Screening and Bioinformatics Analysis of Crucial Gene of Heart Failure and Atrial Fibrillation Based on GEO Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Preprocessing
2.3. Identification of Differentially Expressed Genes
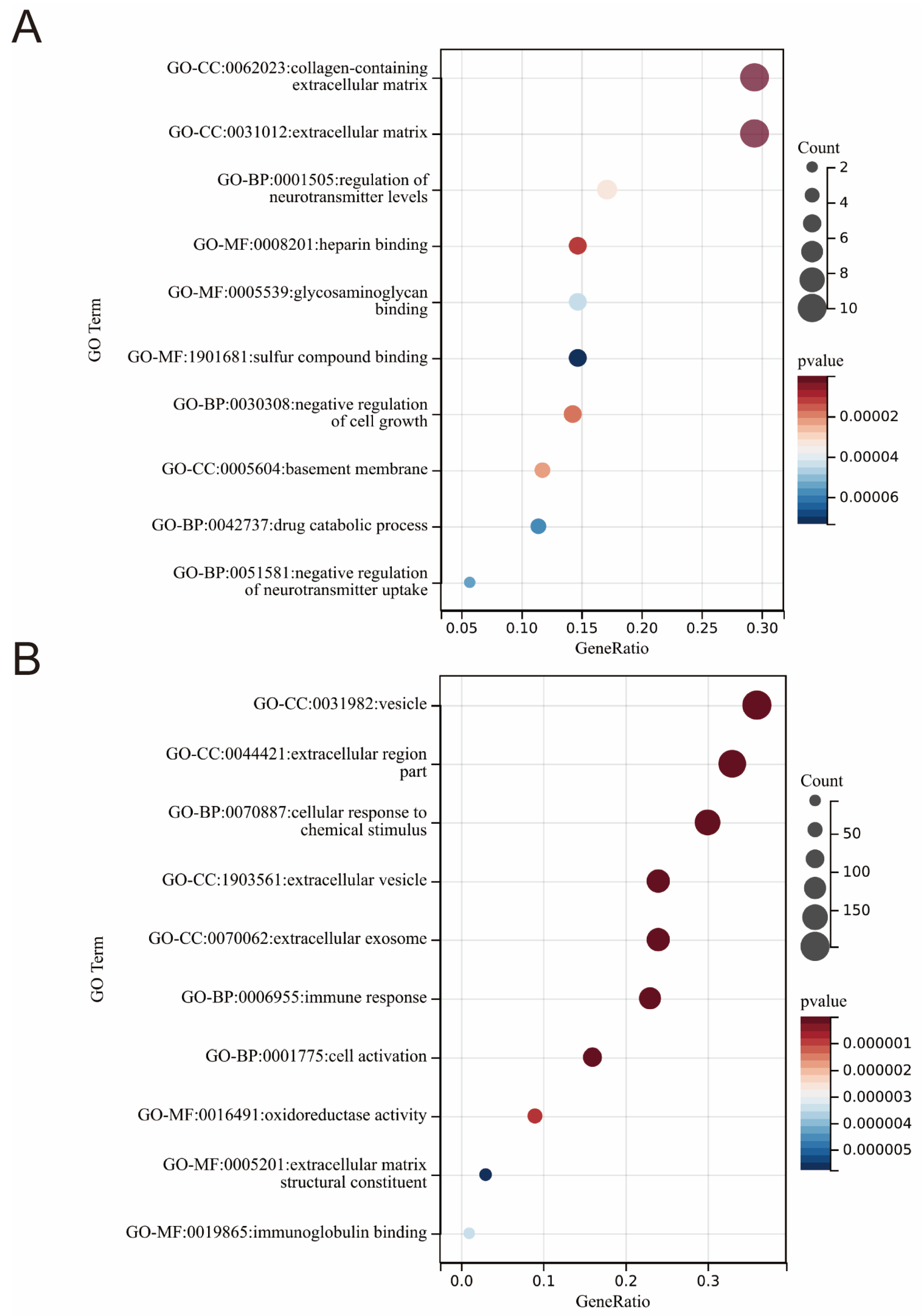
2.4. GO and KEGG Enrichment Analysis
3. Results
3.1. Common Up- and Down-Regulated Genes in HF and AF Datasets
3.2. GO Enrichment Terms in HF and AF Datasets
3.3. KEGG Pathways Identified in HF and AF Datasets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure with Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.; Exner, D.; Gersh, B.; Domanski, M.; Waclawiw, M.; Stevenson, L. Atrial Fibrillation Is Associated with an Increased Risk for Mortality and Heart Failure Progression in Patients with Asymptomatic and Symptomatic Left Ventricular Systolic Dysfunction: A Retrospective Analysis of the Solvd Trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998, 32, 695–703. [Google Scholar] [PubMed]
- Bourassa, M.G.; Gurné, O.; Bangdiwala, S.I.; Ghali, J.K.; Young, J.B.; Rousseau, M.; Johnstone, D.E.; Yusuf, S. Natural History and Patterns of Current Practice in Heart Failure. The Studies of Left Ventricular Dysfunction (Solvd) Investigators. J. Am. Coll. Cardiol. 1993, 22, 14A–19A. [Google Scholar] [CrossRef]
- Middlekauff, R.H.; Stevenson, W.G.; Stevenson, L.W. Prognostic Significance of Atrial Fibrillation in Advanced Heart Failure. A Study of 390 Patients. Circulation 1991, 84, 40–48. [Google Scholar] [CrossRef]
- Doval, H.C.; Nul, D.R.; Grancelli, H.O.; Perrone, S.V.; Bortman, G.R.; Curiel, R. Randomised Trial of Low-Dose Amiodarone in Severe Congestive Heart Failure. Grupo De Estudio De La Sobrevida En La Insuficiencia Cardiaca En Argentina (Gesica). Lancet 1994, 344, 493–498. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; Dilaveris, P.E.; et al. 2020 Esc Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (Eacts): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (Esc) Developed with the Special Contribution of the European Heart Rhythm Association (Ehra) of the Esc. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- John, R.M.; Michaud, G.F.; Stevenson, W.G. Atrial Fibrillation Hospitalization, Mortality, and Therapy. Eur. Heart J. 2018, 39, 3958–3960. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Williams, R.; Hunn, B.; Emdin, C.A. Prognostic Importance of Atrial Fibrillation Timing and Pattern in Adults With Congestive Heart Failure: A Systematic Review and Meta-Analysis. J. Card. Fail. 2016, 23, 56–62. [Google Scholar] [CrossRef]
- Karnik, A.A.; Gopal, D.M.; Ko, D.; Benjamin, E.J.; Helm, R.H. Epidemiology of Atrial Fibrillation and Heart Failure: A Growing and Important Problem. Cardiol. Clin. 2019, 37, 119–129. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal Relations of Atrial Fibrillation and Congestive Heart Failure and Their Joint Influence on Mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.C.; Blackshear, J.L.; McBride, R.; Kronmal, R.A.; Halperin, J.L.; Hart, R.G. Antiarrhythmic Drug Therapy and Cardiac Mortality in Atrial Fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J. Am. Coll. Cardiol. 1992, 20, 527–532. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Gautier, L.; Cope, L.; Bolstad, B.M.; Irizarry, R.A. Affy—Analysis of Affymetrix Genechip Data at the Probe Level. Bioinformatics 2004, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Koniari, I.; Artopoulou, E.; Velissaris, D.; Ainslie, M.; Mplani, V.; Karavasili, G.; Kounis, N.; Tsigkas, G. Biomarkers in the Clinical Management of Patients with Atrial Fibrillation and Heart Failure. J. Geriatr. Cardiol. JGC 2021, 18, 908–951. [Google Scholar]
- Kemp, C.D.; Conte, J.V. The Pathophysiology of Heart Failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Corradi, D. Atrial Fibrillation from the Pathologist’s Perspective. Cardiovasc. Pathol. 2014, 23, 71–84. [Google Scholar] [CrossRef]
- Leyns, L.; Bouwmeester, T.; Kim, S.-H.; Piccolo, S.; De Robertis, E.M. Frzb-1 Is a Secreted Antagonist of Wnt Signaling Expressed in the Spemann Organizer. Cell 1997, 88, 747–756. [Google Scholar] [CrossRef]
- Dann, C.E.; Hsieh, J.-C.; Rattner, A.; Sharma, D.; Nathans, J.; Leahy, D.J. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 2001, 412, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2017, 70, 68–141. [Google Scholar] [CrossRef] [PubMed]
- Askevold, E.T.; Aukrust, P.; Nymo, S.H.; Lunde, I.G.; Kaasbøll, O.J.; Aakhus, S.; Florholmen, G.; Ohm, I.K.; Strand, M.E.; Attramadal, A.; et al. The Cardiokine Secreted Frizzled-Related Protein 3, a Modulator of Wnt Signalling, in Clinical and Experimental Heart Failure. J. Intern. Med. 2014, 275, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Luo, J.; Ouyang, F.; Cheng, L.; Chen, X.; Zhou, H.; Huang, W.; Zhang, W. Polymorphism Is Associated with an Increased Risk of Essential Hypertension and Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 675222. [Google Scholar] [CrossRef]
- Matsushima, K.; Suyama, T.; Takenaka, C.; Nishishita, N.; Ikeda, K.; Ikada, Y.; Sawa, Y.; Jakt, L.M.; Mori, H.; Kawamata, S. Secreted Frizzled Related Protein 4 Reduces Fibrosis Scar Size and Ameliorates Cardiac Function after Ischemic Injury. Tissue Eng. Part A 2010, 16, 3329–3341. [Google Scholar] [CrossRef]
- Zeng, W.; Cao, Y.; Jiang, W.; Kang, G.; Huang, J.; Xie, S. Knockdown of Sfrp4 attenuates apoptosis to protect against myocardial ischemia/reperfusion injury. J. Pharmacol. Sci. 2019, 140, 14–19. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, J.; Du, Y.; Zhu, E.; Wang, Z.; Que, B.; Miao, H.; Shi, S.; Qin, X.; Zhao, Y.; et al. Human epicardial adipose tissue-derived and circulating secreted frizzled-related protein 4 (SFRP4) levels are increased in patients with coronary artery disease. Cardiovasc. Diabetol. 2017, 16, 133. [Google Scholar] [CrossRef]
- Wolke, C.; Antileo, E.; Lendeckel, U. Wnt Signaling in Atrial Fibrillation. Exp. Biol. Med. 2021, 246, 1112–1120. [Google Scholar] [CrossRef]
- Lin, R.; Wu, S.; Zhu, D.; Qin, M.; Liu, X. Osteopontin induces atrial fibrosis by activating Akt/GSK-3β/β-catenin pathway and suppressing autophagy. Life Sci. 2020, 245, 117328. [Google Scholar] [CrossRef]
- Xiong, Y.; Wen, S.; Li, Y.; Wei, Y.; Fang, B.; Li, C.; Huang, Q.; Lin, X. Comprehensive Analysis of Transcriptomics and Metabolomics to Illustrate the Underlying Mechanism of Helenalin against Hepatic Fibrosis. Eur. J. Pharmacol. 2022, 919, 174770. [Google Scholar] [CrossRef]
- Leventoux, N.; Augustus, M.; Azar, S.; Riquier, S.; Villemin, J.P.; Guelfi, S.; Falha, L.; Bauchet, L.; Gozé, C.; Ritchie, W.; et al. Transformation Foci in Idh1-Mutated Gliomas Show Stat3 Phosphorylation and Downregulate the Metabolic Enzyme Etnppl, a Negative Regulator of Glioma Growth. Sci. Rep. 2020, 10, 5504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Chen, W.C.; Li, K.; Wu, G.; Zhang, W.; Ma, P.Z.; Feng, S.Q. Tissue-based metabolomics reveals metabolic signatures and major metabolic pathways of gastric cancer with help of transcriptomic data from TCGA. Biosci. Rep. 2021, 41, BSR20211476. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Yang, P.C. Myocardial Edema on T2-Weighted Mri: New Marker of Ischemia Reperfusion Injury and Adverse Myocardial Remodeling. Circ. Res. 2017, 121, 326–328. [Google Scholar] [CrossRef]
- Ivanova, V.; Sotnikov, A.; Melnikov, M.; Karpov, A. Ultrastructural changes in the endocardium and endocrine cardiomyocytes in the wall of the left atrial appendage in patients with atrial fibrillation. Arkhiv Patol. 2020, 82, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zeng, J.; Wu, G.; Zheng, L.; Huang, M.; Huang, X. Xinshuitong Capsule extract attenuates doxorubicin-induced myocardial edema via regulation of cardiac aquaporins in the chronic heart failure rats. Biomed. Pharmacother. 2021, 144, 112261. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, X.; Gao, Y.; Liu, L.; Li, Z.; Chen, X.; Zeng, J.; Ye, Z.; Li, G. Identification of potential crucial genes in atrial fibrillation: A bioinformatic analysis. BMC Med. Genom. 2020, 13, 104. [Google Scholar] [CrossRef]
- Harrison, S.C.; Zabaneh, D.; Asselbergs, F.W.; Drenos, F.; Jones, G.T.; Shah, S.; Gertow, K.; Sennblad, B.; Strawbridge, R.J.; Gigante, B.; et al. A gene-centric study of common carotid artery remodelling. Atherosclerosis 2013, 226, 440–446. [Google Scholar] [CrossRef]
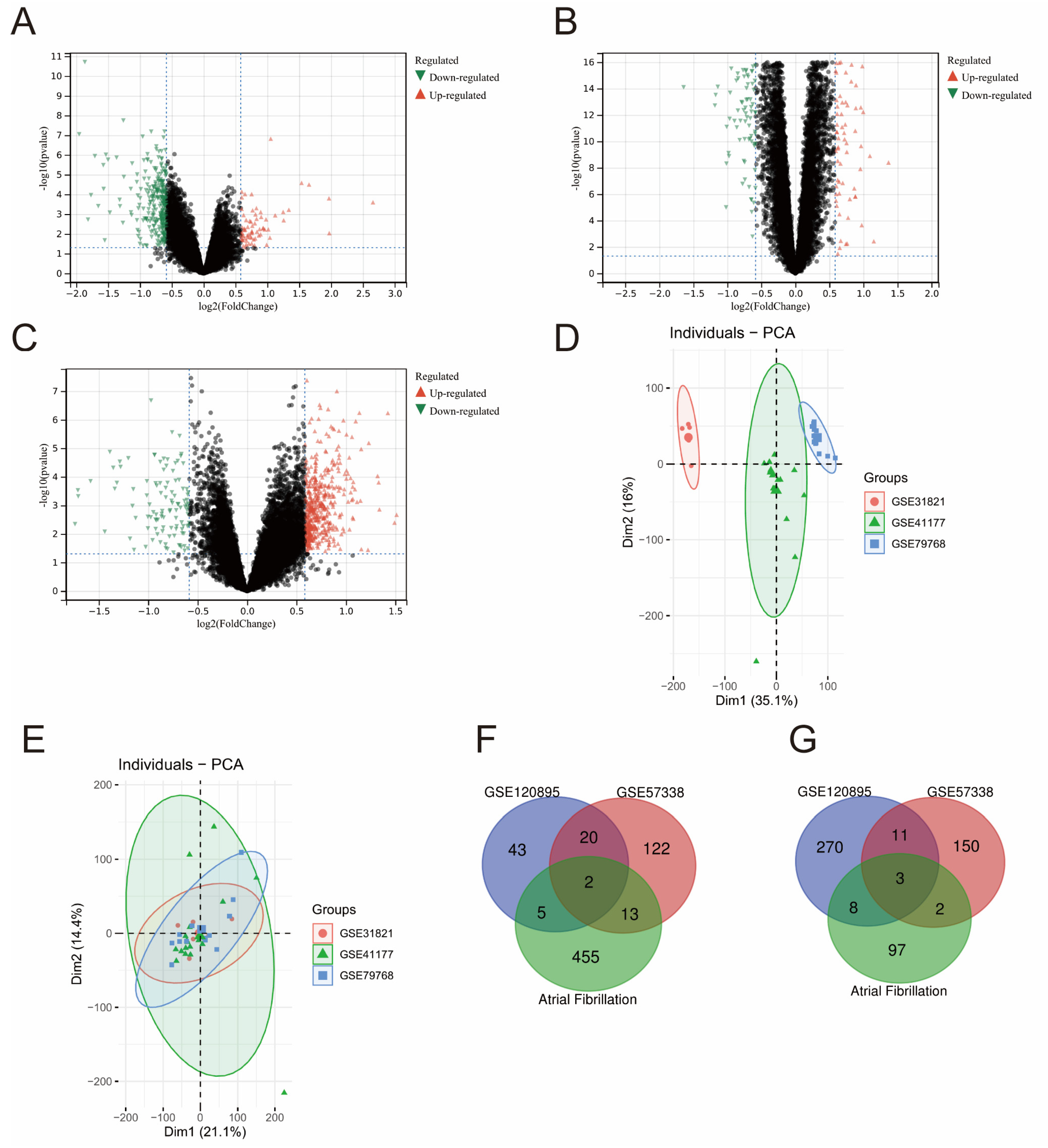
| GEO Serial Number | Diseases | Platform | Number of Subjects | Samples | Study Site |
|---|---|---|---|---|---|
| GSE120895 | Heart Failure | GPL570 | 55 (47 HF + 8 Controls) | Endocardium tissue | Germany |
| GSE57338 | Heart Failure | GPL11532 | 218 (82 HF + 136 Controls) | Left ventricle tissue | the United States |
| GSE31821 | Atrial Fibrillation | GPL570 | 6 (4 AF + 2 Controls) | left atrial appendage | France |
| GSE41177 | Atrial Fibrillation | GPL570 | 19 (16 AF + 3 Controls) | Left atrial appendage | Taiwan |
| GSE79768 | Atrial Fibrillation | GPL570 | 13 (7 AF + 6 Controls) | Left atrial appendage | Taiwan |
| GO Term | Gene Ratio | Count | p Value | Q Value |
|---|---|---|---|---|
| GO-MF:0017147: Wnt-protein binding | 0.40 | 2.00 | 2.43 × 10−5 | 3.33 × 10−4 |
| GO-BP:0030856: regulation of epithelial cell differentiation | 0.50 | 2.00 | 4.19 × 10−4 | 1.86 × 10−2 |
| GO-BP:0090090: negative regulation of canonical Wnt signaling pathway | 0.50 | 2.00 | 5.25 × 10−4 | 1.86 × 10−2 |
| KEGG Term | Gene Ratio | Count | p Value | Q Value |
|---|---|---|---|---|
| hsa04022: cGMP-PKG signaling pathway | 0.19 | 3 | 4.22 × 10−3 | 1.44 × 10−1 |
| hsa04976: Bile secretion | 0.13 | 2 | 9.02 × 10−3 | 1.44 × 10−1 |
| hsa01230: Biosynthesis of amino acids | 0.13 | 2 | 9.76 × 10−3 | 1.44 × 10−1 |
| KEGG Term | Gene Ratio | Count | p Value | Q Value |
|---|---|---|---|---|
| hsa05140: Leishmaniasis | 0.05 | 16 | 1.46 × 10−8 | 3.54 × 10−6 |
| hsa05150: Staphylococcus aureus infection | 0.05 | 15 | 2.43 × 10−6 | 2.94 × 10−4 |
| hsa04145: Phagosome | 0.06 | 18 | 1.41 × 10−5 | 1.14 × 10−3 |
| hsa00190: Oxidative phosphorylation | 0.05 | 16 | 3.49 × 10−5 | 2.11 × 10−3 |
| hsa04672: Intestinal immune network for IgA production | 0.03 | 9 | 7.15 × 10−5 | 3.14 × 10−3 |
| hsa05012: Parkinson disease | 0.05 | 16 | 7.79 × 10−5 | 3.14 × 10−3 |
| hsa05016: Huntington disease | 0.06 | 18 | 3.23 × 10−4 | 1.05 × 10−2 |
| hsa05416: Viral myocarditis | 0.03 | 9 | 3.58 × 10−4 | 1.05 × 10−2 |
| hsa04514: Cell adhesion molecules (CAMs) | 0.05 | 15 | 3.91 × 10−4 | 1.05 × 10−2 |
| hsa05010: Alzheimer disease | 0.05 | 16 | 6.66 × 10−4 | 1.49 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Qiao, Z.; Bi, X.; Han, D.; Jiang, Q.; Zhang, Y.; Wang, F.; Liu, M.; An, Q.; Shangguan, J.; et al. Screening and Bioinformatics Analysis of Crucial Gene of Heart Failure and Atrial Fibrillation Based on GEO Database. Medicina 2022, 58, 1319. https://doi.org/10.3390/medicina58101319
Zhuang Y, Qiao Z, Bi X, Han D, Jiang Q, Zhang Y, Wang F, Liu M, An Q, Shangguan J, et al. Screening and Bioinformatics Analysis of Crucial Gene of Heart Failure and Atrial Fibrillation Based on GEO Database. Medicina. 2022; 58(10):1319. https://doi.org/10.3390/medicina58101319
Chicago/Turabian StyleZhuang, Yuansong, Zhentao Qiao, Xuanye Bi, Dongjian Han, Qingjiao Jiang, Yi Zhang, Fuhang Wang, Miaomiao Liu, Quanxu An, Jiahong Shangguan, and et al. 2022. "Screening and Bioinformatics Analysis of Crucial Gene of Heart Failure and Atrial Fibrillation Based on GEO Database" Medicina 58, no. 10: 1319. https://doi.org/10.3390/medicina58101319
APA StyleZhuang, Y., Qiao, Z., Bi, X., Han, D., Jiang, Q., Zhang, Y., Wang, F., Liu, M., An, Q., Shangguan, J., & Shen, D. (2022). Screening and Bioinformatics Analysis of Crucial Gene of Heart Failure and Atrial Fibrillation Based on GEO Database. Medicina, 58(10), 1319. https://doi.org/10.3390/medicina58101319







