||||||||
| Item 5 | r | 0.534 | 0.385 | 0.605 | 0.858 | 1 | ||||||
| p | <0.001 | 0.001 | <0.001 | <0.001 | ||||||||
| Item 6 | r | 0.346 | 0.192 | 0.392 | 0.189 | 0.062 | 1 | |||||
| p | 0.004 | 0.114 | 0.001 | 0.121 | 0.610 | |||||||
| Item 7 | r | 0.645 | 0.543 | 0.684 | 0.533 | 0.428 | 0.484 | 1 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Item 8 | r | 0.465 | 0.333 | 0.382 | 0.314 | 0.216 | 0.549 | 0.440 | 1 | |||
| p | <0.001 | 0.005 | 0.001 | 0.009 | 0.074 | <0.001 | <0.001 | |||||
| Item 9 | r | 0.659 | 0.598 | 0.374 | 0.304 | 0.245 | 0.493 | 0.455 | 0.558 | 1 | ||
| p | <0.001 | <0.001 | 0.002 | 0.011 | 0.042 | <0.001 | <0.001 | <0.001 | ||||
| Item 10 | r | 0.598 | 0.747 | 0.432 | 0.261 | 0.184 | 0.347 | 0.389 | 0.439 | 0.731 | 1 | |
| p | <0.001 | <0.001 | <0.001 | 0.030 | 0.131 | 0.003 | 0.001 | <0.001 | <0.001 | |||
| Item 11 | r | 0.493 | 0.461 | 0.611 | 0.440 | 0.456 | 0.408 | 0.730 | 0.450 | 0.397 | 0.398 | 1 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.001 | 0.001 | ||
| Correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BCTQSR FSS | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | |
| Item 1 | r | 1 | |||||||
| p | |||||||||
| Item 2 | r | 0.666 | 1 | ||||||
| p | <0.001 | ||||||||
| Item 3 | r | 0.512 | 0.731 | 1 | |||||
| p | <0.001 | <0.001 | |||||||
| Item 4 | r | 0.457 | 0.596 | 0.777 | 1 | ||||
| p | <0.001 | <0.001 | <0.001 | ||||||
| Item 5 | r | 0.462 | 0.556 | 0.591 | 0.592 | 1 | |||
| p | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Item 6 | r | 0.471 | 0.708 | 0.687 | 0.646 | 0.702 | 1 | ||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Item 7 | r | 0.443 | 0.654 | 0.631 | 0.622 | 0.547 | 0.833 | 1 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Item 8 | r | 0.681 | 0.798 | 0.609 | 0.507 | 0.597 | 0.662 | 0.675 | 1 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulatovic, D.; Nikolic, D.; Hrkovic, M.; Filipovic, T.; Cirovic, D.; Radosavljevic, N.; Lazovic, M. Reliability, Validity and Temporal Stability of the Serbian Version of the Boston Carpal Tunnel Questionnaire. Medicina 2022, 58, 1531. https://doi.org/10.3390/medicina58111531
Bulatovic D, Nikolic D, Hrkovic M, Filipovic T, Cirovic D, Radosavljevic N, Lazovic M. Reliability, Validity and Temporal Stability of the Serbian Version of the Boston Carpal Tunnel Questionnaire. Medicina. 2022; 58(11):1531. https://doi.org/10.3390/medicina58111531
Chicago/Turabian StyleBulatovic, Darko, Dejan Nikolic, Marija Hrkovic, Tamara Filipovic, Dragana Cirovic, Natasa Radosavljevic, and Milica Lazovic. 2022. "Reliability, Validity and Temporal Stability of the Serbian Version of the Boston Carpal Tunnel Questionnaire" Medicina 58, no. 11: 1531. https://doi.org/10.3390/medicina58111531
APA StyleBulatovic, D., Nikolic, D., Hrkovic, M., Filipovic, T., Cirovic, D., Radosavljevic, N., & Lazovic, M. (2022). Reliability, Validity and Temporal Stability of the Serbian Version of the Boston Carpal Tunnel Questionnaire. Medicina, 58(11), 1531. https://doi.org/10.3390/medicina58111531






