Cutaneous Permeation of a Percutaneously Applied Glucocorticoid Using Plant-Based Anionic Phospholipids in Hydrogenated Vegetable Oil: A Preliminary Study
Abstract
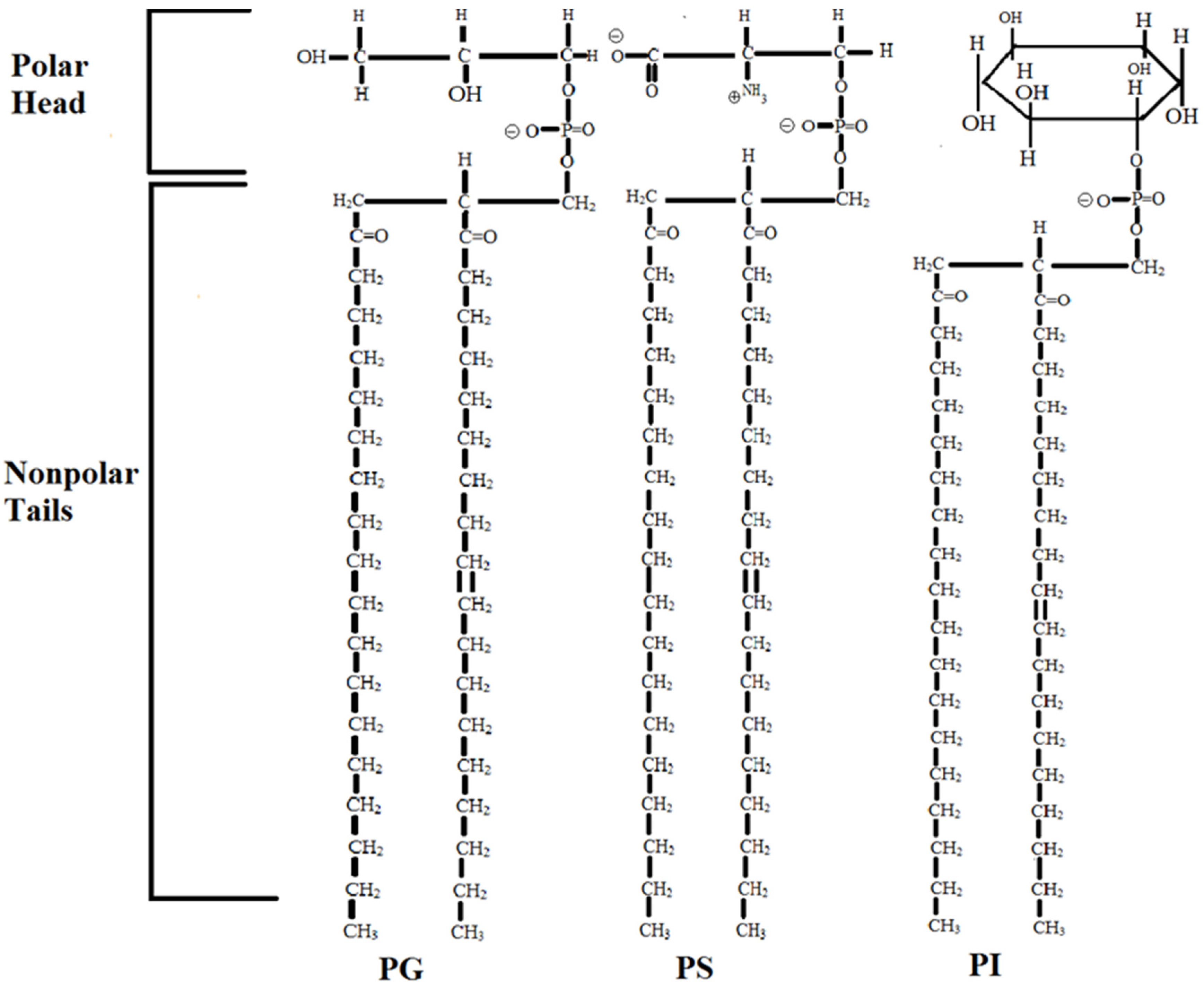
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hydrocortisone. 2016. Available online: http://www.pdr.net (accessed on 21 September 2022).
- Korb, D.R.; Glonek, T.; Greiner, J.V. Skin Cream Preparation and Method. U.S. Patent 5,738,856, 14 April 1998. [Google Scholar]
- Korb, D.R.; Glonek, T.; Greiner, J.V. Skin Care Preparation and Method. U.S. Patent 5,851,543, 22 December 1998. [Google Scholar]
- Bosley, C.; Smith, J.; Baratti, P.; Pritchard, D.L.; Xiong, X.; Li, C.; Merchant, T.E. A phrase III trial comparing an anionic phospholipid-based (APP) cream and aloe vera-based gel in the prevention and treatment of radiation dermatitis. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, S4738. [Google Scholar] [CrossRef]
- Merchant, T.E.; Bosley, C.; Smith, J.; Baratti, P.; Pritchard, D.; Davis, T.; Li, C.; Xiong, X. A phase III trial comparing an anionic phospholipid-based (APP) cream and aloe vera-based gel in the prevention and treatment of radiation dermatitis in pediatric patients. Radiat. Oncol. 2007, 21, 45. [Google Scholar] [CrossRef]
- Glonek, T.; Greiner, J.V.; Oliver, P.J.; Baker, T.L. Implications of a diabetic foot xerosis treatment with an emulsion containing the plant-based anionic phospholipoids. J. Prim. Care Community Health 2022, 13, 215103192110686. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Glonek, T.; Korb, D.R.; Booth, R.; Leahy, C.D. Phospholipids in Meibomian gland secretion. Ophthalmic Res. 1996, 28, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Glonek, T.; Korb, D.R.; Leahy, C.D. Meibomian gland phospholipids. Curr. Eye Res. 1996, 15, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Glonek, T.; Townsend, D.J.; Hearn, S.L. Epidermal and dermal phospholipids of the human eyelid. A 31P nuclear magnetic resonance spectroscopy study. Arch. Dermatol. Res. 1998, 290, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Greiner, J.V.; Glonek, T. The effects of anionic and zwitterionic phospholipids on the tear film lipid layer. Adv. Exp. Med. Biol. 2002, 506, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Korb, D.R.; Baron, D.F.; Herman, J.P.; Finnemore, V.M.; Exford, J.M.; Hermosa, J.L.; Leahy, C.D.; Glonek, T.; Greiner, J.V. Tear film lipid layer thickness as a function of blinking. Cornea 1994, 13, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.; Lehman, P. Percutaneous absorption. In Encyclopedia of Pharmaceutical Technology; Nuclear medicine and pharmacy to permeation enhancement through, skin; Swarbrick, J., Boyland, J., Eds.; CRC Press: New York, NY, USA, 1995; Volume 11. [Google Scholar]
- Greiner, J.V.; Oliver, P.J.; Baker, T.L.; Lindsay, M.E.; McPherson, A.T.; Bedarf, G.E.; Glonek, T. A recalcitrant skin fissure treated with an anionic polar phospholipid emulsion. Fam. Med. Prim. Care Open Access 2022, 6, 168. [Google Scholar] [CrossRef]
- Tan, G.; Xu, P.; Lawson, L.B.; He, J.; Freytag, L.C.; Clements, J.D.; John, V.T. Hydration effects on skin microstructure as probed by high-resolution cryo-scannning electron microscopy and mechanistic implications to enhanced transcutaneous delivery of biomacromolecules. J. Pharm. Sci. 2010, 99, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Larsson, R. Lipids-Molecular Organization. In Physical Functions and Technical Applications; The Oily Press, Limited: Dundee, Scotland, 1994; Chapter 11; pp. 147–154. [Google Scholar]
- Rawlings, A.V. Skin waxes: Their composition, properties, structures and biological significance. In Waxes: Chemistry, Molecular Biology and Functions; Hamilton, R.J., Ed.; The Oil Press: Dundee, Scotland, 1995; p. 226. [Google Scholar]
- Miller, W.; Bose, H. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, D.E.; Schafer, R.M. Nonspecific and metabolic interactions between steroid hormones and human plasma lipoproteins. Lipids 1990, 25, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Scanu, A. Ultrastructure of Serum High Density Lipoproteins: Facts and Models. Lipids 1978, 13, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lhomme, M.; Chapman, M. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, J.V.; Bhargava, H.N.; Glonek, T.; Korb, D.R.; Lindsay, M.E.; Oliver, P.J. Cutaneous Permeation of a Percutaneously Applied Glucocorticoid Using Plant-Based Anionic Phospholipids in Hydrogenated Vegetable Oil: A Preliminary Study. Medicina 2022, 58, 1334. https://doi.org/10.3390/medicina58101334
Greiner JV, Bhargava HN, Glonek T, Korb DR, Lindsay ME, Oliver PJ. Cutaneous Permeation of a Percutaneously Applied Glucocorticoid Using Plant-Based Anionic Phospholipids in Hydrogenated Vegetable Oil: A Preliminary Study. Medicina. 2022; 58(10):1334. https://doi.org/10.3390/medicina58101334
Chicago/Turabian StyleGreiner, Jack V., Hridaya N. Bhargava, Thomas Glonek, Donald R. Korb, Michael E. Lindsay, and Paula J. Oliver. 2022. "Cutaneous Permeation of a Percutaneously Applied Glucocorticoid Using Plant-Based Anionic Phospholipids in Hydrogenated Vegetable Oil: A Preliminary Study" Medicina 58, no. 10: 1334. https://doi.org/10.3390/medicina58101334
APA StyleGreiner, J. V., Bhargava, H. N., Glonek, T., Korb, D. R., Lindsay, M. E., & Oliver, P. J. (2022). Cutaneous Permeation of a Percutaneously Applied Glucocorticoid Using Plant-Based Anionic Phospholipids in Hydrogenated Vegetable Oil: A Preliminary Study. Medicina, 58(10), 1334. https://doi.org/10.3390/medicina58101334






