The Role of miR-375-3p, miR-210-3p and Let-7e-5p in the Pathological Response of Breast Cancer Patients to Neoadjuvant Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Cancer Patients and Samples Collection
2.2. RNA Extraction
2.3. miRNA Expression Evaluation
2.4. TCGA Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient and Tumour Characteristics
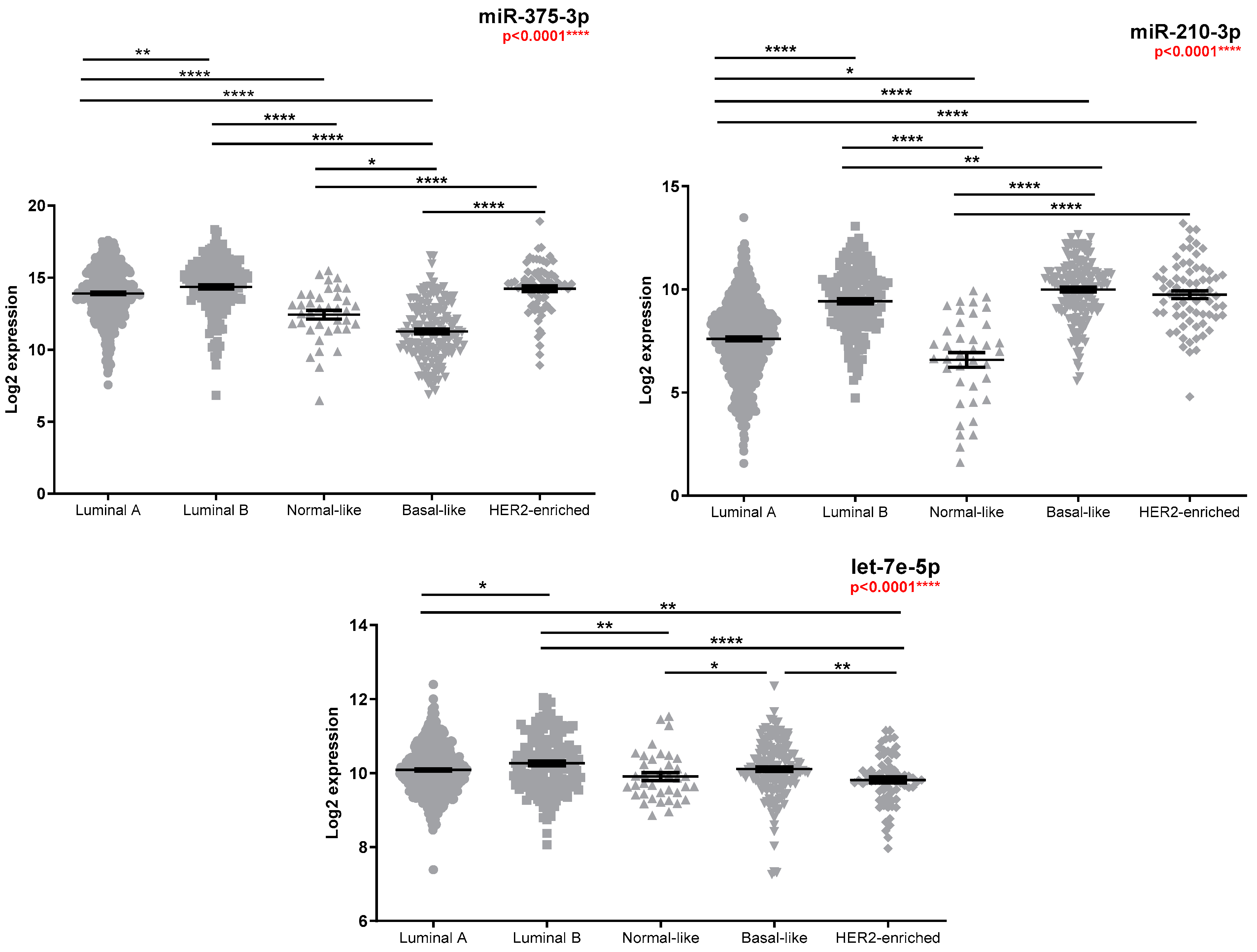
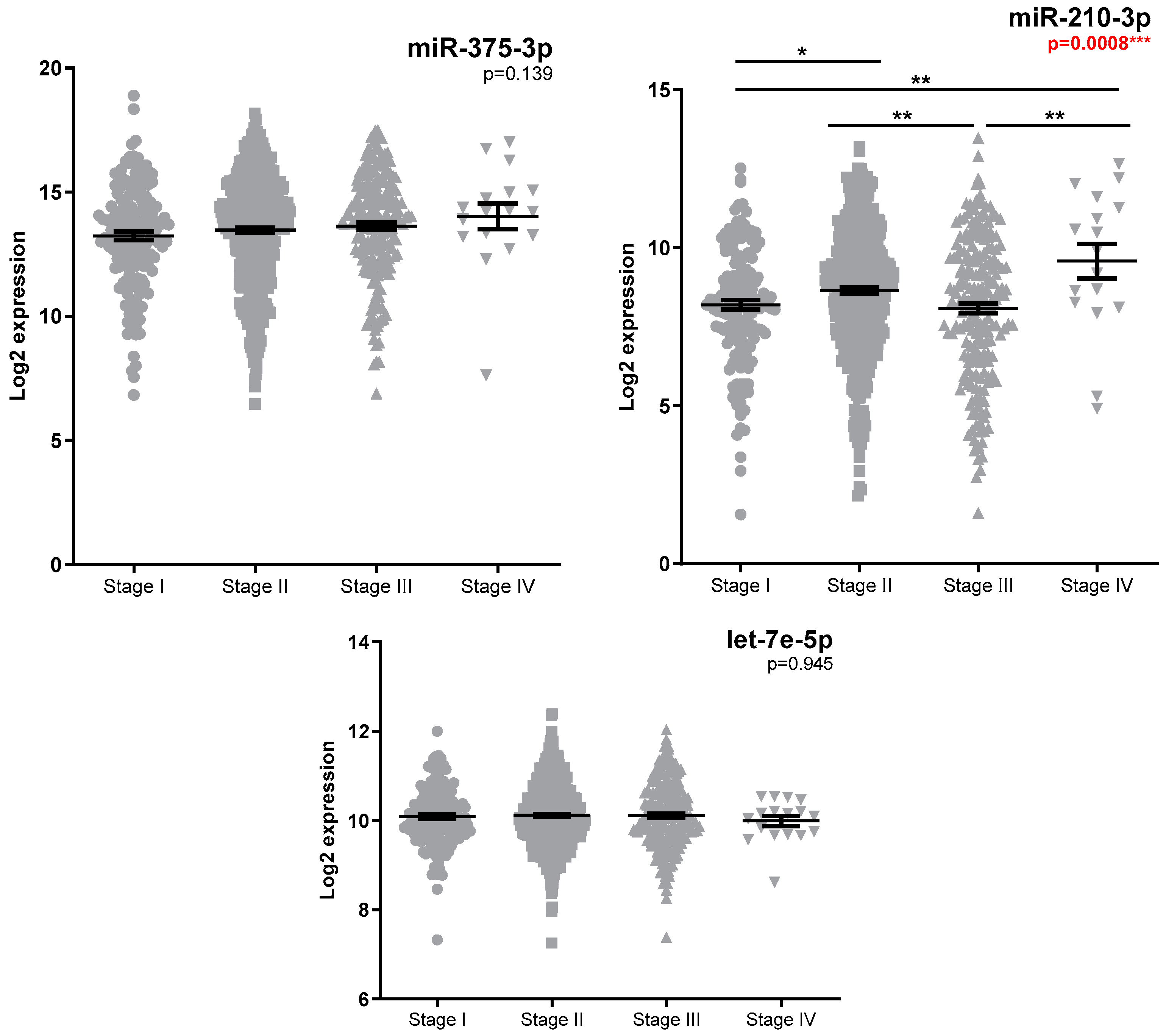
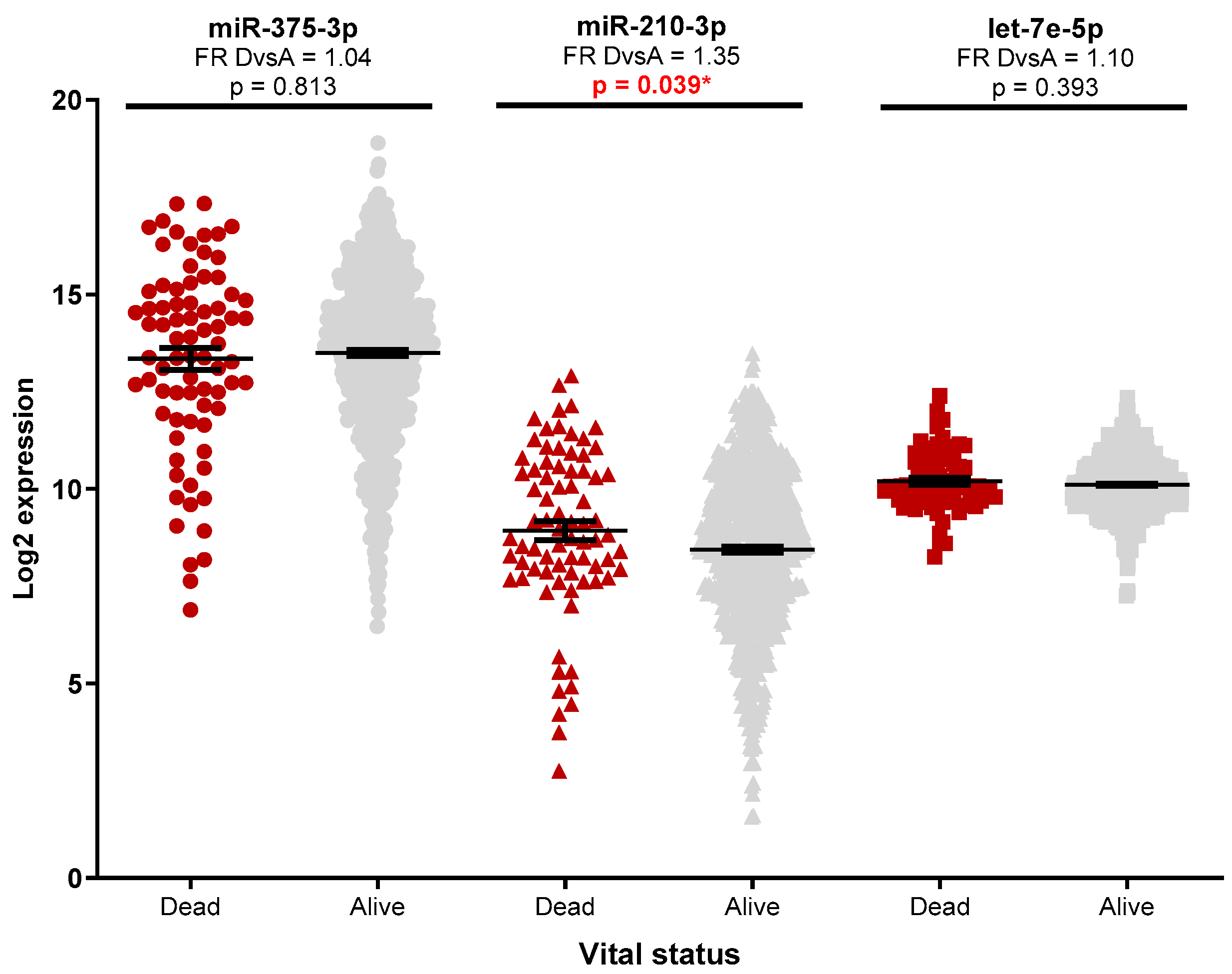
3.2. Investigation of miRNA Expression in Breast Cancer Specimens
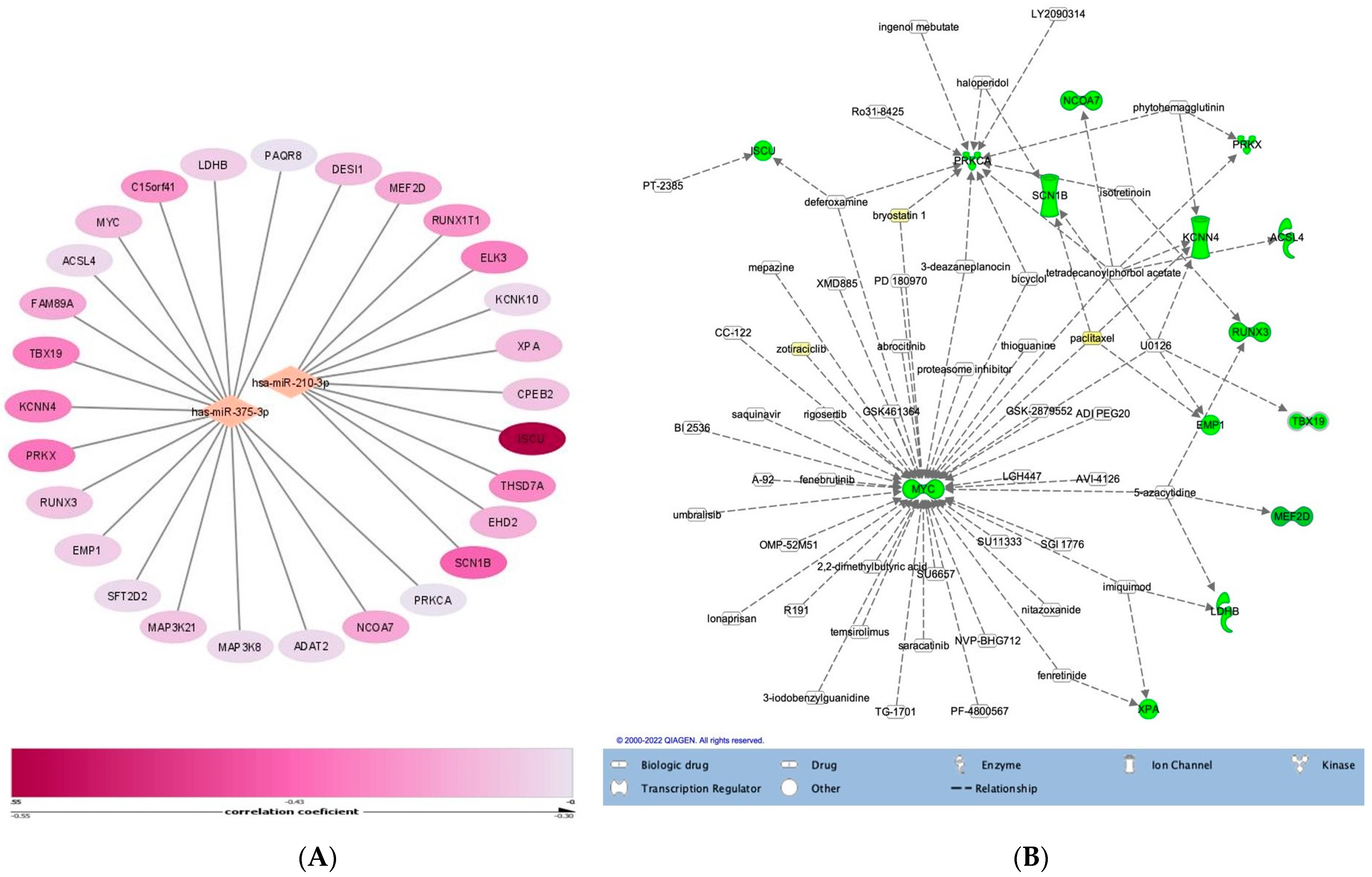
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davey, M.G.; Lowery, A.J.; Miller, N.; Kerin, M.J. MicroRNA expression profiles and breast cancer Chemotherapy. Int. J. Mol. Sci. 2021, 22, 10812. Available online: https://pubmed.ncbi.nlm.nih.gov/34639152/ (accessed on 5 May 2022).
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 9S–16S. Available online: https://jnm.snmjournals.org/content/57/Supplement_1/9S (accessed on 21 April 2022). [CrossRef]
- Thompson, A.M.; Moulder-Thompson, S.L. Neoadjuvant treatment of breast cancer. Ann. Oncol. 2012, 23 (Suppl. 10). [Google Scholar] [CrossRef] [PubMed]
- Sikov, W.; Boughey, J.; Al-Hilli, Z. UpToDate: General Principles of Neoadjuvant Management of Breast Cancer. 2021, pp. 1–21. Available online: https://www.uptodate.com/contents/general-principles-of-neoadjuvant-management-of-breast-cancer. (accessed on 21 August 2022).
- Sahoo, S.; Lester, S.C. Pathology of Breast Carcinomas After Neoadjuvant Chemotherapy: An Overview With Recommendations on Specimen Processing and Reporting. Arch. Pathol. Lab. Med. 2009, 133, 633–642. Available online: https://meridian.allenpress.com/aplm/article/133/4/633/460740/Pathology-of-Breast-Carcinomas-After-Neoadjuvant (accessed on 5 May 2022). [CrossRef] [PubMed]
- Park, C.K.; Jung, W.H.; Koo, J.S. Pathologic Evaluation of Breast Cancer after Neoadjuvant Therapy. J. Pathol. Transl. Med. 2016, 50, 173. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4876080/ (accessed on 5 May 2022). [CrossRef]
- Shintia, C.; Endang, H.; Diani, K. Assessment of pathological response to neoadjuvant chemotherapy in locally advanced breast cancer using the Miller-Payne system and TUNEL. Malays. J. Pathol. 2016, 38, 25–32. Available online: https://pubmed.ncbi.nlm.nih.gov/27126661/ (accessed on 14 April 2022). [PubMed]
- Wang, W.; Liu, Y.; Zhang, H.; Zhang, S.; Duan, X.; Ye, J.; Xu, L.; Zhao, J.; Cheng, Y.; Liu, Q. Prognostic value of residual cancer burden and Miller-Payne system after neoadjuvant chemotherapy for breast cancer. Gland. Surg. 2021, 10, 3211. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. Available online: https://pubmed.ncbi.nlm.nih.gov/15944708/ (accessed on 11 May 2022). [CrossRef]
- Petrović, N.; Nakashidze, I.; Nedeljković, M. Breast Cancer Response to Therapy: Can microRNAs Lead the Way? J. Mammary Gland Biol. Neoplasia 2021, 26, 157–178. Available online: https://pubmed.ncbi.nlm.nih.gov/33479880/ (accessed on 10 February 2022). [CrossRef]
- Zhang, Z.; Zhang, H.; Yu, J.; Xu, L.; Pang, X.; Xiang, Q.; Liu, Q.; Cui, Y. miRNAs as therapeutic predictors and prognostic biomarkers of neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2022, 194, 483–505. Available online: https://pubmed.ncbi.nlm.nih.gov/35727379/ (accessed on 24 September 2022). [CrossRef]
- To, N.H.; Nguyen, H.Q.; Thiolat, A.; Liu, B.; Cohen, J.; Radosevic-Robin, N.; Belkacemi, Y. Radiation therapy for triple-negative breast cancer: Emerging role of microRNAs as biomarkers and radiosensitivity modifiers. A systematic review. Breast Cancer Res. Treat. 2022, 193, 265–279. Available online: https://pubmed.ncbi.nlm.nih.gov/35397079/ (accessed on 24 September 2022). [CrossRef] [PubMed]
- Davey, M.G.; Davey, M.S.; Richard, V.; Wyns, W.; Soliman, O.; Miller, N.; Lowery, A.; Kerin, M. Overview of MicroRNA Expression in Predicting Response to Neoadjuvant Therapies in Human Epidermal Growth Receptor-2 Enriched Breast Cancer—A Systematic Review. Breast Cancer 2022, 16. Available online: https://pubmed.ncbi.nlm.nih.gov/35340888/ (accessed on 24 September 2022). [CrossRef] [PubMed]
- Jackisch, C.; Harbeck, N.; Huober, J.; Von Minckwitz, G.; Gerber, B.; Kreipe, H.H.; Liedtke, C.; Marschner, N.; Möbus, V.; Scheithauer, H.; et al. 14th St. Gallen International Breast Cancer Conference 2015: Evidence, Controversies, Consensus—Primary Therapy of Early Breast Cancer: Opinions Expressed by German Experts. Breast Care 2015, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, G.S.; Li JDi Pan, W.Y.; Shi, Q.; Xiong, D.D.; Mo, C.H.; Zeng, J.J.; Chen, G.; Feng, Z.B. The role of upregulated miR-375 expression in breast cancer: An in vitro and in silico study. Pathol. Res. Pract. 2020, 216, 152754. Available online: https://pubmed.ncbi.nlm.nih.gov/31787478/ (accessed on 9 June 2022). [CrossRef]
- Fu, H.; Fu, L.; Xie, C.; Zuo, W.S.; Liu, Y.S.; Zheng, M.Z.; Yu, J.M. CancermiR-375 inhibits cancer stem cell phenotype and tamoxifen resistance by degrading HOXB3 in human ER-positive breast cancer. Oncol. Rep. 2017, 37, 1093–1099. Available online: http://www.spandidos-publications.com/10.3892/or.2017.5360/abstract (accessed on 11 May 2022). [CrossRef] [PubMed]
- Jung, E.J.; Santarpia, L.; Kim, J.; Esteva, F.J.; Moretti, E.; Buzdar, A.U.; Di Leo, A.; Le, X.F.; Bast, R.C.; Park, S.T.; et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 2012, 118, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Fujiwara, T.; Sato, F.; Shimada, Y.; Tanaka, E.; Sakai, Y.; Shimziu, K.; Tsujimoto, G. MicroRNA-210 Regulates Cancer Cell Proliferation through Targeting Fibroblast Growth Factor Receptor-like 1 (FGFRL1). J. Biol. Chem. 2011, 286, 420. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xia, K.; Xu, P.; Sun, E.; Ma, J.; Gao, S.; Zhou, Q.; Zhang, M.; Wang, F.; Chean, F.; et al. miRNA expression patterns in chemoresistant breast cancer tissues. Biomed. Pharmacother. 2014, 68, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.H.; Hayes, D.F.; Wolff, A.C.; Mangu, P.B.; Temin, S. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Oncol. Pract. 2010, 6, 195. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2206–2223. Available online: https://pubmed.ncbi.nlm.nih.gov/23917950/ (accessed on 9 August 2022). [CrossRef] [PubMed]
- McGuire, A.; Casey, M.C.; Waldron, R.M.; Heneghan, H.; Kalinina, O.; Holian, E.; McDermott, A.; Lowery, A.J.; Newell, J.; Dwyer, R.M.; et al. Prospective Assessment of Systemic MicroRNAs as Markers of Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2020, 12, 1820. Available online: https://www.mdpi.com/2072-6694/12/7/1820/htm (accessed on 13 February 2022). [CrossRef]
- Sheri, A.; Smith, I.E.; Johnston, S.R.; A’hern, R.; Nerurkar, A.; Jones, R.L.; Hills, M.; Detre, S.; Pinder, S.E.; Symmans, W.F.; et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 75–80. Available online: https://pubmed.ncbi.nlm.nih.gov/25361988/ (accessed on 16 April 2022). [CrossRef]
- Wei, J.; Lu, Y.; Wang, R.; Xu, X.; Liu, Q.; He, S.; Pan, H.; Liu, X.; Yuan, B.; Ding, Y. MicroRNA-375: Potential cancer suppressor and therapeutic drug. Biosci. Rep. 2021. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, W.; He, X.; Zhang, L.; Li, C.; Huang, H.; Nace, G.; Geller, D.A.; Lin, J.; Tsung, A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 2012, 143, 177-87.e8. Available online: https://pubmed.ncbi.nlm.nih.gov/22504094/ (accessed on 29 September 2022). [CrossRef]
- Rocha Simonini, P.D.S.; Breiling, A.; Gupta, N.; Malekpour, M.; Youns, M.; Omranipour, R.; Malekpour, F.; Volinia, S.; Croce, C.M.; Naimabadi, H. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor α in breast cancer cells. Cancer Res. 2010, 70, 9175–9184. Available online: https://aacrjournals.org/cancerres/article/70/22/9175/559828/Epigenetically-Deregulated-microRNA-375-Is (accessed on 9 June 2022). [CrossRef]
- Liu, J.; Wang, P.; Zhang, P.; Zhang, X.; Du, H.; Liu, Q.; Huang, B.; Qian, C.; Zhang, S.; Zhu, W.; et al. An integrative bioinformatics analysis identified miR-375 as a candidate key regulator of malignant breast cancer. J. Appl. Genet. 2019, 60, 335–346. Available online: https://pubmed.ncbi.nlm.nih.gov/31372832/ (accessed on 9 June 2022). [CrossRef] [PubMed]
- Jonsdottir, K.; Janssen, S.R.; Da Rosa, F.C.; Gudlaugsson, E.; Skaland, I.; Baak, J.P.A.; Janssen, E.A.M. Validation of Expression Patterns for Nine miRNAs in 204 Lymph-Node Negative Breast Cancers. PLoS ONE 2012, 7. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3492447/ (accessed on 9 June 2022). [CrossRef] [PubMed]
- Liu, S.L.; Sui, Y.F.; Lin, M.Z. MiR-375 is epigenetically downregulated due to promoter methylation and modulates multi-drug resistance in breast cancer cells via targeting YBX1. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3223–3229. Available online: https://www.europeanreview.org/article/11237 (accessed on 12 May 2022). [PubMed]
- Zhao, Q.; Liu, Y.; Wang, T.; Yang, Y.; Ni, H.; Liu, H.; Guo, Q.; Xi, T.; Zheng, L. MiR-375 inhibits the stemness of breast cancer cells by blocking the JAK2/STAT3 signaling. Eur. J. Pharmacol. 2020, 884, 173359. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Balwierz, A.; Zhang, J.D.; Küblbeck, M.; Pawitan, Y.; Hielscher, T.; Wiemann, S.; Sahin, Ö. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene 2012, 32, 1173–1182. Available online: https://www.nature.com/articles/onc2012128 (accessed on 11 May 2022). [CrossRef] [PubMed]
- Ye, X.M.; Zhu, H.Y.; Bai, W.D.; Wang, T.; Wang, L.; Chen, Y.; Yang, A.G.; Jia, L.T. Epigenetic silencing of miR-375 induces trastuzumab resistance in HER2-positive breast cancer by targeting IGF1R. BMC Cancer 2014, 14, 134. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-14-134 (accessed on 11 May 2022). [CrossRef] [PubMed]
- Guan, X.; Shi, A.; Zou, Y.; Sun, M.; Zhan, Y.; Dong, Y.; Fan, Z. EZH2-Mediated microRNA-375 Upregulation Promotes Progression of Breast Cancer via the Inhibition of FOXO1 and the p53 Signaling Pathway. Front. Genet. 2021, 12, 633756. Available online: https://pubmed.ncbi.nlm.nih.gov/33854524/ (accessed on 10 June 2022). [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, C.; Xiang, Q.; Xu, L.; Liu, Q.; Pang, X.; Zhang, W.; Zhang, H.; Zhang, S.; et al. Circulating microRNAs as indicators in the prediction of neoadjuvant chemotherapy response in luminal B breast cancer. Thorac. Cancer 2021, 12, 3396–3406. Available online: https://pubmed.ncbi.nlm.nih.gov/34751517/ (accessed on 5 May 2022). [CrossRef] [PubMed]
- Wu, X.; Somlo, G.; Yu, Y.; Palomares, M.R.; Li, A.X.; Zhou, W.; Chow, A.; Yen, Y.; Rossi, J.J.; Gao, H.; et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J. Transl. Med. 2012, 10, 42. Available online: https://tmu.pure.elsevier.com/en/publications/de-novo-sequencing-of-circulating-mirnas-identifies-novel-markers-2 (accessed on 5 May 2022). [CrossRef] [PubMed]
- Chekhun, V.F.; Borikun, T.V.; Bazas, V.M.; Andriiv, A.V.; Klyusov, O.M.; Yalovenko, T.M.; Lukianova, N.Y. Association of Circulating MIR-21,-205, and-182 with Response of Luminal Breast Cancers to Neoadjuvant Fac and Ac Treatment. Exp. Oncol. 2020, 42, 162–166. Available online: https://doi.org/10.32471/exp-oncology.2312-8852.vol-42-no-3.14805 (accessed on 5 May 2022). [PubMed]
- Evangelista, A.F.; Oliveira, R.J.; Viviane, V.A.; Rene, R.A.; Reis, R.M.; Marcia, M.M. Integrated analysis of mRNA and miRNA profiles revealed the role of miR-193 and miR-210 as potential regulatory biomarkers in different molecular subtypes of breast cancer. BMC Cancer 2021, 21, 76. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-020-07731-2 (accessed on 16 April 2022). [CrossRef]
- Chen, X.; Lu, P.; Wang D dan Yang S jin Wu, Y.; Shen, H.Y.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. The role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissues. Gene 2016, 595, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Gade, S.; Steinbach, B.; Loibl, S.; Von Minckwitz, G.; Untch, M.; Schwedler, K.; Lübbe, K.; Schem, C.; Fasching, P.A.; et al. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: A translational research project within the Geparquinto trial. Breast Cancer Res. Treat. 2014, 147, 61–68. Available online: https://link.springer.com/article/10.1007/s10549-014-3079-3 (accessed on 6 May 2022). [CrossRef] [PubMed]
- Davey, M.G.; Davies, M.; Lowery, A.J.; Miller, N.; Kerin, M.J. The Role of MicroRNA as Clinical Biomarkers for Breast Cancer Surgery and Treatment. Int. J. Mol. Sci. 2021, 22, 8290. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8346977/ (accessed on 10 September 2022). [CrossRef] [PubMed]
- Egeland, N.G.; Lunde, S.; Jonsdottir, K.; Lende, T.H.; Cronin-Fenton, D.; Gilje, B.; Janssen, E.A.M.; Søiland, H. The Role of MicroRNAs as Predictors of Response to Tamoxifen Treatment in Breast Cancer Patients. Int. J. Mol. Sci. 2015, 16, 24243. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4632748/ (accessed on 10 February 2022). [CrossRef]
- Qattan, A.; Al-Tweigeri, T.; Alkhayal, W.; Suleman, K.; Tulbah, A.; Amer, S. Clinical identification of dysregulated circulating microRNAs and their implication in drug response in triple negative breast cancer (TNBC) by target gene network and meta-analysis. Genes 2021, 12, 549. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8068962/ (accessed on 11 May 2022). [CrossRef] [PubMed]
- Pasculli, B.; Barbano, R.; Rendina, M.; Fontana, A.; Copetti, M.; Mazza, T.; Valori, V.M.; Morritti, M.; Mariello, E.; Graziano, P.; et al. Hsa-miR-210-3p expression in breast cancer and its putative association with worse outcome in patients treated with Docetaxel. Sci. Rep. 2019, 9, 14913. [Google Scholar] [CrossRef] [PubMed]
- Bar, I.; Merhi, A.; Abdel-Sater, F.; Ben Addi, A.; Sollennita, S.; Canon, J.L.; Delrée, P. The MicroRNA miR-210 Is Expressed by Cancer Cells but Also by the Tumor Microenvironment in Triple-Negative Breast Cancer. J. Histochem. Cytochem. 2017, 65, 335. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5625853/ (accessed on 9 June 2022). [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.L.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C.; Li, T.; Ding, Y.; Tu, T.; Zhou, F.; Qi, W.; Chen, H.; Sun, X. Let-7a inhibits growth and migration of breast cancer cells by targeting HMGA1. Int. J. Oncol. 2015, 46, 2526–2534. Available online: http://www.spandidos-publications.com/10.3892/ijo.2015.2949/abstract (accessed on 16 April 2022). [CrossRef]
- Gezer, U.; Keskin, S.; Iǧci, A.; Tükenmez, M.; Tiryakioǧlu, D.; Çetinkaya, M.; Dişci, R.; Dalay, N.; Eralp, Y. Abundant circulating microRNAs in breast cancer patients fluctuate considerably during neoadjuvant chemotherapy. Oncol. Lett. 2014, 8, 845–848. Available online: http://www.spandidos-publications.com/10.3892/ol.2014.2188/abstract (accessed on 6 May 2022). [CrossRef]
| Variable | Patients Characteristics |
|---|---|
| n = 60 | |
| Age | |
| ≤50 | 16 (26.67%) |
| >50 | 44 (73.33%) |
| Grading | |
| G1 | 7 (11.67 %) |
| G2 | 32 (53.33%) |
| G3 | 20 (33.33%) |
| NA | 1 (1.67%) |
| Ki-67 | |
| ≤20 | 22 (36.67%) |
| >20 | 37 (61.67%) |
| NA | 1(1.67%) |
| Molecular Subtype | |
| Luminal A | 18 (30%) |
| Luminal B | 28 (46.67%) |
| HER2+ | 3 (5%) |
| TNBC 1 | 8 (13.33%) |
| NA 2 | 3(5%) |
| Tumour size (c 3) | |
| cT1 | 6 (10%) |
| cT2 | 29 (8.33%) |
| cT3 | 8 (13.33%) |
| cT4 | 11 (18.33%) |
| NA | 6 (10%) |
| Lymph nodes (c) | |
| cN0 | 15 (25%) |
| cN1 | 15 (25%) |
| cN2 | 21 (35%) |
| cN3 | 3 (5%) |
| NA | 6 (10%) |
| Metastasis (c) | |
| cM0 | 49 (81.67%) |
| cM1 | 1 (1.67%) |
| NA | 10 (16.67%) |
| Clinical stage | |
| Stage I | 5 (8.33%) |
| Stage II | 19 (31.67%) |
| Stage III | 27 (45%) |
| Stage IV | 1 (1.67%) |
| NA | 8 (13.33%) |
| Tumour size (p 4) | |
| pT0 | 7 (11.67%) |
| pT1 | 23 (38.33%) |
| pT2 | 18 (30%) |
| pT3 | 1 (1.67) |
| NA | 11 (18.33%) |
| Lymph nodes (p) | |
| pN0 | 26 (43.33%) |
| pN1 | 13 (21.67%) |
| pN2 | 8 (13.33%) |
| pN3 | 4 (6.67%) |
| NA | 9 (15%) |
| Lymphatic invasion (p) | |
| L0 | 30 (50%) |
| L1 | 21 (35%) |
| NA | 9 (15%) |
| n = 49 | |
| Neoadjuvant therapy | |
| Only CT 5 | 32 (65.31%) |
| Only ET 6 | 7 (14.29%) |
| CT + HT | 3 (6.12%) |
| Combinatory CT/ET/RTE 7 | 2 (4.08%) |
| Her2+ TT 8 | 4 (8.16%) |
| NA | 1 (2.04%) |
| Miller–Payne system | |
| Grade 1 | 14 (28.57%) |
| Grade 2 | 4 (8.16%) |
| Grade 3 | 13 (26.53%) |
| Grade 4 | 5 (10.2%) |
| Grade 5 | 8 (16.33%) |
| NA | 5 (10.2%) |
| RCB 9 | |
| RCB 0 | 8 (16.33%) |
| RCB-I | 5 (10.2%) |
| RCB-II | 15 (30.61%) |
| RCB-III | 16 (32.65%) |
| NA | 5 (10.2%) |
| Clinicopathological Features | miR-375-3p | miR-210-3p | let-7e-5p |
|---|---|---|---|
| p-Value | p-Value | p-Value | |
| Age ≤50 vs. >50 | 0.116 | 0.709 | 0.682 |
| Grading_Biopsy G1 vs. 1 G2 vs. G3 | 0.007 ** FR 2 G3 vs. G1 * = −2.42 FR G3 vs. G2 ** = −3.08 | 0.783 | 0.118 |
| ER 3 [21] Negative (<1) vs. Positive (≥1) | 0.0008 *** FR ER+ vs. ER- = 6.13 | 0.970 | 0.209 |
| PgR 4 [21] Negative (<1) vs. Positive (≥1) | 0.009 ** FR PgR+ vs. PgR- = 2.28 | 0.384 | 0.305 |
| Ki-67 [22] Negative (≤20) vs. Positive (>20) | 0.042 * FR Ki-67+ vs. Ki-67- = −1.49 | 0.187 | 0.052 |
| Molecular Subtype LuminalA (LumA) vs. LuminalB (LumB) vs. TNBC 5 | 0.004 ** FR TNBC vs. LumA *** = −9.80 FR TNBC vs. LumB ** = −7.28 | 0.332 | 0.296 |
| cT 6 T1 vs. T2 vs. T3 vs. T4 | 0.341 | 0.278 | 0.885 |
| cN 7 Negative (N0) vs. Positive (N1 + N2 + N3) | 0.799 | 0.879 | 0.350 |
| Clinical Stage Early (Stage I + II) vs. Advanced (Stage III + Stage IV) | 0.343 | 0.834 | 0.463 |
| pT 8 T0 vs. T1 vs. T2 | 0.261 | 0.555 | 0.749 |
| pN 9 Negative (N0) vs. Positive (N1 + N2 + N3) | 0.469 | 0.815 | 0.929 |
| Lymphatic Invasion L0 vs. L1 | 0.043 * FR L1 vs. L0 = 1.94 | 0.064 | 0.853 |
| Miller–Payne Low (1+2) vs. Intermediate (3) vs. High (4 + 5) | 0.019 * FR High vs. Intermediate * = −3.03 FR Intermediate vs. Low * = 3.14 | 0.016 * FR High vs. Low * = 2.54 FR Intermediate vs. Low * = 2.06 | 0.409 |
| RCB 10 0/I vs. II vs. III | 0.416 | 0.023 * FR 0/I vs. III ** = 2.87 | 0.475 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisencu, L.A.; Roman, A.; Visan, S.; Bonci, E.-A.; Pașca, A.; Grigorescu, E.; Mustea, E.; Cismaru, A.; Irimie, A.; Lisencu, C.; et al. The Role of miR-375-3p, miR-210-3p and Let-7e-5p in the Pathological Response of Breast Cancer Patients to Neoadjuvant Therapy. Medicina 2022, 58, 1494. https://doi.org/10.3390/medicina58101494
Lisencu LA, Roman A, Visan S, Bonci E-A, Pașca A, Grigorescu E, Mustea E, Cismaru A, Irimie A, Lisencu C, et al. The Role of miR-375-3p, miR-210-3p and Let-7e-5p in the Pathological Response of Breast Cancer Patients to Neoadjuvant Therapy. Medicina. 2022; 58(10):1494. https://doi.org/10.3390/medicina58101494
Chicago/Turabian StyleLisencu, Lorena Alexandra, Andrei Roman, Simona Visan, Eduard-Alexandru Bonci, Andrei Pașca, Emilia Grigorescu, Elena Mustea, Andrei Cismaru, Alexandru Irimie, Cosmin Lisencu, and et al. 2022. "The Role of miR-375-3p, miR-210-3p and Let-7e-5p in the Pathological Response of Breast Cancer Patients to Neoadjuvant Therapy" Medicina 58, no. 10: 1494. https://doi.org/10.3390/medicina58101494
APA StyleLisencu, L. A., Roman, A., Visan, S., Bonci, E.-A., Pașca, A., Grigorescu, E., Mustea, E., Cismaru, A., Irimie, A., Lisencu, C., Balacescu, L., Balacescu, O., & Tudoran, O. (2022). The Role of miR-375-3p, miR-210-3p and Let-7e-5p in the Pathological Response of Breast Cancer Patients to Neoadjuvant Therapy. Medicina, 58(10), 1494. https://doi.org/10.3390/medicina58101494








