Percutaneous N-Butyl-Cyanoacrylate Embolization for Treating Ruptured Pancreaticoduodenal Aneurysm: A Case Report
Abstract
:1. Introduction
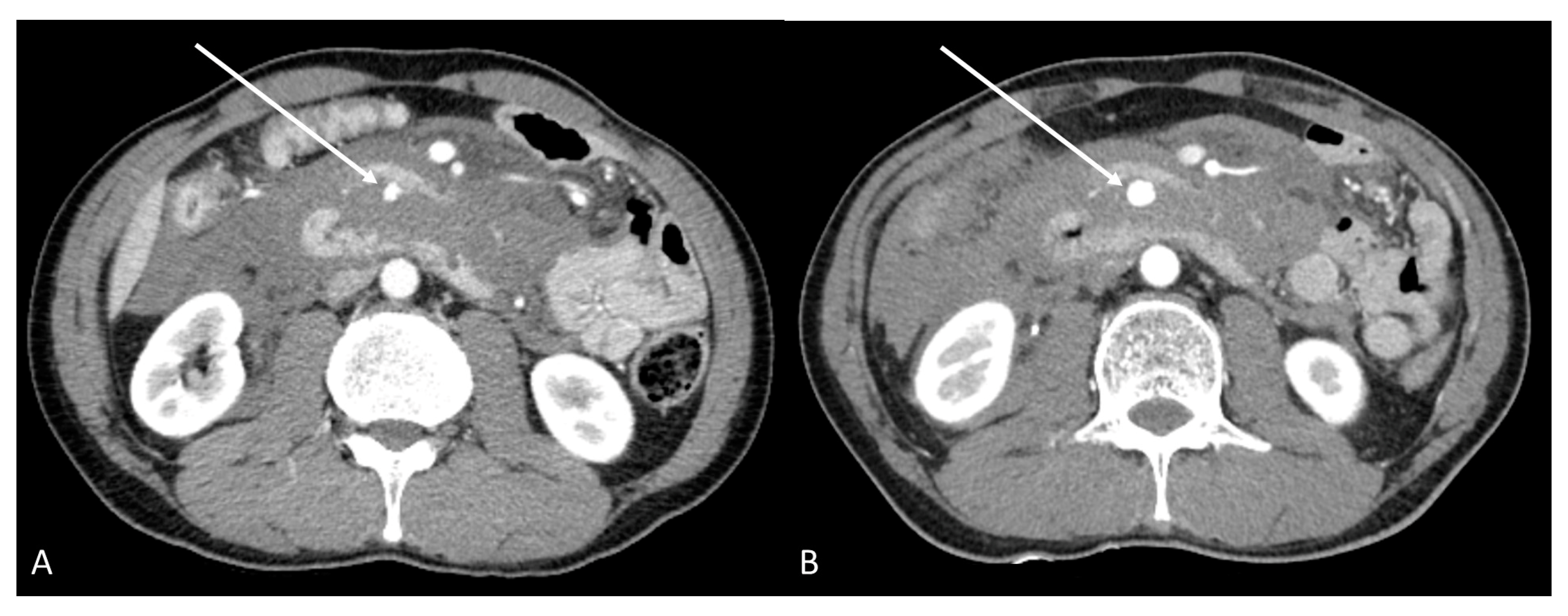
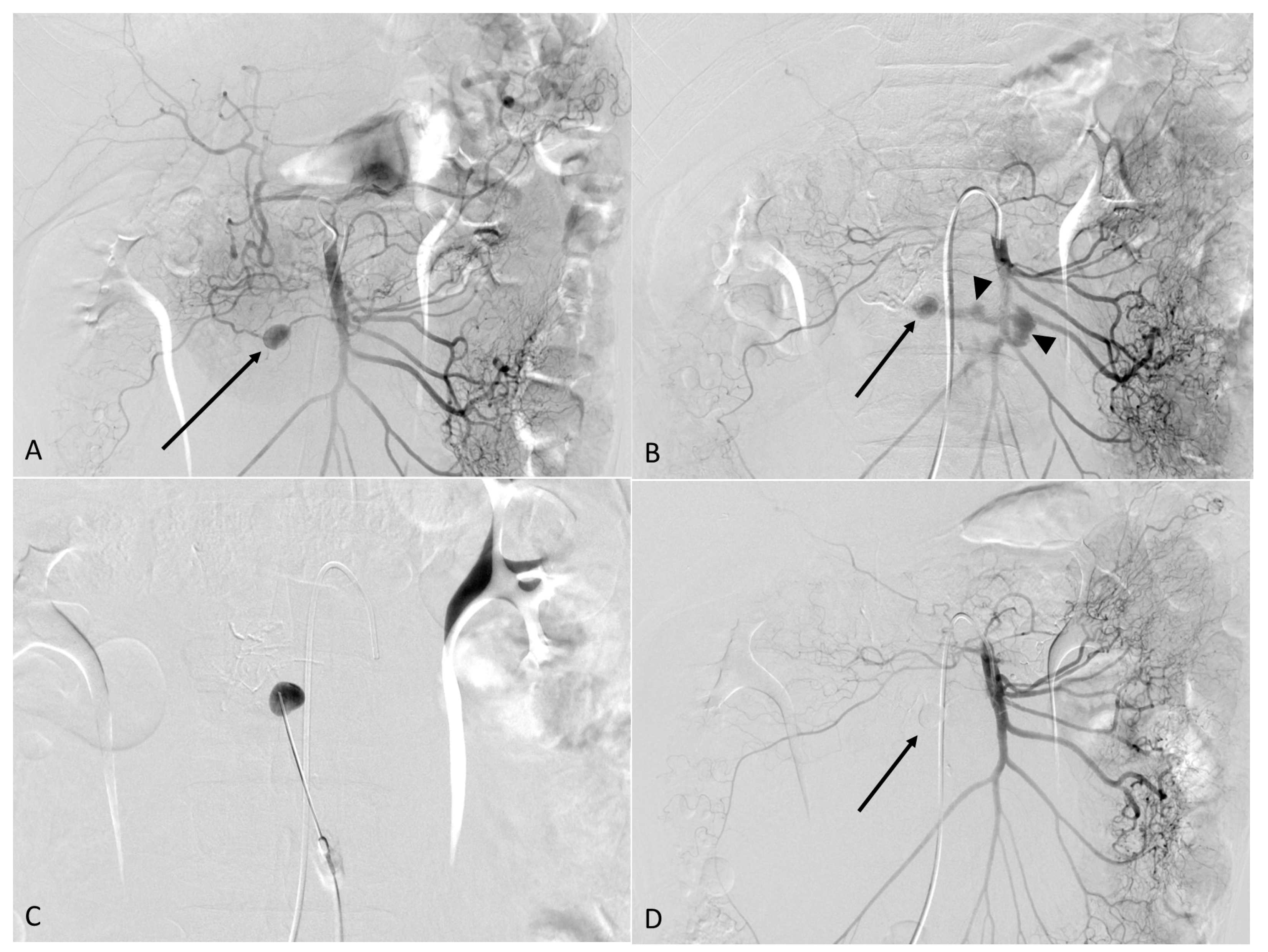
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carmeci, C.; McClenathan, J. Visceral artery aneurysms as seen in a community hospital. Am. J. Surg. 2000, 179, 486–489. [Google Scholar] [CrossRef]
- Carr, S.C.; Pearce, W.H.; Vogelzang, R.L.; McCarthy, W.J.; Nemcek, A.A., Jr.; Yao, J.S. Current management of visceral artery aneurysms. Surgery 1996, 120, 627–634. [Google Scholar] [CrossRef]
- Bradley, S.; Quenzer, F.; Wittler, M. Ruptured visceral artery aneurysms: A deadly cause of epigastric pain. Clin. Pract. Cases Emerg. Med. 2019, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Jesinger, R.A.; Thoreson, A.A.; Lamba, R. Abdominal and pelvic aneurysms and pseudoaneurysms: Imaging review with clinical, radiologic, and treatment correlation. Radiographics 2013, 33, E71–E96. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Weiss, C.; Fishman, E.K.; Johnson, P.T.; Verde, F. Review of visceral aneurysms and pseudoaneurysms. J. Comput. Assist. Tomogr. 2015, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Matthews, M.R.; Minion, D.J.; Quick, R.; Schwarcz, T.H.; Loh, F.K.; Endean, E.D. Surgical management of peripancreatic arterial aneurysms. J. Vasc. Surg. 2004, 40, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Pulli, R.; Dorigo, W.; Troisi, N.; Pratesi, G.; Innocenti, A.A.; Pratesi, C. Surgical treatment of visceral artery aneurysms: A 25-year experience. J. Vasc. Surg. 2008, 48, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Balderi, A.; Antonietti, A.; Ferro, L.; Peano, E.; Pedrazzini, F.; Fonio, P.; Grosso, M. Endovascular treatment of visceral artery aneurysms and pseudoaneurysms: Our experience. Radiol. Med. 2012, 117, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, O.; Giglio, A.; Irace, L.; di Girolamo, A.; Gossetti, B.; Gattuso, R. Single-Center Experience in the Treatment of Visceral Artery Aneurysms. Ann. Vasc. Surg. 2019, 60, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Flood, K.; Nicholson, A.A. Inferior pancreaticoduodenal artery aneurysms associated with occlusive lesions of the celiac axis: Diagnosis, treatment options, outcomes, and review of the literature. Cardiovasc. Interv. Radiol. 2013, 36, 578–587. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Kim, J.H.; Kim, T.U.; Ryu, H.; Lee, T.B.; Ryu, J.H.; Jeon, U.B. Percutaneous N-Butyl-Cyanoacrylate Embolization for Treating Ruptured Pancreaticoduodenal Aneurysm: A Case Report. Medicina 2022, 58, 1320. https://doi.org/10.3390/medicina58101320
Jang JY, Kim JH, Kim TU, Ryu H, Lee TB, Ryu JH, Jeon UB. Percutaneous N-Butyl-Cyanoacrylate Embolization for Treating Ruptured Pancreaticoduodenal Aneurysm: A Case Report. Medicina. 2022; 58(10):1320. https://doi.org/10.3390/medicina58101320
Chicago/Turabian StyleJang, Joo Yeon, Jin Hyeok Kim, Tae Un Kim, Hwaseong Ryu, Tae Beom Lee, Je Ho Ryu, and Ung Bae Jeon. 2022. "Percutaneous N-Butyl-Cyanoacrylate Embolization for Treating Ruptured Pancreaticoduodenal Aneurysm: A Case Report" Medicina 58, no. 10: 1320. https://doi.org/10.3390/medicina58101320
APA StyleJang, J. Y., Kim, J. H., Kim, T. U., Ryu, H., Lee, T. B., Ryu, J. H., & Jeon, U. B. (2022). Percutaneous N-Butyl-Cyanoacrylate Embolization for Treating Ruptured Pancreaticoduodenal Aneurysm: A Case Report. Medicina, 58(10), 1320. https://doi.org/10.3390/medicina58101320





