Abstract
Background and Objectives: Vitamin D (Vit. D) is known for its role in the skeletal system. Vit. D deficiency is also widely researched for its effects on the healing of fractures, bone defects, and osseointegration of implants. In the literature, there are studies that investigated the effects of dietary supplementation with Vit. D to reduce Vit. D deficiency, but increasing the serum level of this vitamin takes time. Therefore, an attempt has been made to combat the effect of Vit. D deficiency through topical applications. The aim of this article was to conduct a review of the existing bibliographic data that investigate the effect of Vit. D on bone regeneration. Materials and Methods: In order to carry out this review, an electronic search was made in several databases and the articles found were selected and analyzed. Results: The in vitro studies’ results demonstrated that Vit. D has a high therapeutic potential by enhancing the differentiation of stem cells in osteoblasts. Human and animal studies were conducting using various methods, but most of them revealed that Vit. D has a positive influence on the process of bone regeneration. Conclusions: The overall results of the research showed that, indeed, Vit. D is beneficial for bone regeneration; however, most of the studies imply that a thorough research is still needed for finding the most effective mode of administration and the dose needed in order to achieve the desired effect.
1. Introduction
One of the challenges of maxillofacial and dentoalveolar surgery is bone regeneration. In the maxillofacial area, bone can be lost due to trauma, tooth loss, periodontal disease, and other pathologies [1].
Research in this area pays special attention to finding ways to regenerate lost bone mass.
Vitamin D (Vit. D) is a steroid hormone obtained mainly from sun exposure, diet, and dietary supplements. Vit. D is the name of two compounds, Vit. D2 (ergocalciferol) and Vit. D3 (cholecalciferol). Vit. D2 is produced by ultraviolet irradiation of ergosterol, and Vit. D3 results from ultraviolet irradiation of 7-dehydrocholesterol [2,3].
Because Vit. D is involved in bone metabolism and controls the immune system, implantology pays special attention to this vitamin. At the right concentration, the effect of this prohormone positively correlates with the process of osseointegration. Studies have shown that Vit. D has an important potential in the processes of regeneration of postoperative wounds, in dental implants osseointegration, and bone homeostasis around the implant [4].
Clinical studies have shown that Vit. D3 intake of 10 μg/day and calcium supplements (adjusted calcium intake of 1000 mg/day) not only reduce bone resorption and fracture rate, but also increase bone density and the total calcium in the body. However, studies exploring the distinct effect of systemic Vit. D3 supplementation and local application of Vit. D3 on bone tissue after traumatic fracture, pathological defects, or surgically created defects in large animals are rare [3,5].
The purpose of this review is to determine the possible link between the presence/level of Vit. D and bone regeneration.
2. Materials and Methods
In order to identify the relevant articles for this review, one author (G.C.M.) chose the search terms and performed an electronic search in the following databases: PubMed, Embase, Web of Science, and Google Scholar. The research included the articles published from 1963 until July 2022.
The search terms were as follows (Extended search terms are available in the Supplementary Materials):
- –
- (bone regeneration) AND (Vit. D)
- –
- ((Vit. D) AND (osseointegration)) AND (bone regeneration)
The screening of all the articles found in all the databases was performed by two independent authors. They screened the titles and abstracts of the articles and obtained full texts and performed further screening if the studies were deemed eligible. Disagreements were resolved by re-evaluating the articles or by consulting a third person.
Exclusion criteria: reviews and meta-analysis, articles not related to the topic, articles evaluating the effects of Vit. D on the articular or muscular system, and articles regarding the protein that binds Vit. D.
Inclusion criteria: articles evaluating the effect of Vit. D on bone regeneration, osseointegration, bone healing, studies on animals and humans, in vitro and in vivo studies, case–control clinical trials, and randomized controlled trials.
In order to assess the quality of the studies included in this review, the Newcastle–Ottawa scale (NOS) [6] was used, which assesses such quality characteristics of studies as selection, comparability and exposure; studies can receive a score between zero and nine stars (Table 1).

Table 1.
The Newcastle–Ottawa scale.
3. Results
The research preliminarily identified 1758 references to bone regeneration and the effects of Vit. D, of which 290 were found in PubMed, 325—in Web of Science, 965—in Embase, and 178—in Google Scholar.
After the exclusion of 900 duplicates/articles on another topic, the remaining 858 references were verified by examining the titles and abstracts in accordance with the inclusion and exclusion criteria described above. The remaining 58 articles were examined in full, and only 27 were eligible for our review (Figure 1).
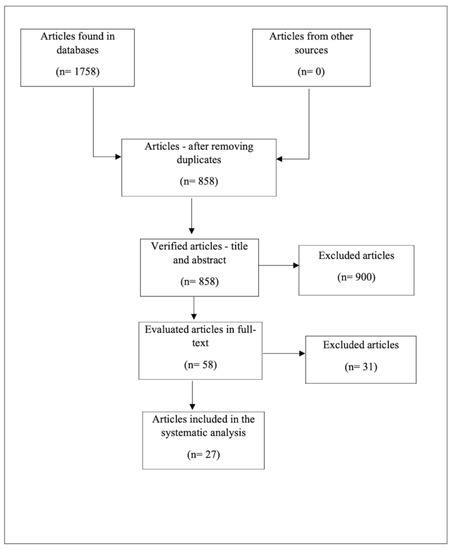
Figure 1.
Diagram representing the inclusion of studies.
Of the 27 articles included in the analysis, three studies were performed on patients, 17—on animals, and seven were in vitro studies.
Fifteen of the articles evaluated the regenerative effect of Vit. D, nine—osseointegration, three—the healing effect, and only one article reported that Vit. D does not have an effect on bone regeneration.
3.1. In Vitro Studies
The in vitro studies in this review aimed to evaluate the influence of Vit. D on fetal or adult osteoblasts, stem cells, whether administered per se or loaded onto nanoparticles and other nanostructures (Table 2).

Table 2.
Characteristics of the in vitro studies and their results.
In 2014, Rekha et al. [7] compared fetal and adult osteoblasts under the influence of Vit. D. The results of the study showed that fetal osteoblasts compared to adult osteoblasts undergo a significant increase in mineralization under the action of Vit. D, reflecting a great therapeutic potential in bone regeneration.
In 2018, Kim et al. [8] prepared osteoblast cells, to which they added 1.25-dihydroxyvitamin D3 in order to evaluate the effect of this active form of Vit. D on osteoblasts. The research results showed that 1.25-dihydroxyvitamin D3 had a positive effect on these cells; more precisely, it could function as a stimulating factor in bone regeneration.
In 2020, Abdelgawad et al. and Petrescu et al. [12,13] showed the positive effects of Vit. D alone or in combination with other substances or under the action of a laser on stem cells (dental or from the periodontal ligament), stimulating their differentiation to odontoblasts.
In 2019–2020, Nah et al., Chen et al., and Mahdavi et al. [9,10,11] conjugated Vit. D onto nanoparticles and other functional nanocarriers to enhance its bioactivity. The results of the studies showed the potential of these nanomaterials to improve osteogenic differentiation and bone regeneration.
3.2. Studies Carried out on Animals
In our review, we included 17 studies on animal subjects, specifically on rats, mice, and dogs (Table 3).

Table 3.
Characteristics of the studies on animals and their results.
In 1980, Galus et al. [14] demonstrated that Vit. D derivatives 24R,25-dihydroxyvitamin D and 1 alpha-hydroxyvitamin D accelerate the rate of appositional growth, increase the percentage of existing osteoid connections, and decrease the number, the width, and the perimeter of new osteoid connections. The authors also reported that 1 alpha-hydroxyvitamin D results in multidirectional bone remodelling that increases mineralization in concert with resorption while 24R,25-dihydroxyvitamin D stimulates bone formation and mineralization, reducing bone resorption.
In 2009, Kelly et al. [15] demonstrated that Vit. D deficiency affects the osseointegration of implants.
In the same year, Uysal et al. [16] showed the beneficial effect of a Vit. D analog in bone regeneration. They expanded the palatal median suture and applied ED-71 locally in a single dose of 0.8 μg/kg body weight.
In 2012, Hong et al. [17] showed through research on dogs that the post-extraction use of a combination of biphasic phosphate alloplastic material applied topically and Vit. D and calcium administered orally resulted in positive effects in terms of bone regeneration.
Dvorak et al. and Zhou et al. [18,19] published in 2012 the results of a study performed on ovariectomized rats. Their aim was to demonstrate the negative effects of Vit. D deficiency and the beneficial effect of Vit. D supplementation on the osseointegration of implants.
In 2014, Liu W et al. [20] investigated the effect of Vit. D on implant fixation in subjects with chronic kidney disease. The authors showed that by injecting Vit. D 8 weeks after the second intervention, they obtained an improved bone-to-implant ratio and an increased bone volume around the implant.
In 2015, Fügl et al. [22] showed in their results that Vit. D deficiency would not affect bone regeneration, and the administration of a single dose of calcitriol did not improve bone regeneration. A few months later, in the same year, Liu H et al. [21] used calcitriol-soaked collagen membranes which they applied over bone defects. The results of their research showed that these membranes accelerated bone formation and maturation.
In the same year, Hong et al. [23] supported the positive effect of Vit. D on bone regeneration. They evaluated the effects of the combination of topical application of calcitriol and oral administration of Vit. D on regeneration and bone density. The results showed that topical application of calcitriol accelerated the formation of new bone, increased bone density, but, compared to systemic Vit. D administration, topically applied calcitriol had a lower effect.
Another study that evaluated the effects of Vit. D was that of Salomó-Coll et al. [24,27] from 2016 along with that of 2018 by the same authors. They showed that soaking the implant surface with Vit. D before intraosseous application reduced the loss of crestal bone and increased the contact of the implant with the bone by 10%.
In 2017, Fischer et al. [25] evaluated whether Vit. D deficiency compromised bone regeneration and whether early Vit. D administration had beneficial effects on healing. Their results suggested that Ca/Vit. D supplementation initiated at the time of fracture offset the negative effects of Vit. D deficiency.
Another study conducted in 2017 by Han et al. [26] analyzed histological changes after systemic administration of eldecalcitol (ELD) to rats in correlation with bone regeneration. Their research found that taking ELD improved the growth of new bone mass.
In 2019, Wang et al. [29] designed two new Vit. D derivatives, 25-dihydroxyvitamin D3 and α,α-difluoro-cyclohexanone, which, according to the research, greatly improved the parameters related to bone mass and density.
In 2020, Cignachi et al. [30] assessed whether the administration of Vit. D, insulin, or Vit. D and insulin influenced bone regeneration in mice with type 1 diabetes. Their research showed that all mice regardless of sex had a similar detriment in terms of bone regeneration, and the administration of Vit. D or insulin improved bone healing.
3.3. Clinical Studies
The clinical trials included in the review consisted of three prospective randomized controlled trials, one of which was double-blind, and the other two had convenience sampling (Table 4).

Table 4.
Characteristics of the studies on patients and their results.
In 1989, Zarubina et al. [31] investigated the efficacy and differences in the action of some Vit. D derivatives (1-alpha-hydroxycholecalciferol and 1-alpha,25-dihydroxycholecalciferol) in bone regeneration in patients with osteoporosis/osteomalacia. The results demonstrated the positive effects of the two derivatives in terms of regeneration, their effectiveness being equal.
In 2019, Grønborg et al. [32] assessed the effect of eating Vit. D-fortified foods in a clinical study. That study showed that Vit. D-fortified foods did not improve bone markers, and muscle strength did not change significantly.
In 2021, Kwiatek et al. [33] presented the results of their study which evaluated the effect of 25-hydroxycholecalciferol along with the treatment of Vit. D deficiency on changes in bone levels around the implant. The study found that a correct dose on the day of surgery and adequate treatment of Vit. D deficiency had a positive effect on the increase in bone levels around the implant during osseointegration.
4. Discussion
The studies on animals showed positive effects of the administration of Vit. D, either systemically or locally. Four of these studies showed the impact of Vit. D on implants osseointegration. Salomó-Coll et al. [24,27] showed that by soaking the implants in 10% Vit. D solution before their intraosseous application, the rate of increase of the contact surface between the implant and the bone could be more than 10%. Kelly et al. and Dvorak et al. [15,18] showed that Vit. D deficiency has a negative influence on the osseointegration of implants, an effect that can be reversed by the administration of Vit. D. Zhou et al. [19] demonstrated that by administering 0.1 μg/kg/day 1.25(OH)2D3 by oral gavage for 8 weeks in rats with induced osteoporosis, they obtained an improvement in the osseointegration of the implants. The rest of the animal studies highlighted the positive effect that Vit. D has on bone regeneration, either by oral administration or by local application [5,15,21,23,25]. One single study by Fügl et al. [22] stated that Vit. D deficiency does not necessarily affect bone regeneration and that a single topical application of calcitriol does not improve healing. The study conducted by Cignachi et al. [30] showed that Vit. D improves bone regeneration even in rats with induced diabetes.
The studies on patients represented by three clinical trials evaluated the effects of Vit. D. Zarubina et al. [31] showed that Vit. D derivatives 1-alpha-hydroxycholecalciferol and 1-alpha,25-dihydroxycholecalciferol have positive effects on bone regeneration, with no differences between them. The study by Kwiatek et al. [33] reported that Vit. D administration before implant surgery associated with the treatment of Vit. D deficiency increased bone levels in implants, improving osseointegration. On the other hand, Grønborg et al. [32] showed that the consumption of foods fortified with Vit. D for twelve weeks did not bring significant changes in terms of markers of bone turnover and muscle strength.
The in vitro studies provided further evidence of the positive effects of Vit. D on osteoblasts and stem cell differentiation. These studies showed that osteoblasts together with Vit. D have a great potential in bone regeneration and, in the case of stem cells, the presence of Vit. D improved the differentiation to osteoblasts [11]. All the in vitro studies brought us useful information on the use of nanostructures/nanoparticles loaded with Vit. D acting as reservoirs of the active substance, thus improving bone regeneration [9,10].
The main limitation of this review was the small number of articles available in the literature and their heterogeneity—clinical studies, animal model or human patient studies, in vitro studies, different methods of investigation, different design, etc.
Heterogeneity and different study designs prevented us from making a clear statement about the influence of Vit. D on bone regeneration because its effects might be influenced by the presence or deficiency of other vitamins or elements (vitamin K).
Epidemiological studies suggest that the absence of vitamin K in the organism is correlated with bone disease and mineralization issues. [34] It is known that vitamin K is a catalyst for the process of carboxylation of certain proteins. Osteocalcin and matrix Gla protein are two calcium-binding vitamin K-dependent proteins [35]. The necessity of these proteins for bone health has been proven in previous studies [36], but there is also evidence that bone mineralization is not influenced by the absence of osteocalcin [37]. Thus, future studies need to be conducted, particularly on organisms deficient in several elements considered important for bone formation, in order to identify the exact elements required in the process.
In vitro studies also provide valuable information about the effects of Vit. D, but they are carried out in a controlled environment, without other interferences that would be found in the human body (such as vitamin deficiency). Since bone health depends on various vitamins and nutrients (calcium, magnesium, phosphorus, fluorides, copper, zinc, boron, manganese, potassium, vitamin A, vitamin C, B vitamins) [38], additional studies are needed with and without these elements besides Vit. D that have been proven in other studies to influence the bone regeneration process.
As shown above, to assess the risk of bias, the NOS was used to assess the items as follows: low risk of bias (7–9 NOS score), high risk of bias (4–6 NOS score), and very high risk of bias (0–3 NOS score), the vast majority of the articles included in this review have a score of 7,8—low risk of bias, and only seven had high risk of bias.
5. Conclusions
According to the evidence in the studies included in this review, we concluded that Vit. D has a major role in bone regeneration, for proper bone mineralization. In the context in which today’s society has a growing need for bone reconstruction and dental implants, for the modern patients who want fast results, Vit. D administration could increase the rate of osseointegration using a minimally invasive and inexpensive technique.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/medicina58101337/s1, The research strategy.
Author Contributions
Conceptualization, O.L., M.H., G.C.M. and N.P.; methodology, O.L., G.C.M. and N.P.; validation, O.L., M.H. and S.B.; formal analysis, G.C.M., O.L. and N.P.; investigation, G.C.M., O.L. and N.P.; resources, G.C.M., O.L. and N.P.; data curation, G.C.M., O.L. and N.P.; writing—original draft preparation, G.C.M. and N.P.; writing—review and editing, M.H., O.L. and S.B.; visualization, M.H., O.L. and S.B.; supervision, M.H., O.L. and S.B.; project administration, G.C.M. and O.L.; funding acquisition, G.C.M. and O.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant CNCS-UEFISCDI of the Ministry of Research, Innovation, and Digitization, project No. PN-III-P1-1.1-TE-2021-0531, within PNCDI III. This work was funded by the Doctoral Research Project (PCD 2022) of the Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca (contract No. 881/13/12.01.2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Diachkova, E.; Trifonova, D.; Morozova, E.; Runova, G.; Ashurko, I.; Ibadulaeva, M.; Fadeev, V.; Tarasenko, S. Vitamin D and Its Role in Oral Diseases Development. Scoping Review. Dent. J. 2021, 9, 129. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’to authors’ assessments. BMC Med. Res. Methodol. 2014, 1, 14–45. [Google Scholar] [CrossRef] [PubMed]
- Rekha, P.; Amarpal; Tamilmahan, P.; Kuldeep, D.; Netrapal, S. Evaluation of in vitro Efficacy of Vitamin D3 on the Osteogenic Differentiation and Mineralization Capabilities of Fetal and Adult Osteoblasts of Rabbit Reflects Therapeutic Potential. Int. J. Pharmacol. 2014, 10, 440–450. [Google Scholar] [CrossRef]
- Kim, H.S.; Zheng, M.; Kim, D.K.; Lee, W.P.; Yu, S.J.; Kim, B.O. Effects of 1,25-dihydroxyvitamin D3 on the differentiation of MC3T3-E1 osteoblast-like cells. J. Periodontal. Implant. Sci. 2018, 48, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.; Lee, D.; Heo, M.; Lee, J.S.; Lee, S.J.; Heo, D.N.; Seong, J.; Lim, H.N.; Lee, Y.H.; Moon, H.J.; et al. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci. Technol. Adv. Mater. 2019, 20, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, L.; Shen, X.; Li, M.; Luo, Z.; Cai, K.; Hu, Y. Construction of multilayered molecular reservoirs on a titanium alloy implant for combinational drug delivery to promote osseointegration in osteoporotic conditions. Acta Biomater. 2020, 105, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Belgheisi, G.; Haghbin-Nazarpak, M.; Omidi, M.; Khojasteh, A.; Solati-Hashjin, M. Bone tissue engineering gelatin-hydroxyapatite/graphene oxide scaffolds with the ability to release vitamin D: Fabrication, characterization, and in vitro study. J. Mater. Sci. Mater. Med. 2020, 31, 97. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S.; et al. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, L.M.; Abdelaziz, A.M.; Sabry, D.; Abdelgwad, M. Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression. Biomol. Concepts 2020, 11, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Galus, K.; Szymendera, J.; Zaleski, A.; Schreyer, K. Effects of 1 alpha-hydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3 on bone remodeling. Calcif. Tissue Int. 1980, 31, 209–213. [Google Scholar] [CrossRef]
- Kelly, J.; Lin, A.; Wang, C.J.; Park, S.; Nishimura, I. Vitamin D and bone physiology: Demonstration of vitamin D deficiency in an implant osseointegration rat model. J. Prosthodont. 2009, 18, 473–478. [Google Scholar] [CrossRef]
- Uysal, T.; Amasyali, M.; Enhos, S.; Sonmez, M.F.; Sagdic, D. Effect of ED-71, a New Active Vitamin D Analog, on Bone Formation in an Orthopedically Expanded Suture in Rats. A Histomorphometric Study. Eur. J. Dent. 2009, 3, 165–172. [Google Scholar] [CrossRef]
- Hong, H.H.; Chou, T.A.; Yang, J.C.; Chang, C.J. The potential effects of cholecalciferol on bone regeneration in dogs. Clin. Oral. Implants Res. 2012, 23, 1187–1192. [Google Scholar] [CrossRef]
- Dvorak, G.; Fügl, A.; Watzek, G.; Tangl, S.; Pokorny, P.; Gruber, R. Impact of dietary vitamin D on osseointegration in the ovariectomized rat. Clin. Oral. Implants Res. 2012, 23, 1308–1313. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Y.; Wang, X.; Shui, X.; Hu, J. 1,25Dihydroxy vitamin D(3) improves titanium implant osseointegration in osteoporotic rats. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 114, 174–178. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Zhao, D.; Zou, H.; Sun, N.; Liang, X.; Dard, M.; Lanske, B.; Yuan, Q. Vitamin D supplementation enhances the fixation of titanium implants in chronic kidney disease mice. PLoS ONE 2014, 9, 95689. [Google Scholar] [CrossRef]
- Liu, H.; Cui, J.; Feng, W.; Lv, S.; Du, J.; Sun, J.; Han, X.; Wang, Z.; Lu, X.; Yimin Oda, K.; et al. Local administration of calcitriol positively influences bone remodeling and maturation during restoration of mandibular bone defects in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Fügl, A.; Gruber, R.; Agis, H.; Lzicar, H.; Keibl, C.; Schwarze, U.Y.; Dvorak, G. Alveolar bone regeneration in response to local application of calcitriol in vitamin D deficient rats. J. Clin. Periodontol. 2015, 42, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.H.; Yen, T.H.; Hong, A.; Chou, T.A. Association of vitamin D3 with alveolar bone regeneration in dogs. J. Cell. Mol. Med. 2015, 19, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Salomó-Coll, O.; Maté-Sánchez de Val, J.E.; Ramírez-Fernandez, M.P.; Hernández-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: A pilot study in dogs part II. Clin. Oral. Implants Res. 2016, 27, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Haffner-Luntzer, M.; Prystaz, K.; Vom Scheidt, A.; Busse, B.; Schinke, T.; Amling, M.; Ignatius, A. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci. Rep. 2017, 7, 7223. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Du, J.; Liu, D.; Liu, H.; Amizuka, N.; Li, M. Histochemical examination of systemic administration of eldecalcitol combined with guided bone regeneration for bone defect restoration in rats. J. Mol. Histol. 2017, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Salomó-Coll, O.; Maté-Sánchez de Val, J.E.; Ramírez-Fernandez, M.P.; Hernández-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Osseoinductive elements around immediate implants for better osteointegration: A pilot study in foxhound dogs. Clin. Oral. Implants Res. 2018, 29, 1061–1069. [Google Scholar] [CrossRef]
- Won, D.J.; Seong, K.S.; Jang, C.H.; Lee, J.S.; Ko, J.A.; Bae, H.; Park, H.J. Effects of vitamin D2-fortified shiitake mushroom on bioavailability and bone structure. Biosci. Biotechnol. Biochem. 2019, 83, 942–951. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, G.D.; Cui, Y.J.; Li, M.Q.; Liu, Z.P. Synthesis of 1α,25-dihydroxyvitamin D3 analogues with α,α-difluorocycloketone at the CD-ring side chains and their biological properties in ovariectomized rats. J. Steroid. Biochem. Mol. Biol. 2019, 186, 66–73. [Google Scholar] [CrossRef]
- Cignachi, N.P.; Ribeiro, A.; Machado, G.D.B.; Cignachi, A.P.; Kist, L.W.; Bogo, M.R.; Silva, R.B.M.; Campos, M.M. Bone regeneration in a mouse model of type 1 diabetes: Influence of sex, vitamin D3, and insulin. Life Sci. 2020, 263, 118593. [Google Scholar] [CrossRef]
- Zarubina, N.A.; Rozhinskaia, L.I.; Bukhman, A.I.; Dorokhova, I.I.; Konnova, E.V. Sravnitel’naia otsenka éffektivnosti preparatov vitamina D3 (1-alpha- i 1-alpha,25-dihydroxycholecalciferol) pri razlichnykh vidakh osteoporoza i osteomaliatsii [Comparative evaluation of the effectiveness of vitamin D3 preparations (1-alpha-hydroxy- and 1-alpha,25-dihydroxycholecalciferol in various forms of osteoporosis and osteomalacia]. Probl. Endokrinol. 1989, 35, 15–19. [Google Scholar] [PubMed]
- Grønborg, I.M.; Tetens, I.; Andersen, E.W.; Kristensen, M.; Larsen, R.E.K.; Tran, T.L.L.; Andersen, R. Effect of vitamin D fortified foods on bone markers and muscle strength in women of Pakistani and Danish origin living in Denmark: A randomised controlled trial. Nutr. J. 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, J.; Jaroń, A.; Trybek, G. Impact of the 25-Hydroxycholecalciferol Concentration and Vitamin D Deficiency Treatment on Changes in the Bone Level at the Implant Site during the Process of Osseointegration: A Prospective, Randomized, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Mereu, M.C.; Aghi, A.; Iervasi, G.; Gallieni, M. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 2017, 14, 200–206. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Reid, M.L. Timed appearance of a calcium-binding protein containing gamma-carboxyglutamic acid in developing chick bone. Dev. Biol. 1978, 65, 426–434. [Google Scholar] [CrossRef]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; La Manna, G.; et al. Vitamin K and Osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Palacios, C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).