Crohn’s Disease and Jejunal Artery Aneurysms: A Report of the First Case and a Review of the Literature
Abstract
1. Introduction
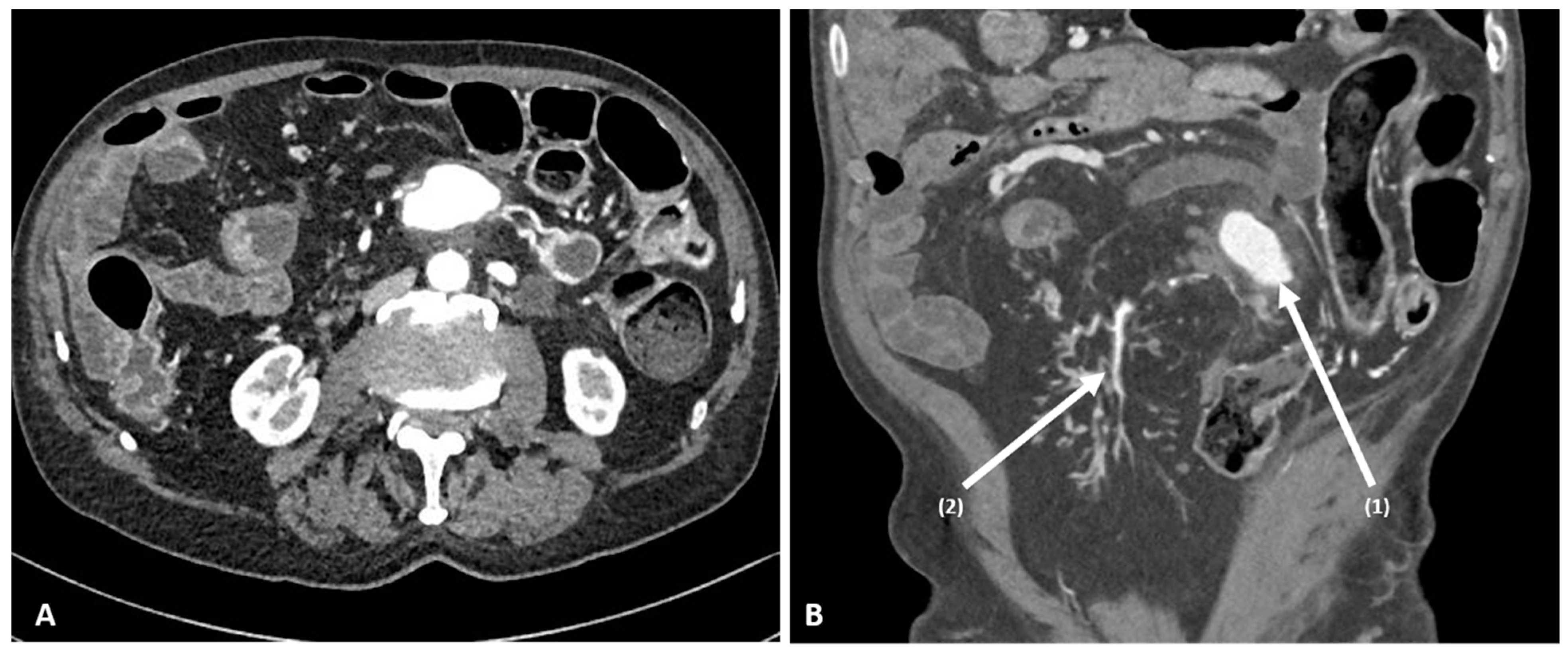
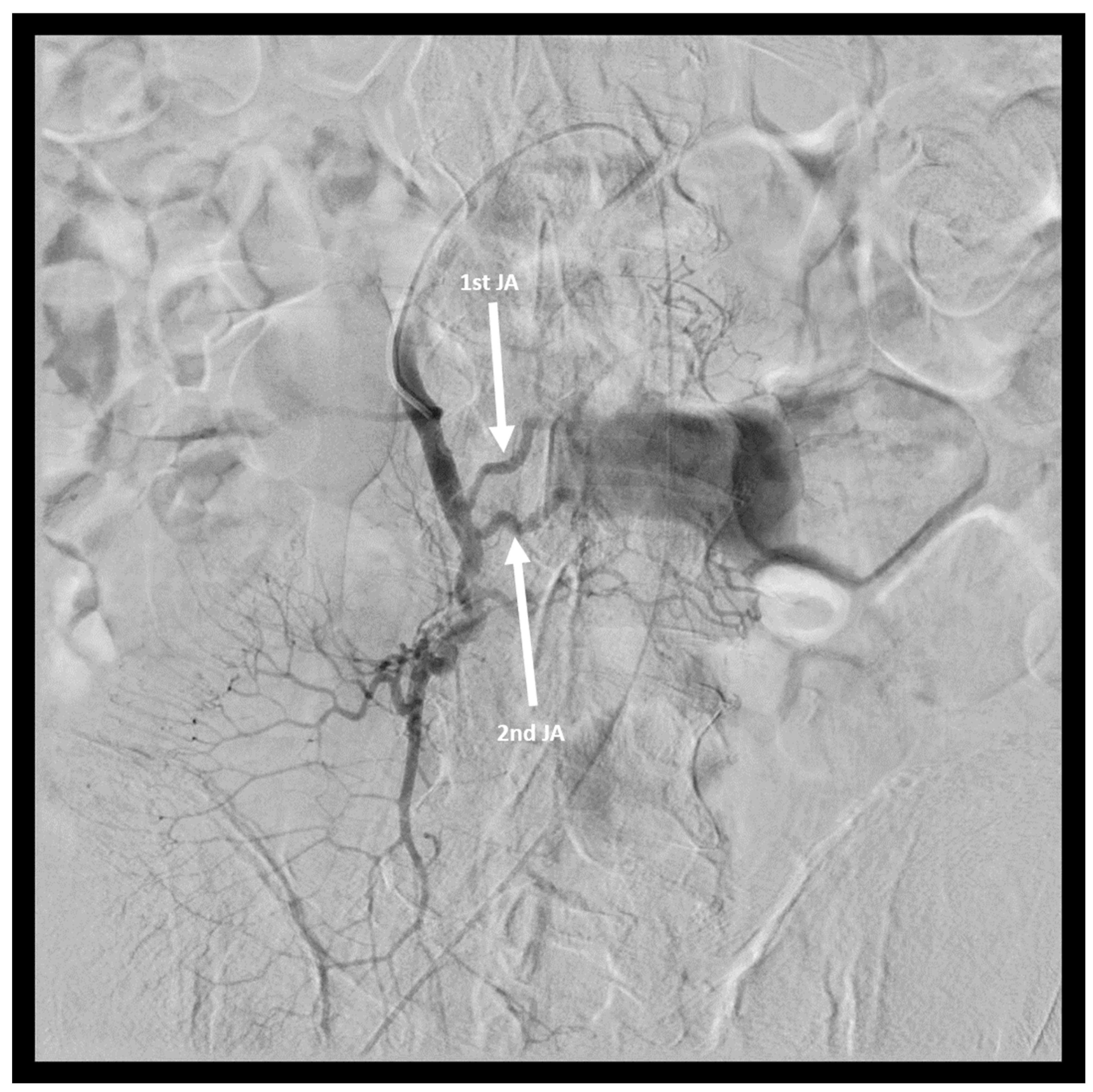
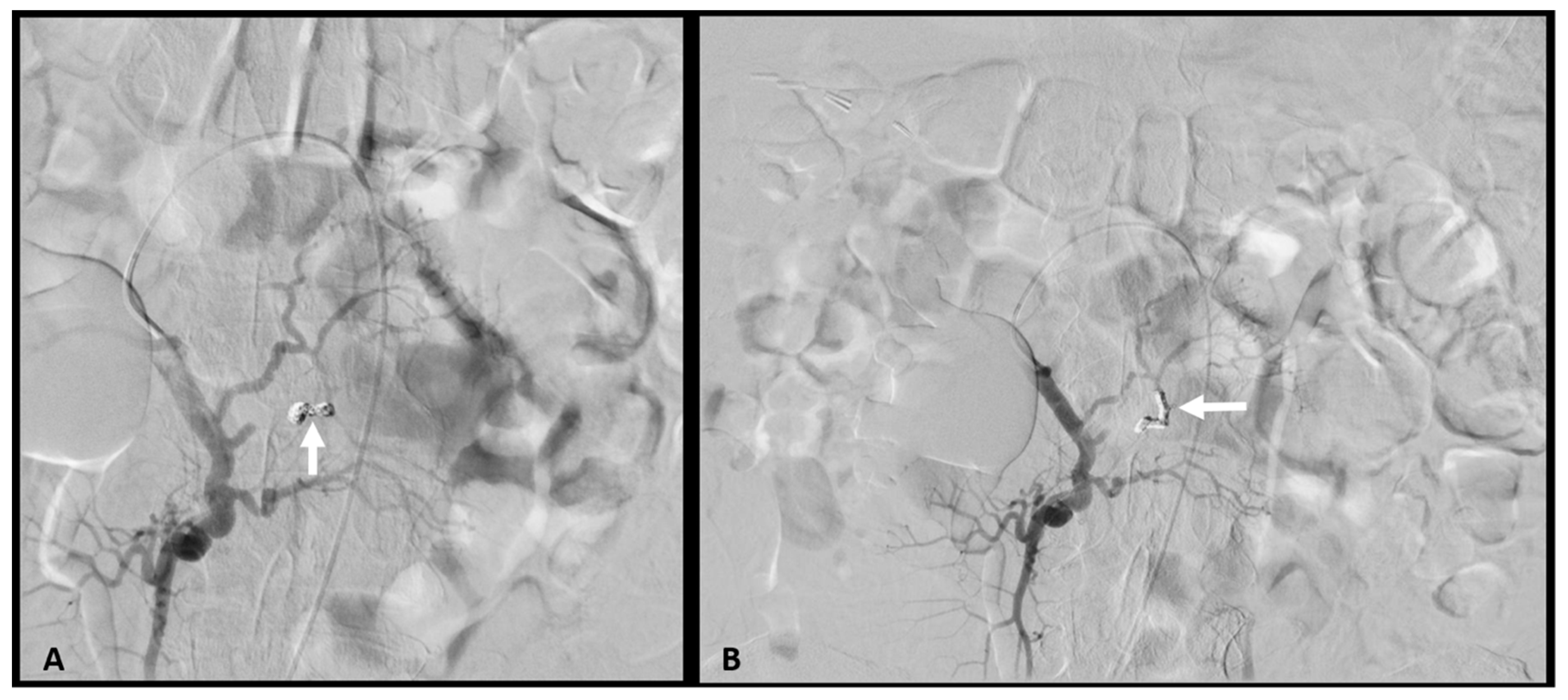
2. Case Report
3. Materials and Methods
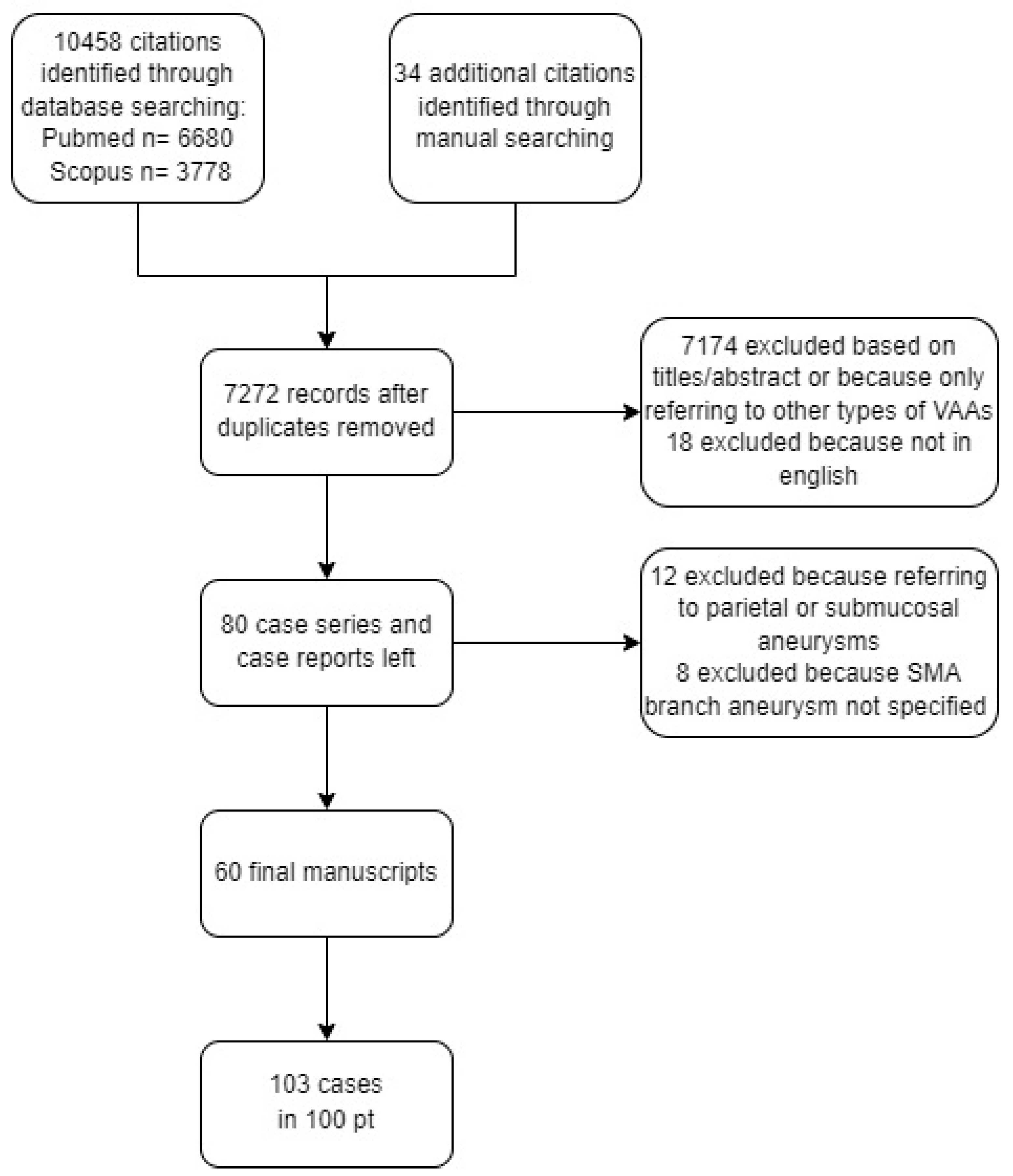
3.1. Literature Review
3.2. Statistical Analysis
4. Results
4.1. Study Population
4.2. Rupture Group
4.3. Non-Rupture Group
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johal, M.; Kalaravy, M.; Ali, F.; Barve, R.; Ahmed, A.; Francis, C.T.; Harky, A. Evolving Diagnostic and Therapeutic Options for Visceral Artery Aneurysms. Ann. Vasc. Surg. 2021, 76, 488–499. [Google Scholar] [CrossRef]
- Obara, H.; Kentaro, M.; Inoue, M.; Kitagawa, Y. Current management strategies for visceral artery aneurysms: An overview. Surg. Today 2020, 50, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Pulli, R.; Dorigo, W.; Troisi, N.; Pratesi, G.; Innocenti, A.A.; Pratesi, C. Surgical treatment of visceral artery aneurysms: A 25-year experience. J. Vasc. Surg. 2008, 48, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Thompson, N.W.; Fry, W.J. Splanchnic Artery Aneurysms. Arch. Surg. 1970, 101, 689–697. [Google Scholar] [CrossRef]
- Deterling, R.A. Aneurysm of the visceral arteries. J. Cardiovasc. Surg. 1971, 12, 309–322. [Google Scholar]
- Corey, M.R.; Ergul, E.A.; Cambria, R.P.; English, S.J.; Patel, V.I.; Lancaster, R.T.; Kwolek, C.J.; Conrad, M.F. The natural history of splanchnic artery aneurysms and outcomes after operative intervention. J. Vasc. Surg. 2016, 63, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Erben, Y.; Brownstein, A.J.; Rajaee, S.; Li, Y.; Rizzo, J.A.; Mojibian, H.; Ziganshin, B.A.; Elefteriades, J.A. Natural history and management of splanchnic artery aneurysms in a single tertiary referral center. J. Vasc. Surg. 2018, 68, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Pitton, M.B.; Dappa, E.; Jungmann, F.; Kloeckner, R.; Schotten, S.; Wirth, G.M.; Mittler, J.; Lang, H.; Mildenberger, P.; Kreitner, K.-F.; et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur. Radiol. 2015, 25, 2004–2014. [Google Scholar] [CrossRef]
- Habib, N.; Hassan, S.; Abdou, R.; Torbey, E.; Alkaied, H.; Maniatis, T.; Azab, B.; Chalhoub, M.; Harris, K. Gastroduodenal artery aneurysm, diagnosis, clinical presentation and management: A concise review. Ann. Surg. Innov. Res. 2013, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Ng, S.C.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 1–19. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis.-A-Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, T.K.; Horwell, R.J.; Greeley, J.P. Congenital aneurysm of the jejunum producing fatal intestinal hemorrhage. A.M.A. Arch. Pathol. 1951, 51, 461–465. [Google Scholar]
- Horton, R.E. Ruptured mycotic aneurysm of the superior mesenteric artery. Br. J. Surg. 1959, 46, 541–542. [Google Scholar] [CrossRef]
- Hug, H.; Branch, C.D. Congenital aneurysm of the jejunum. Am. J. Surg. 1961, 102, 859–860. [Google Scholar] [CrossRef]
- Reuter, S.R. Mesenteric Artery Branch Aneurysms. Arch. Surg. 1968, 97, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, J.G.; Bartholomew, L.G.; Osmundson, P.J.; Wallace, R.B. Aneurysms of the mesenteric artery. Am. J. Surg. 1968, 115, 832–834. [Google Scholar] [CrossRef]
- Gueco, J.M.; Rosenberg, N.; Hart, J.T.; Smith, D.W. Ruptured aneurysm of jejunum with massive intestinal bleeding. Am. Surg. 1969, 35, 643–647. [Google Scholar]
- Han, S.Y.; Jander, H.P.; Laws, H.L. Polyarteritis nodosa causing severe intestinal bleeding. Gastrointest. Radiol. 1976, 1, 285–287. [Google Scholar] [CrossRef]
- Keehan, M.F.; Kistner, R.L.; Banis, J. Angiography as an Aid in Extra-enteric Gastrointestinal Bleeding Due to Visceral Artery Aneurysm. Ann. Surg. 1978, 187, 357–361. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.F.; Griska, L.B. Superior mesenteric artery branch aneurysms. Surgery 1980, 88, 625–630. [Google Scholar]
- Skudder, P.A.; Craver, W.L. Mesenteric Hematoma Suggests Rupture of Visceral Artery Aneurysm. Arch. Surg. 1984, 119, 863. [Google Scholar] [CrossRef]
- Wilson, J.W.; Ellis, D.; Leyden, M.J.; Thomas, R.; Sullivan, J.R. Mycotic aneurysm of the small bowel presenting as gastrointestinal haemorrhage. Med. J. Aust. 1984, 141, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Bleichrodt, R.P.; Smulders, T.A.; Schreuder, F.; Tinbergen, W.; Müller, W.F. Aneurysms of the jejunal artery. J. Cardiovasc. Surg. 1984, 25, 376–377. [Google Scholar]
- Diettrich, N.A.; Cacioppo, J.C.; Ying, D.P.W. Massive gastrointestinal hemorrhage caused by rupture of a jejunal branch artery aneurysm. J. Vasc. Surg. 1988, 8, 187–189. [Google Scholar] [CrossRef][Green Version]
- Ku, A.; Kadir, S. Embolization of a mesenteric artery aneurysm: Case report. Cardiovasc. Interv. Radiol. 1990, 13, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, C.-G.; Stridbeck, H. Aneurysms of the superior mesenteric artery and its branches. Gastrointest. Radiol. 1992, 17, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Namimoto, T.; Morishita, S.; Saitoh, R.; Oguni, T.; Makita, O.; Takahashi, M.; Tanaka, M.; Okamoto, M.; Kaneko, Y.; et al. Embolization for ruptured superior mesenteric artery aneurysms. Br. J. Radiol. 1996, 69, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Kubota, J.; Tsunemura, M.; Amano, S.; Tokizawa, S.; Oowada, S.; Shinkai, H.; Maehara, Y.; Endo, K. Non-Marfan idiopathic medionecrosis (cystic medial necrosis) presenting with multiple visceral artery aneurysms and diffuse connective tissue fragility: Two brothers. Cardiovasc. Interv. Radiol. 1997, 20, 225–227. [Google Scholar] [CrossRef]
- Røkke, O.; Søndenaa, K.; Amundsen, S.R.; Larssen, T.B.; Jensen, D. Successful management of eleven splanchnic artery aneurysms. Eur. J. Surg. 1997, 163, 411–417. [Google Scholar]
- Weinstock, L.B.; Wu, J.S.; Malden, E.S.; Garcia, K.M.; Rubin, B.G.; Brunt, L. Small bowel obstruction resulting from mesenteric hematoma caused by spontaneous rupture of a jejunal branch artery. Gastrointest. Endosc. 1999, 49, 537–540. [Google Scholar] [CrossRef]
- Dongola, N.A.; Foord, K.D. Angiographic features associated with antiphospholipid syndrome. Br. J. Radiol. 2000, 73, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.C.; Mahvi, D.M.; Hoch, J.R.; Archer, C.W.; Turnipseed, W.D. Visceral artery aneurysm rupture. J. Vasc. Surg. 2001, 33, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Oran, I.; Parildar, M.; Memis, A. Mesenteric artery aneurysms in intestinal tuberculosis as a cause of lower gastrointestinal bleeding. Gastrointest. Radiol. 2001, 26, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Matsumoto, M.; Ahn, T.; Adachi, S.; Oku, K.; Takagi, M.; Fukui, H.; Yoshikawa, M. Microscopic polyangiitis complicated with massive intestinal bleeding. J. Gastroenterol. 2001, 36, 264–270. [Google Scholar] [CrossRef]
- Tessier, D.J.; Abbas, M.A.; Fowl, R.J.; Stone, W.M.; Bower, T.C.; McKusick, M.A.; Gloviczki, P. Management of Rare Mesenteric Arterial Branch Aneurysms. Ann. Vasc. Surg. 2002, 16, 586–590. [Google Scholar] [CrossRef]
- Gabelmann, A.; Görich, J.; Merkle, E.M. Endovascular Treatment of Visceral Artery Aneurysms. J. Endovasc. Ther. 2002, 9, 38–47. [Google Scholar] [CrossRef]
- Chiu, H.-M.; Wang, H.-P.; Lin, M.-T.; Lee, Y.-C.; Wu, M.-S.; Lin, J.-T. Color Doppler sonography for preoperative diagnosis of an aneurysm of the ileal branch of the superior mesenteric artery. J. Clin. Ultrasound 2002, 30, 308–311. [Google Scholar] [CrossRef]
- Morra, A.; Rimondini, A.; Adovasio, R. Jejunal artery aneurysm: Diagnostic efficacy of spiral CT angiography. A case report. La Radiol. Med. 2002, 104, 95–98. [Google Scholar]
- Lorelli, D.R.; Cambria, R.A.; Seabrook, G.R.; Towne, J.B. Diagnosis and Management of Aneurysms Involving the Superior Mesenteric Artery and Its Branches. Vasc. Endovasc. Surg. 2003, 37, 59–66. [Google Scholar] [CrossRef]
- Kahn, S.A.; Kirschner, B.S. Massive Intestinal Bleeding in a Child With Superior Mesenteric Artery Aneurysm and Gastrointestinal Tuberculosis. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Shimohira, M.; Ogino, H.; Kitase, M.; Takeuchi, M.; Shibamoto, Y. Embolization for asymptomatic aneurysms of the first jejunal artery. Vasa 2006, 35, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Bavunoğlu, I.; Ayan, F.; Karabicak, I.; Ledamo, Y.; Sayilgan, C.; Numan, F.; Sirin, F. Selective jejunal artery pseudoaneurysm embolization in a patient with massive gastrointestinal bleeding due to intestinal tuberculosis. J. Emerg. Med. 2006, 31, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.E.; Stonerock, C.E.; Dalsing, M.C. Multiple Giant Superior Mesenteric Artery Branch Aneurysms. Ann. Vasc. Surg. 2007, 21, 280–283. [Google Scholar] [CrossRef]
- Yan, S.-L.; Wu, H.-S.; Chou, D.-A.; Kuo, C.-L.; Huang, H.-T.; Lee, Y.-T.; Huang, M.-H. Pseudoaneurysm of Superior Mesentery Artery Branch After Renal Extracorporeal Shock Wave Lithotripsy: Case Report and Review. J. Trauma Inj. Infect. Crit. Care 2007, 62, 770–774. [Google Scholar] [CrossRef]
- Asano, M.; Nushida, H.; Nagasaki, Y.; Tatsuno, Y.; Ueno, Y. Rupture of a jejunal artery aneurysm. Leg. Med. 2008, 10, 268–273. [Google Scholar] [CrossRef]
- Turkbey, B.; Peynircioglu, B.; Akpinar, E.; Cil, B.E.; Karcaaltincaba, M. Isolated Aneurysm of the Distal Branch of the Jejunal Artery: MDCT Angiographic Diagnosis and Endovascular Management. Cardiovasc. Interv. Radiol. 2007, 31, 34–37. [Google Scholar] [CrossRef]
- Garwood, E.R.; Kumar, A.S.; Hirvela, E. Spontaneous hemoperitoneum from a ruptured mesenteric branch arterial aneurysm: Report of a case. Surg. Today 2009, 39, 721–724. [Google Scholar] [CrossRef]
- Yamasaki, A.; Tomita, K.; Fujii, Y.; Hasegawa, Y.; Watanabe, M.; Sano, H.; Okazaki, R.; Ouchi, Y.; Nakamura, S.; Shimizu, E. Repressed ileal artery aneurysms in Churg-Strauss syndrome following combination treatment with glucocorticoid and cyclophosphamide. Rheumatol. Int. 2008, 29, 335–337. [Google Scholar] [CrossRef]
- Kurdal, A.T.; Cerrahoglu, M.; Iskesen, I.; Sirin, H. Superior mesenteric artery branch—Jejunal artery aneurysm. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 859–861. [Google Scholar] [CrossRef]
- Rossi, U.G.; Seitun, S.; Ferro, C. Endovascular embolization of a third jejunal artery aneurysm: Isolation technique using the amplatzer vascular plug 4. Catheter. Cardiovasc. Interv. 2012, 81, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Costa, A.; Pereira, T.; Maciel, J. Ruptured jejunal artery aneurysm. BMJ Case Rep. 2013, 2013, bcr2013008772. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; McCulloch, N.; Forde, C.; Mahon, B.; Mangat, K.; Olliff, S.; Jones, R. Emergency Treatment of Haemorrhaging Coeliac or Mesenteric Artery Aneurysms and Pseudoaneurysms in the Era of Endovascular Management. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 382–389. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Wei, J.-P.; Zhao, X.-Y.; Wang, Y.; Wu, H.-H.; Shi, T.; Liu, T.; Liu, G. Spontaneous Intra-Abdominal Hemorrhage Due to Rupture of Jejunal Artery Aneurysm in Behcet Disease. Medicine 2015, 94, e1979. [Google Scholar] [CrossRef] [PubMed]
- Lo, Z.; Leow, J.; Tan, K.; Tan, G. Successful endovascular embolisation of a jejunal artery aneurysm. Singap. Med. J. 2015, 56, e46–e48. [Google Scholar] [CrossRef] [PubMed]
- Breguet, R.; Pupulim, L.F.; Terraz, S. Embolization of a Jejunal Artery Pseudoaneurysm via Collateral Vessels. Case Rep. Surg. 2015, 2015, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ray, S.; Khamrui, S. Ruptured Jejunal Artery Aneurysm. Am. Surg. 2015, 81, 130–131. [Google Scholar] [CrossRef]
- Arer, I.M.; Gedikoglu, M.; Yabanoglu, H.; Noyan, M.T. Rupture of an Aneurysm of a Small Branch of the Superior Mesenteric Artery: A Case Report. Pol. J. Radiol. 2016, 81, 354–356. [Google Scholar] [CrossRef]
- Guirgis, M.; Xu, J.H.; Kaard, A.; Mwipatayi, B.P. Spontaneous Superior Mesenteric Artery Branch Pseudoaneurysm: A Rare Case Report. EJVES Short Rep. 2017, 37, 1–4. [Google Scholar] [CrossRef]
- Kaihara, M.; Ono, S.; Shibutani, S.; Funabiki, T.; Egawa, T. A Rare Surgical Case of Giant Jejunal Artery Aneurysm in a Young Patient. Ann. Vasc. Surg. 2018, 50, 297.e5–297.e8. [Google Scholar] [CrossRef]
- Toya, T.; Uehara, K.; Ito, Y.; Sasaki, H.; Matsuda, H. Surgical Treatment of Jejunal Artery Aneurysm. EJVES Short Rep. 2018, 40, 15–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minaya-Bravo, A.M.; Vera-Mansilla, C.; Ruiz-Grande, F. Presentation of a large jejunal artery aneurysm: Management and review of the literature. Int. J. Surg. Case Rep. 2018, 48, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Takata, N.; Watanabe, A.; Fukai, S.; Yoshikawa, K.; Sasamatsu, S.; Lefor, A.K.; Sakamoto, T.; Mizokami, K.; Kanzaki, M.; et al. Pseudoaneurysm of an ileal mesenteric artery after a stapled anastomosis. Surgery 2018, 163, 968–969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Tang, J. An Unruptured Jejunal Aneurysm in a Female Patient with Melena Caused by Arteriovenous Malformation. J. Gastrointest. Surg. 2020, 25, 1073–1075. [Google Scholar] [CrossRef]
- Gogeneata, I.; Lejay, A. A 39 mm Diameter Aneurysm of a Jejunal Artery. EJVES Vasc. Forum 2021, 52, 25. [Google Scholar] [CrossRef]
- Murakawa, M.; Arai, S.; Kawagoe, M.; Tomomitsu, Y.; Odajima, K.; Ueno, M.; Asakawa, S.; Hirohama, D.; Nagura, M.; Yamazaki, O.; et al. A Ruptured Jejunal Arterial Aneurysm in a Young Woman Undergoing Chronic Hemodialysis Due to Myeloperoxidase-antineutrophil Cytoplasmic Antibody-associated Vasculitis. Intern. Med. 2021, 60, 2939–2945. [Google Scholar] [CrossRef]
- Shimohira, M.; Kondo, H.; Ogawa, Y.; Kawada, H.; Koganemaru, M.; Ikeda, O.; Yamamoto, A.; Komada, T.; Tanoue, S.; Muraoka, N.; et al. Natural History of Unruptured Visceral Artery Aneurysms Due to Segmental Arterial Mediolysis and Efficacy of Transcatheter Arterial Embolization: A Retrospective Multiinstitutional Study in Japan. Am. J. Roentgenol. 2021, 216, 691–697. [Google Scholar] [CrossRef]
- Anwar, M.N.; Anthony, N.; Amin, Q.K.; Yousafzai, Z.A.; Khalil, H. An Undiagnosed Case of Chronic Pancreatitis With Multiple Visceral Arteries Pseudoaneurysm. Cureus 2021, 13, e14789. [Google Scholar] [CrossRef]
- Yadav, A.; Godasu, G.; Buxi, T.B.S.; Sheth, S. Multiple Artery Aneurysms: Unusual Presentation of IgG4 Vasculopathy. J. Clin. Imaging Sci. 2021, 11, 17. [Google Scholar] [CrossRef]
- Pitcher, G.S.; Cirillo-Penn, N.C.; Mendes, B.C.; Shuja, F.; DeMartino, R.R.; Kalra, M.; Bower, T.C.; Harmsen, W.S.; Colglazier, J.J. Aneurysms of the superior mesenteric artery and its branches. J. Vasc. Surg. 2022, 76, 149–157. [Google Scholar] [CrossRef]
- Tipaldi, M.A.; Krokidis, M.; Orgera, G.; Pignatelli, M.; Ronconi, E.; Laurino, F.; Laghi, A.; Rossi, M. Endovascular management of giant visceral artery aneurysms. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Malas, M.B.; Nejim, B.; Haddad, A.; Morrow, A.; Ponce, O.; Hasan, B.; Seisa, M.; Chaer, R.; Murad, M.H. A systematic review and meta-analysis of the management of visceral artery aneurysms. J. Vasc. Surg. 2019, 70, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Chaer, R.A.; Abularrage, C.J.; Coleman, D.M.; Eslami, M.H.; Kashyap, V.S.; Rockman, C.; Murad, M.H. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J. Vasc. Surg. 2020, 72, 3S–39S. [Google Scholar] [CrossRef] [PubMed]
- Sy, A.; Khalidi, N.; Dehghan, N.; Barra, L.; Carette, S.; Cuthbertson, D.; Hoffman, G.S.; Koening, C.L.; Langford, C.A.; McAlear, C.; et al. Vasculitis in patients with inflammatory bowel diseases: A study of 32 patients and systematic review of the literature. Semin. Arthritis Rheum. 2016, 45, 475–482. [Google Scholar] [CrossRef]
- Shukla, A.J.; Eid, R.; Fish, L.; Avgerinos, E.; Marone, L.; Makaroun, M.; Chaer, R.A. Contemporary outcomes of intact and ruptured visceral artery aneurysms. J. Vasc. Surg. 2015, 61, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Total pt n. | JAAs/ IAAs pt n. | Age/ Sex | Site/ n | TRUE | Cause | Size (mm) | Rupture/ Pt n. | Main symptoms | MH/ HPT | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reuter [16] | 1968 | 4 | 4 | 39/F | J | CONG | 6 | no | - | aneurysmectomy | uneventful | ||
| 72/F | I | yes | ATS | 3.5 | no | asymptomatic | - | - | - | ||||
| 57/F | I | ATS | 11 | no | - | - | - | ||||||
| 76/M | I | ATS | 4 | yes | hypotension | MH | RHC | death (MI) | |||||
| Hoehn [17] | 1968 | 2 | 2 | 60/F | J | n.r. | ID | n.r. | yes | shock | MH | e-lap | uneventful |
| 50/F | J | yes | CONG | 12 | no | chronic pain | - | aneurysmectomy | uneventful | ||||
| Stanley [4] | 1970 | 45 | 3 | 71/M | J | - | - | ||||||
| 68/M | I | yes | ATS | n.r. | no | asymptomatic | - | - | - | ||||
| 74/M | I | aneurysmectomy | uneventful | ||||||||||
| Tessier [36] | 2002 | 12 | 5 | 69/F | J | n.r. | n.r. | 30 | yes | acute pain | no | ligation | uneventful |
| 48/F | J | n.r. | n.r. | 15 | yes | acute pain | no | ligation | uneventful | ||||
| 79/M | J | n.r. | n.r. | 10 | no | asymptomatic | - | - | - | ||||
| 56/M | J/1; I/1 | no | PAN | n.r. | yes | acute pain | no | RHC | uneventful | ||||
| 72/M | I | n.r. | n.r. | n.r. | no | asymptomatic | - | - | - | ||||
| Roberts [53] | 2015 | 48 | 3 | n.r. | n.r. | death (MI) | |||||||
| 26/M | I | n.r. | n.r. | n.r. | no | GI bleeding | - | embolization | uneventful | ||||
| n.r. | n.r. | uneventful | |||||||||||
| Corey [6] | 2016 | 250 | 3 | n.r./M | yes | ATS | 8 | - | - | ||||
| n.r/M | J | no | VASC | 30 | no | asymptomatic | - | aneurysmectomy | uneventful | ||||
| n.r./F | n.r. | n.r. | 30 | CE | uneventful | ||||||||
| Shimohira [67] | 2021 | 45 | 4 | n.r. | n.r. | yes | SAM | n.r. | yes/2 | n.r. | n.r. | CE | uneventful |
| no/2 | asymptomatic | - | - | - | |||||||||
| Pitcher [70] | 2022 | 131 | 24 | n.r. | J | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. | n.r. |
| Author | Year | Age/ Sex | Site/ n | True | Cause | Size (mm) | Clinical Picture | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Rathmell [13] | 1951 | 29/F | J | yes | CONG | 5 | GI bleeding | e-lap | death |
| Horton [14] | 1959 | 31/M | I | no | IE | 50 | acute pain, fever | SBR | uneventful |
| Hug [15] | 1961 | 22/F | J | yes | CONG | 5 | GI bleeding, acute pain, hypotension | SBR | uneventful |
| Gueco [18] | 1969 | 20/F | J | yes | CONG | 8 | GI bleeding | SBR | uneventful |
| Han [19] | 1976 | 50/F | J | no | PAN | n.r. | GI bleeding, acute pain, shock, ME | RHC | death |
| Skudder [22] | 1984 | 61/M | J | n.r. | n.r. | 15 | acute pain, shock, ME, HPT | ligation | death |
| Wilson [23] | 1984 | 65/M | J | no | IE | 15 | GI bleeding | SBR | uneventful |
| Bleichrodt * [24] | 1984 | 16/M | J | no | TR | n.r. | GI bleeding | ligation | uneventful |
| Diettrich [25] | 1988 | 28/M | J | yes | CONG | 6 | GI bleeding shock | SBR | uneventful |
| Ishii [28] | 1996 | 76/F | J/2 | no | AP | 10 both | shock, HPT | CE | uneventful |
| Rokke [30] | 1997 | 73/M | J | no | abscess in PD | 5 | GI bleeding | CE | uneventful |
| Weinstock [31] | 1999 | 57/M | J | n.r. | n.r. | 5 | SBO, ME | GJ | uneventful |
| Carr [33] | 2001 | n.r. | I | yes | ATS | n.r. | GI bleeding | ligation | n.r. |
| Oran [34] | 2001 | 41/F | J | no | GI TB | 5 | GI bleeding | CE | uneventful |
| Ueda [35] | 2001 | 74/M | I | no | MPA | n.r. | GI bleeding | CE | death |
| Kahn [41] | 2006 | 12/F | J | no | GI TB | n.r. | GI bleeding | CE | uneventful |
| Bavunoglu [43] | 2006 | 16/M | J | no | GI TB | n.r. | GI bleeding, acute pain, hypotension | GE | death |
| Asano [46] | 2008 | 66/M | J | n.r. | n.r. | 10 | shock, HPT | aneurysmectomy | death |
| Garwood [48] | 2009 | 54/F | J | yes | ATS | n.r. | shock, HPT, ME | aneurysmectomy | uneventful |
| Yamasaki [49] | 2009 | 51/M | I | no | CSS | n.r. | GI bleeding | SBR | uneventful |
| Costa [52] | 2013 | 76/M | J | yes | SAM | 52 | acute pain, hypotension, HPT | aneurysmectomy followed by SBR | uneventful |
| Wu [54] | 2015 | 35/M | J | no | BD | 4.8 | acute pain, hypotension, HPT | SBR | uneventful |
| Ray [57] | 2015 | 59/F | J | n.r. | n.r. | 23 | acute pain, hypotension, HPT | aneurysmectomy | uneventful |
| Arer [58] | 2016 | 68/M | J | no | ACD | n.r. | GI bleeding | CE | uneventful |
| Kimura [63] | 2018 | 47/F | I | no | stapler anastomosis | n.r. | acute pain | CE | uneventful |
| Murakawa [66] | 2021 | 21/F | J | no | AAV | n.r. | acute pain, GI bleeding | embolization | uneventful |
| Author | Year | Age/ Sex | Site | True | Cause | Size (mm) | Clinical Picture | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Keehan [20] | 1978 | 21/M | J | no | IE | 50 | acute pain | aneurysmectomy | uneventful |
| McNamara [21] | 1980 | 72/F | J | yes | ATS | 40 | acute pain, vomiting | aneurysmectomy | uneventful |
| Bleichrodt * [24] | 1984 | 16/M | J | no | TR | n.r. | n.r. | ligation | uneventful |
| Ku [26] | 1990 | 50/M | J | no | CP | 12 | chronic pain | PE | uneventful |
| Lindberg [27] | 1992 | 44/M | J | no | IE | 5 | asymptomatic | - | - |
| Kubota [29] | 1997 | 32/M | J | yes | IM | n.r. | asymptomatic | - | - |
| Dongola [32] | 2000 | 39/M | J | no | primary APS | n.r. | GI bleeding, acute pain, shock | RPH evacuation | death |
| Gabelmann [37] | 2002 | 78/M | J | no | IE | 15 | asymptomatic | CE | uneventful |
| Chiu [38] | 2002 | 60/M | I | yes | ATS | 30 | acute pain | aneurysmectomy | uneventful |
| Morra [39] | 2002 | 70/F | J | n.r. | n.r. | 50 | acute pain | aneurysmectomy | uneventful |
| Lorelli [40] | 2003 | 58/F | J | no | CP | 20 | chronic pain | CE | uneventful |
| Shimohira [42] | 2006 | 71/M | J | yes | ATS | 10 | asymptomatic | CE | uneventful |
| Sohn [44] | 2007 | 73/F | J | yes | ATS | 45 | asymptomatic | aneurysmectomy+SVIG | uneventful |
| Yan [45] | 2007 | 53/M | J | no | bacteriemia after ESWL | 50 | acute pain | SBR | uneventful |
| Turkbey [47] | 2008 | 85/M | J | yes | ATS | 5 | GI bleeding | CE | uneventful |
| Kurdal [50] | 2010 | 62/F | J | yes | ATS | 45 | acute pain | aneurysmectomy+SVIG | uneventful |
| Rossi [51] | 2013 | 76/M | J | yes | ATS | 12 | asymptomatic | PE | uneventful |
| Lo [55] | 2015 | 57/M | J | n.r. | n.r. | 9 | asymptomatic | CE | uneventful |
| Breguet [56] | 2015 | 34/F | J | no | CP | 17 | chronic pain | CE | uneventful |
| Guirgis [59] | 2017 | 86/M | J | yes | ATS | 19 | chronic pain | CE | uneventful |
| Kaihara [60] | 2018 | 34/M | J | n.r. | n.r. | 35 | chronic pain | aneurysmectomy+SVIG | uneventful |
| Toya [61] | 2018 | 59/M | J | no | heterotopic pancreas | 17 | asymptomatic | aneurysmectomy+SVIG | uneventful |
| Minaya-Bravo [62] | 2018 | 49/F | J | yes | CONG | 50 | asymptomatic | aneurysmectomy | uneventful |
| Chen [64] | 2021 | 56/F | J | no | AVM | 18 | GI bleeding | SBR | uneventful |
| Gogeneata [65] | 2021 | 54/M | J | no | IE | 39 | acute pain, fever | aneurysmectomy | uneventful |
| Anwar [68] | 2021 | 43/F | J | no | CP | n.r. | asymptomatic | - | - |
| Yadav [69] | 2021 | 55/M | J | no | IgG4-vasculopathy | n.r. | asymptomatic | - | - |
| Author | Pt n. | Other VAAs | Site | Other Non VAAs | Site | Rupture/ Site | Treatment/ Site | Outcome |
|---|---|---|---|---|---|---|---|---|
| Hoehn [17] | 1 | yes | SA | no | - | no | no | uneventful |
| 2 | no | - | yes | intracranial | no | no | uneventful | |
| Stanley [4] | 1 | no | - | yes | AA | no | no | uneventful |
| 2 | no | - | yes | AA | no | no | uneventful | |
| Han [19] | 1 | yes | SA, HA, CA | no | - | yes/ SA, CA | splenectomy, RHC | death |
| Ku [26] | 1 | yes | GDA | no | - | no | no | uneventful |
| Lindberg [27] | 1 | yes | SMA | no | - | no | no | uneventful |
| Kubota [29] | 1 | yes | HA, SA, SMA, CA | yes | CIA | no | no | uneventful |
| Dongola [32] | 1 | yes | PDA CA | no | - | yes/PDA | RPH evacuation | death |
| Oran [34] | 1 | yes | jejunal vasa recta | no | - | no | no | uneventful |
| Tessier [36] | 1 | yes | RA, CTA | yes | thoracic, | no | no | uneventful |
| SMA, IMA, CA | intracranial | |||||||
| 2 | no | - | yes | AA | no | no | uneventful | |
| Rossi [51] | 1 | no | - | yes | AA | no | no | uneventful |
| Wu [54] | 1 | yes | SMA | no | - | no | no | uneventful |
| Anwar [68] | 1 | yes | CTA, PDA, GDA | no | - | no | CE/ PDA, GDA | uneventful |
| Yadav [69] | 1 | yes | HA, PDA, CTA | yes | coronary, intercostal | no | no | uneventful |
| Rupture Group (n = 33 pt) | No-rupture Group (n = 43 pt) | |
|---|---|---|
| Mortality, n (%) | 7 (21.2) | 2 (4.6) |
| Aneurysm-related mortality | 4 (57.1) | 1 (50) |
| Endovascular technical success rate, n (%) | 11 (100) | 12 (92.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenzi, P.; Gaudenzi, D.; Mulazzani, L.; Rebonato, A.; Patriti, A. Crohn’s Disease and Jejunal Artery Aneurysms: A Report of the First Case and a Review of the Literature. Medicina 2022, 58, 1344. https://doi.org/10.3390/medicina58101344
Vincenzi P, Gaudenzi D, Mulazzani L, Rebonato A, Patriti A. Crohn’s Disease and Jejunal Artery Aneurysms: A Report of the First Case and a Review of the Literature. Medicina. 2022; 58(10):1344. https://doi.org/10.3390/medicina58101344
Chicago/Turabian StyleVincenzi, Paolo, Diletta Gaudenzi, Luca Mulazzani, Alberto Rebonato, and Alberto Patriti. 2022. "Crohn’s Disease and Jejunal Artery Aneurysms: A Report of the First Case and a Review of the Literature" Medicina 58, no. 10: 1344. https://doi.org/10.3390/medicina58101344
APA StyleVincenzi, P., Gaudenzi, D., Mulazzani, L., Rebonato, A., & Patriti, A. (2022). Crohn’s Disease and Jejunal Artery Aneurysms: A Report of the First Case and a Review of the Literature. Medicina, 58(10), 1344. https://doi.org/10.3390/medicina58101344






