Clinicopathologic Features of Lymphoproliferative Neoplasms Involving the Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
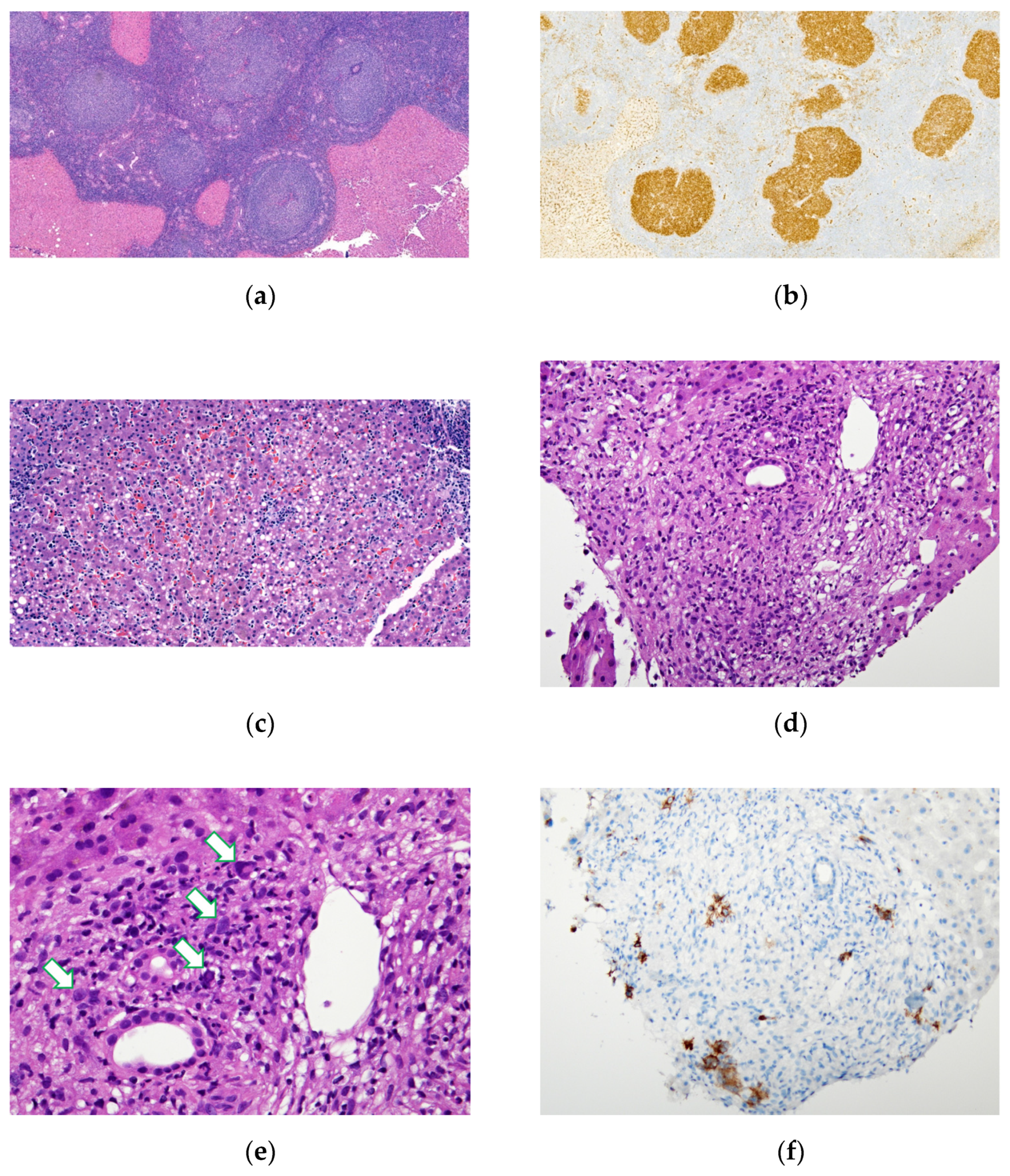
3. Results
3.1. Demographic Details, Clinical Features and Laboratory Findings
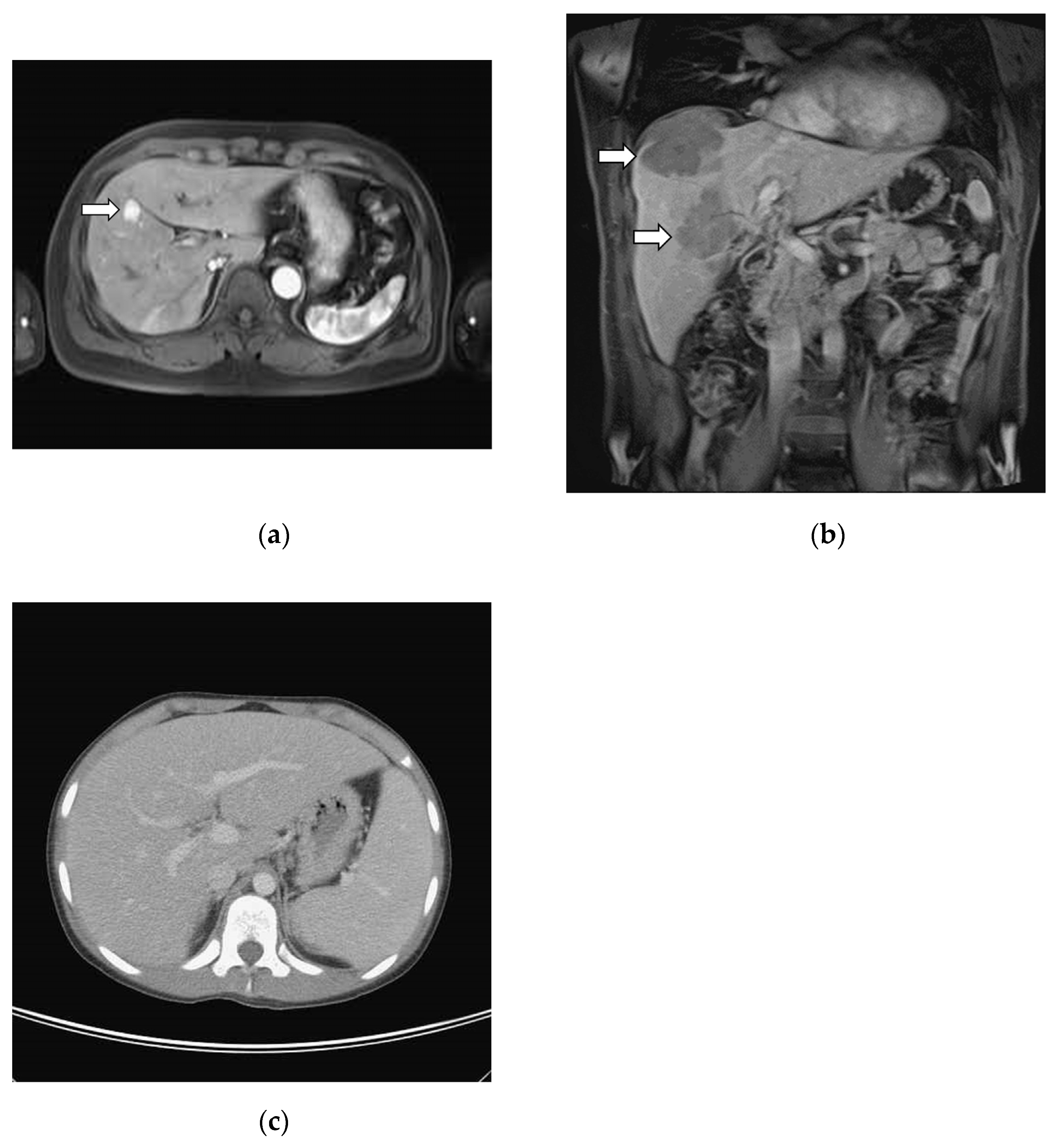
3.2. Image Studies
3.3. Correlation between Radiographic Findings and Histological Patterns
3.4. Prognosis and Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffe, E.S. Malignant lymphomas: Pathology of hepatic involvement. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 1987; pp. 257–268. [Google Scholar]
- Kit Lei, K.I. Primary non-Hodgkin’s lymphoma of the liver. Leuk. Lymphoma 1998, 29, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-F.; Liu, J.-M.; Zhang, X.-M.; Jiang, C.-Z.; Xu, X.; Zheng, S.-S. Percutaneous liver biopsy: Retrospective study of primary and secondary hepatic lymphoma in twenty-one patients. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 58–64. [Google Scholar] [CrossRef]
- Ugurluer, G.; Miller, R.C.; Li, Y.; Thariat, J.; Ghadjar, P.; Schick, U.; Ozsahin, M. Primary hepatic lymphoma: A retrospective, multicenter rare cancer network study. Rare Tumors 2016, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Hepatic manifestations of lymphoproliferative disorders. Clin. Liver Dis. 2019, 23, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Qing, A.C.; Cai, J.; Yue, C.; French, S.W.; Qing, X. Lymphoma of the liver: Clinicopathological features of 19 patients. Exp. Mol. Pathol. 2016, 100, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Wang, R.-C.; Chen, B.-J.; Chen, W.-Y.; Jhuang, J.-Y.; Chang, M.-C.; Wu, Y.-H.; Nakada, N.; Karube, K.; Chuang, S.-S. Granuloma With an Underlying Lymphoma: A Diagnostic Challenge and a Wider Histologic Spectrum Including Adult T-Cell Leukemia/Lymphoma. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.; Campo, E.; Harris, N.L.; Jaffe, E.; Pileri, S.; Stein, H.; Thiele, J.; Arber, D.; Hasserjian, R.; Le Beau, M. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017; Volume 421, p. 439. [Google Scholar]
- Semelka, R.C.; Nimojan, N.; Chandana, S.; Ramalho, M.; Palmer, S.L.; DeMulder, D.; Villavicencio, C.P.; Woosley, J.; Garon, B.L.; Jha, R.C. MRI features of primary rare malignancies of the liver: A report from four university centres. Eur. Radiol. 2018, 28, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Kamimura, K.; Kawai, H.; Kamimura, H.; Domori, K.; Kobayashi, Y.; Nomoto, M.; Aoyagi, Y. Diagnostic imaging of hepatic lymphoma. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Guan, Y.-S. Primary hepatic lymphoma: Is it a disease entity? Liver 2017, 20, 21. [Google Scholar] [CrossRef][Green Version]
- Avlonitis, V.S.; Linos, D. Primary hepatic lymphoma: A review. Eur. J. Surg. 1999, 165, 725–729. [Google Scholar] [PubMed]
- Choi, W.-T.; Gill, R.M. Hepatic lymphoma diagnosis. Surg. Pathol. Clin. 2018, 11, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Page, R.D.; Romaguera, J.E.; Osborne, B.; Medeiros, L.J.; Rodriguez, J.; North, L.; Sanz-Rodriguez, C.; Cabanillas, F. Primary hepatic lymphoma: Favorable outcome after combination chemotherapy. Cancer 2001, 92, 2023–2029. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, H.; Wang, W.-P.; Jin, Y.-J.; Ji, Z.-B. Primary non-Hodgkin’s lymphoma of the liver: Sonographic and CT findings. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 75–81. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Menias, C.; Willatt, J.; Pandya, A.; Wiggins, M. Primary hepatic lymphoma: Imaging findings. J. Med. Imaging Radiat. Oncol. 2009, 53, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Mastoraki, A.; Stefanou, M.I.; Chatzoglou, E.; Danias, N.; Kyriazi, M.; Arkadopoulos, N.; Smyrniotis, V. Primary hepatic lymphoma: Dilemmas in diagnostic approach and therapeutic management. Indian J. Hematol. Blood Transfus. 2014, 30, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Cesaretti, M.; Loustau, M.; Robba, C.; Senescende, L.; Le Bian, A.Z. Reappraisal of primary hepatic lymphoma: Is surgical resection underestimated? Crit. Rev. Oncol./Hematol. 2018, 123, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Colagrande, S.; Calistri, L.; Grazzini, G.; Nardi, C.; Busoni, S.; Morana, G.; Grazioli, L. MRI features of primary hepatic lymphoma. Abdom. Radiol. 2018, 43, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Schechter, S.; Lamps, L. Epstein-Barr virus hepatitis: A review of clinicopathologic features and differential diagnosis. Arch. Pathol. Lab. Med. 2018, 142, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Padhan, R.K.; Das, P. Primary hepatic lymphoma. Trop. Gastroenterol. 2015, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Muazzam, I.; Siddiqui, N.; Muzaffar, N.; Das, K.; Awan, U. Liver Involvement by Lymphoma–Indicator of Poor Response and Mortality. Ann. Oncol. 2012, 23, ix358. [Google Scholar] [CrossRef]
| Pathologic Classification a | PHL (n = 6) | SHL (n = 30) | ||
|---|---|---|---|---|
| Mature B cell lymphoma | 5 | (83%) | 15 | (50%) |
| Diffuse Large B-cell Lymphoma | 4 | (67%) | 12 | (40%) |
| Burkitt’s Lymphoma | 0 | 2 | (7%) | |
| Follicular Lymphoma | 1 | (17%) | 0 | |
| Mature B-cell Lymphoma (Unclassified due to limited tissue) | 0 | 1 | (3%) | |
| Mature T cell lymphoma | 1 | (17%) | 11 | (37%) |
| Peripheral T-cell lymphoma | 0 | 5 | (17%) | |
| NK/T-cell lymphoma, nasal type | 0 | 1 | (3%) | |
| Anaplastic Large-cell Lymphoma | 0 | 1 | (3%) | |
| Hepatosplenic T cell lymphoma | 1 | (17%) | 0 | |
| Mature T-cell Lymphoma (Unclassified due to limited tissue) | 0 | 4 | (13.3%) | |
| Hodgkin lymphoma | 0 | (0%) | 3 | (10%) |
| Classical Hodgkin Lymphoma | ||||
| Other | 0 | (0%) | 1 | (3%) |
| EBV-associated polymorphic posttransplant lymphoproliferative disorder | 0 | 1 | (3%) | |
| PHL (n = 6) | SHL (n = 30) | p Value | |||||
|---|---|---|---|---|---|---|---|
| N # | N # | ||||||
| Age at Diagnosisa (years) Mean ± SD (median) | 6 | 58.1 | ±17.36 (59) | 30 | 59.52 | ±16.57 (63) | 0.349 |
| Sexa | 30 | 1.000 | |||||
| Male | 4 | (66.67%) | 19 | (63.33%) | |||
| Female | 2 | (33.33%) | 11 | (36.67%) | |||
| B symptoma | 6 | 3 | (50%) | 30 | 22 | (73.33%) | 0.343 |
| IPI scorea | 6 | 29 | <0.001 | ||||
| 0–1 | 1 | (16.67%) | 0 | ||||
| 2–3 | 5 | (83.33%) | 5 | (17.24%) | |||
| 4–5 | 0 | 24 | (82.76%) | ||||
| Laba (Mean ± SD) | |||||||
| Hemoglobin (g/L) | 6 | 113.8 | ±23.8 | 30 | 98.4 | ±18.7 | 0.149 |
| Platelet (×109/L) | 6 | 266 | ±57.94 | 30 | 130.87 | ±89.09 | <0.001 |
| AST (U/L) | 6 | 192.17 | ±229.92 | 30 | 146.13 | ±139.04 | 0.893 |
| ALT (U/L) | 6 | 217.46 | ±91.53 | 30 | 91.53 | ±90.75 | 0.246 |
| ALK-P (U/L) | 6 | 499.33 | ±566.57 | 30 | 637.43 | ±688.85 | 0.268 |
| Total Bilirubin (μmol/L) | 6 | 76.11 | ±131.7 | 29 | 78.49 | ±98.5 | 0.542 |
| Direct Bilirubin (μmol/L) | 4 | 65 | ±96.47 | 27 | 48.56 | ±64.3 | 0.898 |
| LDH (U/L) | 6 | 991 | ±610.89 | 29 | 1135.14 | ±1691.41 | 0.589 |
| GGT (U/L) | 2 | 329 | ±173.95 | 10 | 518.50 | ±461.60 | 0.606 |
| Albumin (g/L) | 6 | 32.8 | ±6.6 | 29 | 29.6 | ±6.6 | 0.372 |
| AFP (μg/L) | 3 | 3.6 | ±0.86 | 15 | 3.47 | ±2.71 | 0.426 |
| CEA (μg/L) | 1 | 2.45 | 13 | 3.32 | ±2.29 | 0.857 | |
| Status of Last Follow-upa | 6 | 29 | |||||
| Expired | 0 | (0%) | 21 | (72.41%) | 0.002 | ||
| Follow-up (months) (Mean ± SD) | 44.52 | ±28.94 | 9.46 | ±14.12 | 0.002 | ||
| Image Findings a | PHL | (n = 6) | SHL | (n = 29 #) |
|---|---|---|---|---|
| Mass lesion | ||||
| Single mass | 33% | (2/6) | 7% | (2/29) |
| Multiple masses | 50% | (3/6) | 66% | (19/29) |
| Non-mass lesion | ||||
| Hepatomegaly | 0% | 0 | 14% | (4/29) |
| Negative image findings | 17% | (1/6) | 14% | (4/29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, I.; Wang, R.-C.; Lai, Y.-C.; Chang, C.-C.; Chen, C.-H.; Hsu, C.-Y.; Chen, C.-H. Clinicopathologic Features of Lymphoproliferative Neoplasms Involving the Liver. Medicina 2022, 58, 72. https://doi.org/10.3390/medicina58010072
Chiang I, Wang R-C, Lai Y-C, Chang C-C, Chen C-H, Hsu C-Y, Chen C-H. Clinicopathologic Features of Lymphoproliferative Neoplasms Involving the Liver. Medicina. 2022; 58(1):72. https://doi.org/10.3390/medicina58010072
Chicago/Turabian StyleChiang, I, Ren-Ching Wang, Ying-Ching Lai, Chung-Che Chang, Chuan-Han Chen, Chiann-Yi Hsu, and Chi-Hung Chen. 2022. "Clinicopathologic Features of Lymphoproliferative Neoplasms Involving the Liver" Medicina 58, no. 1: 72. https://doi.org/10.3390/medicina58010072
APA StyleChiang, I., Wang, R.-C., Lai, Y.-C., Chang, C.-C., Chen, C.-H., Hsu, C.-Y., & Chen, C.-H. (2022). Clinicopathologic Features of Lymphoproliferative Neoplasms Involving the Liver. Medicina, 58(1), 72. https://doi.org/10.3390/medicina58010072






