The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences
Abstract
1. Introduction
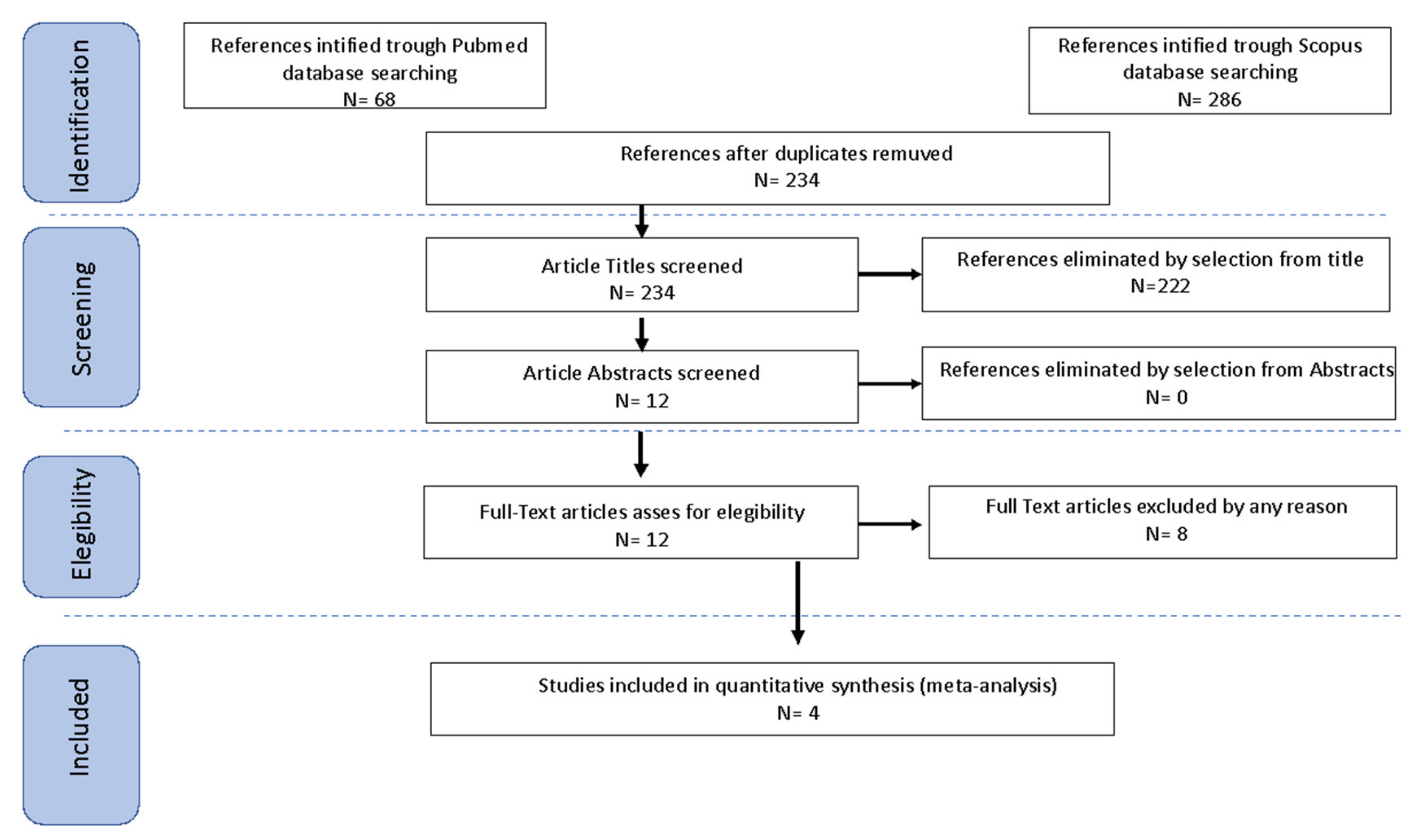
2. Material and Methods
2.1. Search Method
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Quality Assessment
3. Results
3.1. Studies’ Characteristics
3.2. Outcomes
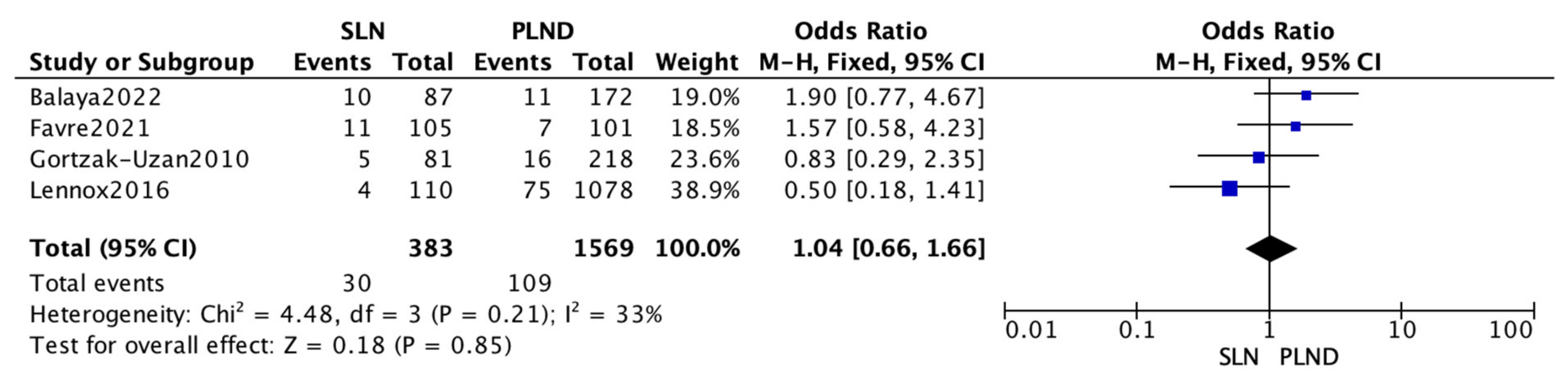
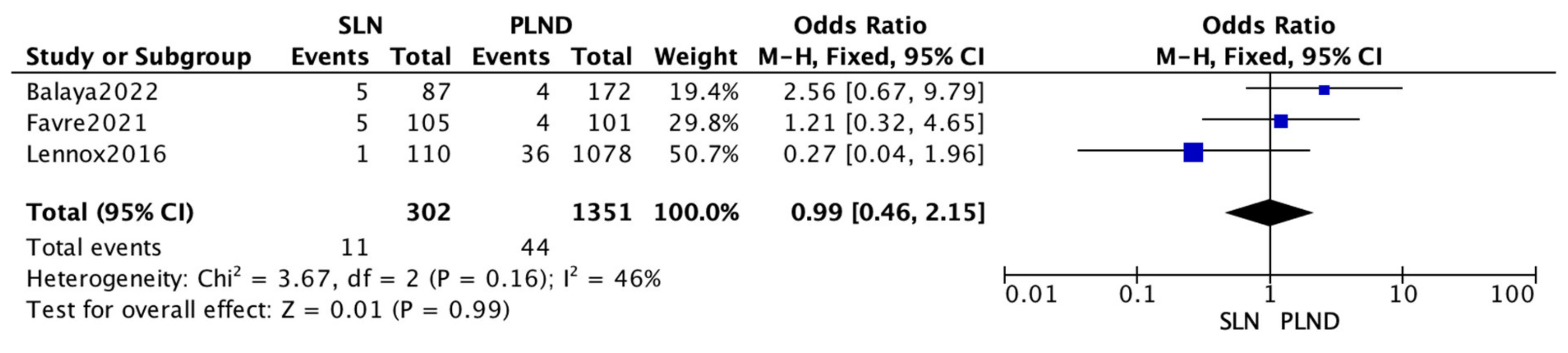
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abbas, K.M.; van Zandvoort, K.; Brisson, M.; Jit, M. Effects of updated demography, disability weights, and cervical cancer burden on estimates of human papillomavirus vaccination impact at the global, regional, and national levels: A PRIME modeling study. Lancet Glob. Health 2020, 8, e536–e544. [Google Scholar] [CrossRef]
- Cibula, V.; Pötter, R.; Chiva, L.; Planchamp, F.; Avall-Lundqvist, E.; Cibula, D.; Raspollini, M. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef]
- Wertheim, E. Die Erweiterte Abdominale Operation bei Carcinoma Colli Uteri (auf Grund von 500 Fällen); Urban und Schwarzenberg: Berlin, Germany; Wien, Austria, 1911. [Google Scholar]
- Octavian Neagoe, C.; Mazilu, O. Pelvic intraoperative iatrogenic oncosurgical injuries: Single-center experience. J. Balk. Union Oncol. 2016, 21, 498–504. [Google Scholar]
- Barbic, M.; Telenta, K.; Noventa, M.; Blaganje, M. Ureteral injuries during different types of hysterecomy: A 7-year series at a single university center. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 1–4. [Google Scholar] [CrossRef]
- Matsuura, Y.; Kawagoe, T.; Toki, N.; Tanaka, M.; Kashimura, M. Long-standing complications after treatment for cancer of the uterine cervix–clinical significance of medical examination at 5 years after treatment. Int. J. Gynecol. Cancer 2006, 16, 294–297. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Xu, T.; Yuan, L.; Yang, X. Sentinel lymph node mapping in early-stage cervical cancer: Meta-analysis. Medicine 2021, 100, e27035. [Google Scholar] [CrossRef]
- Ronsini, C.; Anchora, L.P.; Restaino, S.; Fedele, C.; Arciuolo, D.; Teodorico, E.; Bizzarri, N.; Zannoni, G.F.; Ferrandina, G.; Scambia, G.; et al. The role of semiquantitative evaluation of lympho-vascular space invasion in early stage cervical cancer patients. Gynecol. Oncol. 2021, 162, 299–307. [Google Scholar] [CrossRef]
- Ronsini, C.; Köhler, C.; De Franciscis, P.; La Verde, M.; Mosca, L.; Solazzo, M.C.; Colacurci, N. Laparo-assisted vaginal radical hysterectomy as a safe option for Minimal Invasive Surgery in early stage cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 166, 188–195. [Google Scholar] [CrossRef]
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Zahl Eriksson, A.G.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834. [Google Scholar] [CrossRef]
- Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef]
- Chaimani, A.; Higgins, J.P.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Paynter, R.; et al. [Table], Quality Assessment Criteria for Observational Studies, Based on the Newcastle-Ottawa Scale. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK476448/table/appc.t4/ (accessed on 16 August 2021).
- Balaya, V.; Guani, B.; Morice, P.; Querleu, D.; Fourchotte, V.; Leblanc, E.; Daraï, E.; Baron, M.; Marret, H.; Levêque, J.; et al. SENTICOL Group. Long-term oncological safety of sentinel lymph node biopsy in early-stage cervical cancer: A post-hoc analysis of SENTICOL I and SENTICOL II cohorts. Gynecol. Oncol. 2022, 164, 53–61. [Google Scholar] [CrossRef]
- Favre, G.; Guani, B.; Balaya, V.; Magaud, L.; Lecuru, F.; Mathevet, P. Sentinel Lymph-Node Biopsy in Early-Stage Cervical Cancer: The 4-Year Follow-Up Results of the Senticol 2 Trial. Front. Oncol. 2021, 10, 621518. [Google Scholar] [CrossRef]
- Gortzak-Uzan, L.; Jimenez, W.; Nofech-Mozes, S.; Ismiil, N.; Khalifa, M.A.; Dubé, V.; Rosen, B.; Murphy, J.; Laframboise, S.; Covens, A. Sentinel lymph node biopsy vs. pelvic lymphadenectomy in early stage cervical cancer: Is it time to change the gold standard? Gynecol. Oncol. 2010, 116, 28–32. [Google Scholar] [CrossRef]
- Lennox, G.K.; Covens, A. Can sentinel lymph node biopsy replace pelvic lymphadenectomy for early cervical cancer? Gynecol. Oncol. 2017, 144, 16–20. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Restaino, S.; Ronsini, C.; Finelli, A.; Perrone, E.; Scambia, G.; Fanfani, F. Role of blue dye for sentinel lymph node detection in early endometrial cancer. Gynecol. Surg. 2017, 14, 23. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Slama, J.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: Lowering the false-negative rate and improving the detection of micrometastasis. Gynecol. Oncol. 2012, 127, 462–466. [Google Scholar] [CrossRef]
- Fanfani, F.; Monterossi, G.; Di Meo, M.L.; La Fera, E.; Dell’Orto, F.; Gioè, A.; Lamanna, M.; Ferrari, D.; De Ponti, E.; Perego, P.; et al. Standard ultra-staging compared to one-step nucleic acid amplification for the detection of sentinel lymph node metastasis in endometrial cancer patients: A retrospective cohort comparison. Int. J. Gynecol. Cancer 2020, 30, 372–377. [Google Scholar] [CrossRef]
- Guani, B.; Dorez, M.; Magaud, L.; Buenerd, A.; Lecuru, F.; Mathevet, P. Impact of micrometastasis or isolated tumor cells on recurrence and survival in patients with early cervical cancer: SENTICOL Trial. Int. J. Gynecol. Cancer 2019, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sponholtz, S.E.; Mogensen, O.; Hildebrandt, M.G.; Schledermann, D.; Parner, E.; Markauskas, A.; Frøding, L.P.; Fuglsang, K.; Vilstrup, M.H.; Bjørnholt, S.M.; et al. Sentinel lymph node mapping in early-stage cervical cancer—A national prospective multicenter study (SENTIREC trial). Gynecol. Oncol. 2021, 162, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Al, R.A.; Baykal, C.; Demirtas, E.; Ayhan, A.; Yüce, K. Prognostic factors inFIGO stage IB cervical cancer without lymph node metastasis and the role of adjuvant radiotherapy after radical hysterectomy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 286–292. [Google Scholar] [CrossRef]
- Sartori, E.; Tisi, G.; Chiudinelli, F.; La Face, B.; Franzini, R.; Pecorelli, S. Earlystagecervical cancer: Adjuvant treatment in negative lymph node cases. Gynecol. Oncol. 2007, 107 (Suppl. 1), S170–S174. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R.; Fischerova, D.; Pather, S.; Lavigne, K.; Slama, J.; Alektiar, K.; Ming-Yin, L.; Kocian, R.; Germanova, A.; et al. Surgical treatment of “intermediate risk” lymph node negative cervical cancer patients without adjuvant radiotherapy—A retrospective cohort study and review of the literature. Gynecol. Oncol. 2018, 151, 438–443. [Google Scholar] [CrossRef]
- Yahata, H.; Kobayashi, H.; Sonoda, V.; Kodama, K.; Yagi, H.; Yasunaga, M.; Ohgami, T.; Onoyama, I.; Kaneki, E.; Okugawa, K.; et al. Prognostic outcome and complications of sentinel lymph node navigation surgery for early-stage cervical cancer. Int. J. Clin. Oncol. 2018, 23, 1167–1172. [Google Scholar] [CrossRef]
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer 2020, 137, 69–80. [Google Scholar] [CrossRef]
| Comparative Studies | ||||||
|---|---|---|---|---|---|---|
| Name | Country | Study Design | Study Year | FIGO Stage/Population | No. of Participants | Mean FUP * Months |
| Balaya 2022 [14] | France | Retrospective Case-Control Multicentric study | 2005–2012 | IA1-IIA | 259 | 53 |
| Favre 2021 [15] | France | Prospective Randomized Multicentric study | 2008–2011 | IA1-IB1 | 206 | 51 |
| Gortzak-Uzan 2010 [16] | Canada | Retrospective Case-Control Monocentric study | 2004–2008 | IA-IB1 | 299 | 59 |
| Lennox 2016 [17] | Canada | Retrospective Case-Control Monocentric study | 1984–2015 | IA2-IB1 | 1188 | 59 |
| Name | SLN 3Y DFS * (%) | PLND 3Y DFS * (%) | SLN 4.5Y DFS * (%) | PLND 4.5Y DFS * (%) | p-Value |
|---|---|---|---|---|---|
| Balaya 2022 [14] | - | - | 85.1 | 80.4 | 0.24 |
| Favre 2021 [15] | - | - | 89.5 | 93.1 | 0.53 |
| Gortzak-Uzan 2010 [16] | - | - | 93.8 | 92.7 | 0.72 |
| Lennox 2016 [17] | 97.0 | 95.0 | 93.0 | 92.0 | 0.61 |
| Name | SLN 3Y OS ° (%) | PLND 3Y OS ° (%) | SLN 4.5Y OS ° (%) | PLND 4.5Y OS ° (%) | p-Value |
|---|---|---|---|---|---|
| Balaya 2022 [14] | - | - | 90.8 | 97.2 | 0.22 |
| Favre 2021 [15] | - | - | 95.2 | 96.0 | 0.97 |
| Lennox 2016 [17] | - | - | 100.0 | 97.6 | 0.051 |
| Name | SLN RR § (%) | PLND RR § (%) | p-Value |
|---|---|---|---|
| Balaya 2022 [14] | 11.5 | 6.4 | 0.23 |
| Favre 2021 | 10.5 | 6.9 | 0.37 |
| Gortzak-Uzan 2010 [16] | 6.2 | 7.3 | 0.83 |
| Lennox 2016 [17] | 3.6 | 6.9 | 0.18 |
| Name | Site of Recurrence |
|---|---|
| Balaya 2022 [14] | 1 Vaginal; 2 Pelvic; 4 Distant; 3 Nodal |
| Favre 2021 [15] | 1 Parametrium; 3 Lungs; 1 Pelvic; 1 Inguinal; 1 Peritoneum; 2 Vaginal; 1 Right Iliac Lymph node |
| Gortzak-Uzan 2010 [16] | 3 Centro-pelvic; 1 Sidewall; 1 Distant |
| Lennox 2016 [17] | 1 Vaginal vault; 1 Rectovaginal septum; 1 Sigmoid colon; 1 NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsini, C.; De Franciscis, P.; Carotenuto, R.M.; Pasanisi, F.; Cobellis, L.; Colacurci, N. The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina 2022, 58, 1539. https://doi.org/10.3390/medicina58111539
Ronsini C, De Franciscis P, Carotenuto RM, Pasanisi F, Cobellis L, Colacurci N. The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina. 2022; 58(11):1539. https://doi.org/10.3390/medicina58111539
Chicago/Turabian StyleRonsini, Carlo, Pasquale De Franciscis, Raffaela Maria Carotenuto, Francesca Pasanisi, Luigi Cobellis, and Nicola Colacurci. 2022. "The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences" Medicina 58, no. 11: 1539. https://doi.org/10.3390/medicina58111539
APA StyleRonsini, C., De Franciscis, P., Carotenuto, R. M., Pasanisi, F., Cobellis, L., & Colacurci, N. (2022). The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina, 58(11), 1539. https://doi.org/10.3390/medicina58111539






