Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned
Abstract
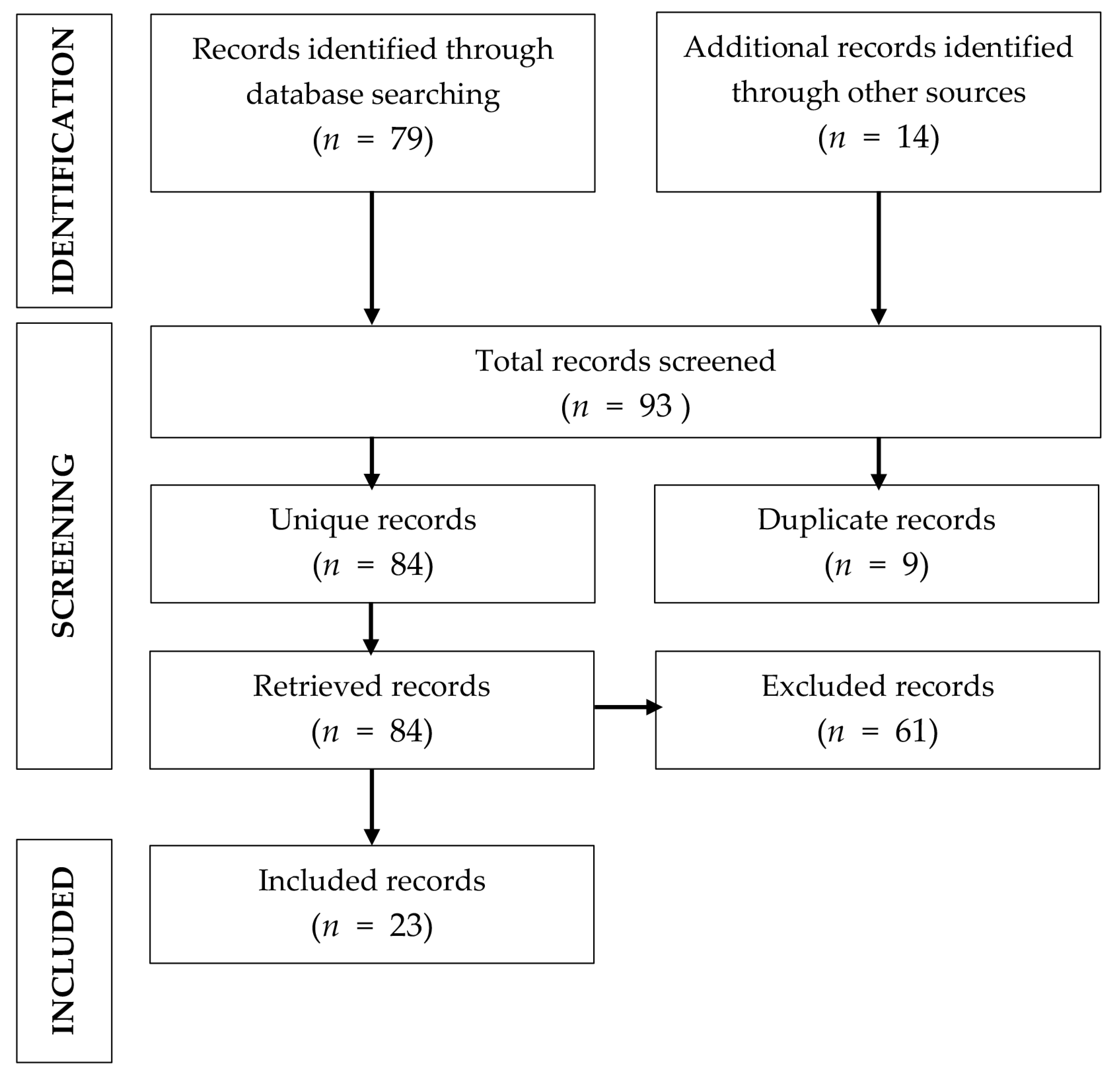
:1. Introduction
2. Literature Review Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection Methodology
2.4. Data Extraction
2.5. Quality Control
2.6. Selection of Studies
3. Impact of the COVID-19 Pandemic on the Rate of Elective Surgical Interventions for Colorectal Cancer
4. Impact of the COVID-19 Pandemic on the Duration of Hospital Stay
5. Waiting Time for Hospital Admission
6. Impact on Postoperative Mortality
7. Discussion
8. Learning Points
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cui, J.; Li, Z.; An, Q.; Xiao, G. Impact of the COVID-19 Pandemic on Elective Surgery for Colorectal Cancer. J. Gastrointest. Cancer 2021, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.; Lin, T.; Zhao, M.L.; Chen, H.; Chen, T.; Guo, W.H.; Zhao, L.Y.; Liu, H.; Hu, Y.F.; Yu, J.; et al. Management strategy for the resumption of regular diagnosis and treatment in gastrointestinal surgery department during the outbreak of coronavirus disease 2019 (COVID-19). Zhonghua Wei Chang Wai Ke Za Zhi 2020, 23, 321–326. [Google Scholar] [PubMed]
- COVID Surg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar]
- Grubic, A.D.; Ayazi, S.; Zebarjadi, J.; Tahmasbi, H.; Ayazi, K.; Jobe, B.A. COVID-19 outbreak and surgical practice: The rationale for suspending non-urgent surgeries and role of testing modalities. World J. Gastrointest. Surg. 2020, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, Z.Y.; Chen, Y.K.; Wang, H.Y.; Chen, J.J.; Song, C.; Gu, J. Effects on the integrated treatment of colorectal cancer patients during COVID-19 epidemic in China: A cross-sectional study. Zhonghua Wei Chang Wai Ke Za Zhi 2020, 23, 795–800. [Google Scholar] [PubMed]
- Antikchi, M.H.; Neamatzadeh, H.; Ghelmani, Y.; Jafari-Nedooshan, J.; Dastgheib, S.A.; Kargar, S.; Noorishadkam, M.; Bahrami, R.; Jarahzadeh, M.H. The Risk and Prevalence of COVID-19 Infection in Colorectal Cancer Patients: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2021, 52, 73–79. [Google Scholar] [CrossRef]
- Margulis, A.V.; Pladevall, M.; Riera-Guardia, N.; Varas-Lorenzo, C.; Hazell, L.; Berkman, N.; Viswanathan, M.; Perez-Gutthann, S. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: The Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014, 6, 359–368. [Google Scholar] [CrossRef]
- Brunner, M.; Krautz, C.; Kersting, S.; Weber, G.F.; Stinner, B.; Benz, S.R.; Grützmann, R. Oncological colorectal surgery during the COVID-19 pandemic-a national survey. Int. J. Colorectal. Dis. 2020, 35, 2219–2225. [Google Scholar] [CrossRef]
- Kuryba, A.; Boyle, J.M.; Blake, H.A.; Aggarwal, A.; van der Meulen, J.; Braun, M.; Walker, K.; Fearnhead, N.S. Surgical Treatment and Outcomes of Colorectal Cancer Patients During the COVID-19 Pandemic: A National Population-based Study in England. Ann. Surg. Open 2021, 2, e071. [Google Scholar] [CrossRef]
- Merchant, J.; Lindsey, I.; James, D.; Symons, N.; Boyce, S.; Jones, O.; George, B.; Cunningham, C. Maintaining Standards in Colorectal Cancer Surgery During the Global Pandemic: A Cohort Study. World J. Surg. 2021, 45, 655–661. [Google Scholar] [CrossRef]
- Morris, E.J.A.; Goldacre, R.; Spata, E.; Mafham, M.; Finan, P.J.; Shelton, J.; Richards, M.; Spencer, K.; Emberson, J.; Hollings, S.; et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: A population-based study. Lancet Gastroenterol. Hepatol. 2021, 6, 199–208. [Google Scholar] [CrossRef]
- Donlon, N.E.; Hayes, C.; Davern, M.; Bolger, J.C.; Irwin, S.C.; Butt, W.T.; McNamara, D.A.; Mealy, K. Impact of COVID-19 on the Diagnosis and Surgical Treatment of Colorectal Cancer: A National Perspective. Dis. Colon Rectum 2021, 64, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Allaix, M.E.; Lo Secco, G.; Velluti, F.; De Paolis, P.; Arolfo, S.; Morino, M. Colorectal surgery during the COVID-19 outbreak: Do we need to change? Updates Surg. 2021, 73, 173–177. [Google Scholar] [CrossRef]
- Caricato, M.; The Italian ColoRectal Anastomotic Leakage (iCral) Study Group; Baiocchi, G.L.; Crafa, F.; Scabini, S.; Brisinda, G.; Clementi, M.; Sica, G.; DelRio, P.; Longo, G.; et al. Colorectal surgery in Italy during the Covid19 outbreak: A survey from the iCral study group. Updates Surg. 2020, 72, 249–257. [Google Scholar] [CrossRef] [PubMed]
- de la Portilla de Juan, F.; Reyes Díaz, M.L.; Ramallo Solía, I. Impact of the pandemic on surgical activity in colorectal cancer in Spain. Results of a national survey. Cir. Esp. (Engl. Ed.) 2021, 99, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Koczkodaj, P.; Sulkowska, U.; Kamiński, M.F.; Didkowska, J. SARS-CoV-2 as a new possible long-lasting determining factor impacting cancer death numbers. Based on the example of breast, colorectal and cervical cancer in Poland. Nowotwory 2021, 71, 42–46. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Bardan, R.; Muntean, C.; Feier, O.; Olariu, A.; Olariu, S. The consequences of the Covid-19 pandemic on elective surgery for colon cancer. Ann. Ital. Chir. 2022, 93, 1–7. [Google Scholar]
- Xu, Y.; Huang, Z.H.; Zheng, C.Z.; Li, C.; Zhang, Y.Q.; Guo, T.A.; Liu, F.Q.; Xu, Y. The impact of COVID-19 pandemic on colorectal cancer patients: A single-center retrospective study. BMC Gastroenterol. 2021, 21, 185. [Google Scholar] [CrossRef]
- He, C.; Li, Y.; Huang, X.; Xiaohui, H.; Yan, Y.; Liu, Y.; Zhao, P.; Lin, H.; Xu, X.; Wang, Y.; et al. How should colorectal surgeons practice during the COVID-19 epidemic? A retrospective single-centre analysis based on real-world data from China. ANZ J. Surg. 2020, 90, 1310–1315. [Google Scholar] [CrossRef]
- Raj Kumar, B.; Pandey, D. An observational study of the demographic and treatment changes in a tertiary colorectal cancer center during the COVID-19 pandemic. J. Surg. Oncol. 2020, 122, 1271–1275. [Google Scholar] [CrossRef]
- Tschann, P.; Girotti, P.N.C.; Lechner, D.; Adler, S.; Feurstein, B.; Szeverinski, P.; Königsrainer, I. How Does the COVID-19 Pandemic Influence Surgical Case Load and Histological Outcome for Colorectal Cancer? A Single-Centre Experience. J. Gastrointest. Surg. 2021, 25, 2957–2960. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Koller, S.; Duldulao, P.M.N.; Ault, G.T.; Lee, S.W.; Cologne, K.G. COVID-19 Impact on Colorectal Daily Practice-How Long Will It Take to Catch Up? J. Gastrointest. Surg. 2021, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, L.F.; Nahas, C.S.R.; Marques, C.F.S.; Cotti, G.C.D.C.; Imperiale, A.R.; Averbach, P.; de Meira, J.D.; Horvat, N.; Ribeiro-Júnior, U.; Cecconello, I.; et al. Is it Safe to Perform Elective Colorectal Surgical Procedures during the COVID-19 Pandemic? A Single Institution Experience with 103 Patients. Clinics 2021, 76, e2507. [Google Scholar] [CrossRef]
- Gurney, J.K.; Millar, E.; Dunn, A.; Pirie, R.; Mako, M.; Manderson, J.; Hardie, C.; Jackson, C.G.; North, R.; Ruka, M.; et al. The impact of the COVID-19 pandemic on cancer diagnosis and service access in New Zealand-a country pursuing COVID-19 elimination. Lancet Reg. Health West. Pac. 2021, 10, 100127. [Google Scholar] [CrossRef]
- Santoro, G.A.; Grossi, U.; Murad-Regadas, S.; Nunoo-Mensah, J.W.; Mellgren, A.; Di Tanna, G.L.; Gallo, G.; Tsang, C.; Wexner, S.D. DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19): Global perspective from an international survey. Surgery 2021, 169, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.U.; Raza, S.S.; Thomas, P.; Vakis, S. Comparison of Colorectal Cancer Surgery Services During COVID-19 First Wave With Pre-COVID Time. Cureus 2021, 13, e17585. [Google Scholar] [CrossRef]
- López-Rojo, I.; Alonso, O.; Ortega-Pérez, G.; Galipienzo-Garcia, J.; González-Moreno, S. Prioritization, patient selection, and multimodal perioperative management of colorectal cancer facing health-care system saturation. Cir. Cir. 2021, 89, 755–762. [Google Scholar] [CrossRef]
- COVID Surg Collaborative. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis. 2020, 23, 732–749. [Google Scholar]
- Kapoor, D.; Perwaiz, A.; Singh, A.; Chaudhary, A. Elective Gastrointestinal Surgery in COVID Times. Indian J. Surg. 2020, 83, 277–283. [Google Scholar] [CrossRef]
- Prachand, V.N.; Milner, R.; Angelos, P.; Posner, M.C.; Fung, J.J.; Agrawal, N.; Jeevanandam, V.; Matthews, J.B. Medically Necessary, Time-Sensitive Procedures: Scoring System to Ethically and Efficiently Manage Resource Scarcity and Provider Risk During the COVID-19 Pandemic. J. Am. Coll. Surg. 2020, 231, 281–288. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, J.; Ammar, H. Impact of COVID-19 on the outcomes of gastrointestinal surgery. Clin. J. Gastroenterol. 2021, 14, 932–946. [Google Scholar] [CrossRef] [PubMed]
- COVID Surg Collaborative. Preoperative nasopharyngeal swab testing and postoperative pulmonary complications in patients undergoing elective surgery during the SARS-CoV-2 pandemic. Br. J. Surg. 2021, 108, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Asbun, H.J.; Abu Hilal, M.; Kunzler, F.; Asbun, D.; Bonjer, J.; Conlon, K.; Demartines, N.; Feldman, L.S.; Morales-Conde, S.; Pietrabissa, A.; et al. International Delphi Expert Consensus on Safe Return to Surgical and Endoscopic Practice: From the Coronavirus Global Surgical Collaborative. Ann. Surg. 2021, 274, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.E.; Scott, A.J.; Markar, S.R.; Clarke, J.M.; Martin, G.; Winter Beatty, J.; Sounderajah, V.; Yalamanchili, S.; Denning, M.; Arulampalam, T.; et al. Insights from a global snapshot of the change in elective colorectal practice due to the COVID-19 pandemic. PLoS ONE 2020, 15, e0240397. [Google Scholar] [CrossRef] [PubMed]
- Foo, F.J.; Ho, L.M.L.; Tan, W.J.; Koh, F.H.; Sivarajah, S.S.; Park, S.Y.; Chen, W.T.; Chew, M.H. Colorectal cancer surgery in Asia during the COVID-19 pandemic: A tale of 3 cities. Asian J. Surg. 2022, 45, 1095–1100. [Google Scholar] [CrossRef]
- Yao, H.; Pang, K.; Xiao, G.; Li, F.; Xiao, Y.; Ye, Y.; Wang, X.; Xiu, D.; Wang, Z.; Du, X.; et al. What Should Surgeons Do in the Face of the Coronavirus Disease 2019 Pandemic? A Beijing Experience. Dis. Colon Rectum 2020, 63, 1020–1022. [Google Scholar] [CrossRef]
- Porpiglia, F.; Checcucci, E.; Amparore, D.; Verri, P.; Campi, R.; Claps, F.; Esperto, F.; Fiori, C.; Carrieri, G.; Ficarra, V.; et al. Slowdown of urology residents’ learning curve during the COVID-19 emergency. BJU Int. 2020, 125, E15–E17. [Google Scholar] [CrossRef]
| Study Authors | Center, Country | Analyzed Period | No. of Patients | No. of Surgeons | No. of Centers | Elective Surgery Decrease |
|---|---|---|---|---|---|---|
| Brunner et al. [8] | Germany | April 2020 | 12,423 | 112 | 101 | 34% |
| Kuryba et al. [9] | England, UK | 1 October 2019–31 May 2020 | 3227 | - | 146 | 26.6% |
| Merchant et al. [10] | Oxford University Hospitals, UK | 23 March 2020–7 June 2020 | 85 | - | 1 | 52.51% |
| Morris et al. [11] | NHS England | April 2020 | 1378 | - | National database | 31% |
| Donlon et al. [12] | Hospital InpatientEnquiry Database, Ireland | 1 March 2020–28 February 2021 | 1072 | - | National database | 30% |
| Allaix et al. [13] | University of Torino, Italy | 9 March 2020–15 April 2020 | 74 | - | 1 | 34% |
| Caricato et al. [14] | Italian ColoRectal Anasto-motic Leakage (iCral) Study Group, Italy | 1 January 2020–27 March 2020 | 1328 | - | 43 | 30.37% |
| de la Portilla et al. [15] | National Survey of Colorectal Surgery Units, Spain | February 2020–April 2020 | - | - | 67 | - |
| Koczkodai et al. [16] | National Institute of Oncology, Warsaw, Poland | January 2020–July 2020 | - | - | - | 51% |
| Feier et al. [17] | 1st Department of Surgery, Timisoara, Romania | 26 February 2020–1 October 2021 | 147 | - | 1 | 42% |
| Xu et al. [18] | Fudan University, Shanghai, China | 1 January 2020–3 May 2020 | 710 | - | 1 | 14.25% |
| Cui et al. [1] | Department of General Surgery, National Center of Gerontology, Beijing, China | 1 February 2020–31 May 2020 | 67 | - | 1 | 34% |
| He et al. [19] | Chinese Army General Hospital, Beijing, China | 20 January 2020–20 March 2020 | 71 | - | 1 | 63% |
| Kumar et al. [20] | Tata Memorial Centre, Mumbai, India | Jane 2020–May 2020 | 257 | - | 1 | 39.88% |
| Tschann et al., [21] | Academic Hospital Feldkirch, Austria | 1 January 2020–31 December 2020 | 63 | - | 1 | 11.26% |
| Yoon et al. [22] | Los Angeles, USA | 16 March 2020–23 April 2020 | 13 | - | 1 | 74% |
| Sobrado et al. [23] | Sao Paulo, Brazil | 10 March 2020–9 September 2020 | 103 | - | 1 | - |
| Gurney et al. [24] | New Zealand | January 2020–August 2020 | - | - | 20 | 1% |
| Santoro et al. [25] | Worldwide (84 countries) | 20 May 2020–10 June 2020 | - | - | 1051 | 58.3% |
| COVIDSurg [3] | Worldwide (71 countries) | 12 weeks in 2020 | - | - | 359 | 37.7% |
| Study Authors | Center, Country | Analyzed Period | No. of Patients | No. of Centers | Hospital Stay Pre-Pandemic (days) | Hospital Stay during Pandemic (days) |
|---|---|---|---|---|---|---|
| Merchant et al. [10] | Oxford University Hospitals, UK | 23 March 2020–7 June 2020 | 85 | 1 | 5 | 4 |
| Rashid et al. [26] | University Hospitals of Derby and Burton, England, UK | 1 March 2020–30 April 2020 | 22 | 1 | 8 ± 9 | 5 ± 2 |
| Allaix et al. [13] | University of Torino, Italy | 9 March 2020–15 April 2020 | 74 | 1 | 5 | 5 |
| Sobrado et al. [23] | Sao Paulo, Brazil | 10 March 2020–9 September 2020 | 103 | 1 | 11.7 ± 9.3 | |
| Cui et al. [1] | National Center of Gerontology, Beijing, China | 1 February 2020–31 May 2020 | 67 | - | 21.3 | 18.5 |
| Xu et al. [18] | Fudan University, Shanghai, China | 1 January 2020–3 May 2020 | 710 | 1 | 11 | 13.2 |
| COVIDSurg [28] | Worldwide (40 countries) | First COVID case to 19 April 2020 | 2073 | 270 hospitals | 7 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feier, C.V.I.; Bardan, R.; Muntean, C.; Olariu, A.; Olariu, S. Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned. Medicina 2022, 58, 1322. https://doi.org/10.3390/medicina58101322
Feier CVI, Bardan R, Muntean C, Olariu A, Olariu S. Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned. Medicina. 2022; 58(10):1322. https://doi.org/10.3390/medicina58101322
Chicago/Turabian StyleFeier, Catalin Vladut Ionut, Razvan Bardan, Calin Muntean, Andra Olariu, and Sorin Olariu. 2022. "Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned" Medicina 58, no. 10: 1322. https://doi.org/10.3390/medicina58101322
APA StyleFeier, C. V. I., Bardan, R., Muntean, C., Olariu, A., & Olariu, S. (2022). Impact of the COVID-19 Pandemic on the Elective Surgery for Colorectal Cancer: Lessons to Be Learned. Medicina, 58(10), 1322. https://doi.org/10.3390/medicina58101322








