A Rare Case of Epithelioid Trophoblastic Tumor Presenting as Hematoma of a Caesarean Scar in the Lower Uterine Segment
Abstract
:1. Introduction
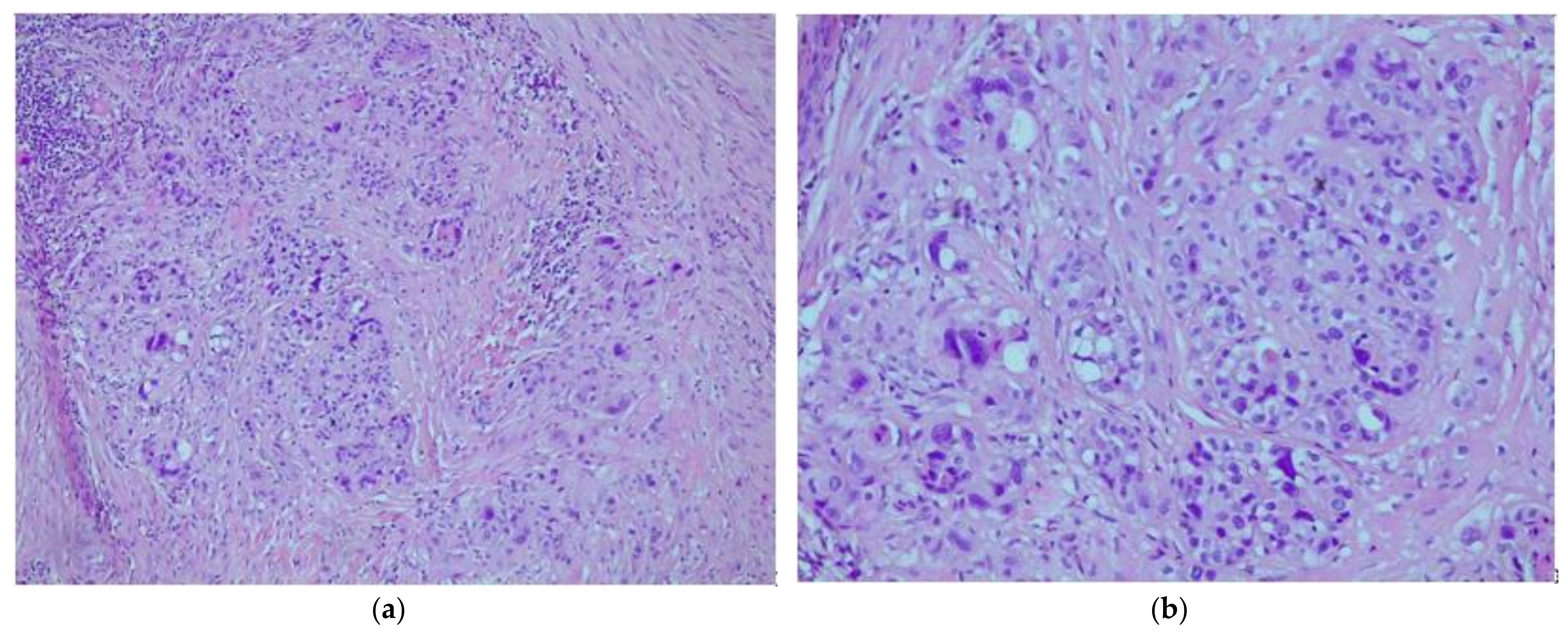
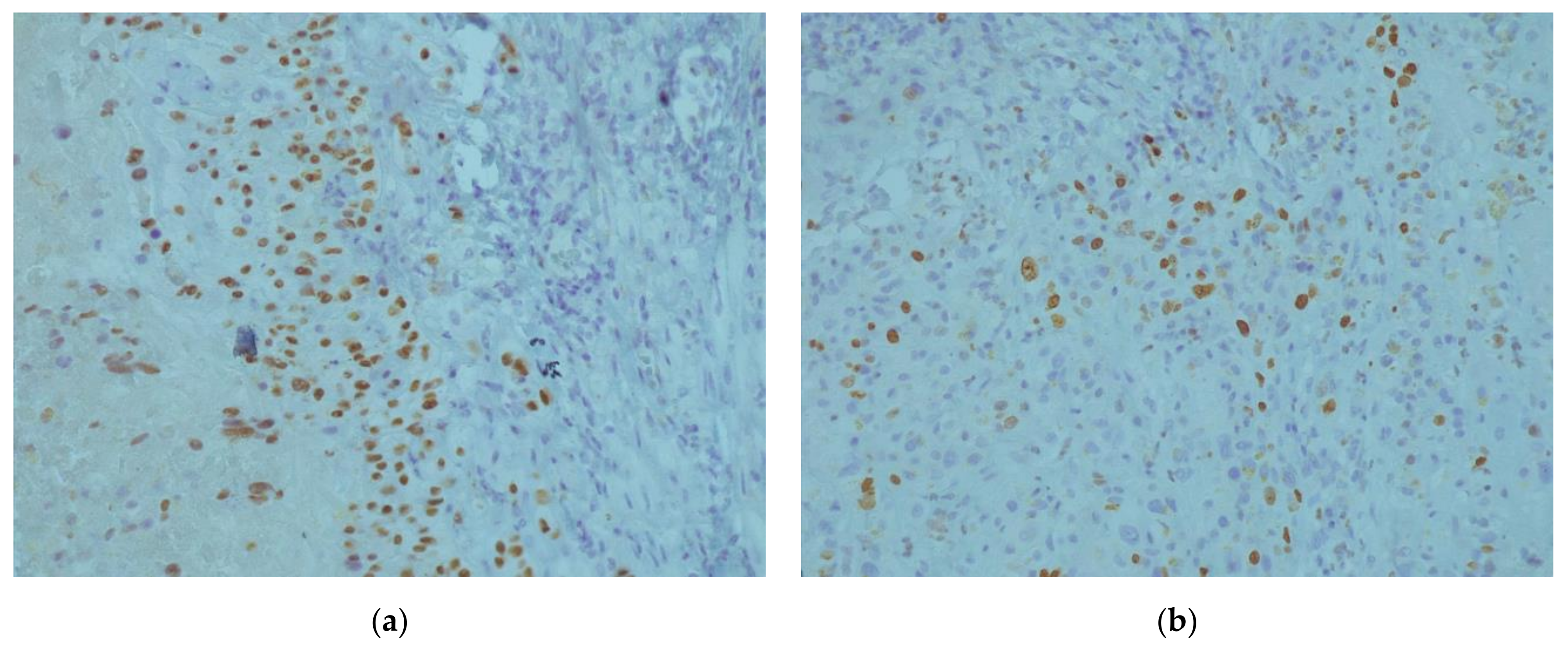
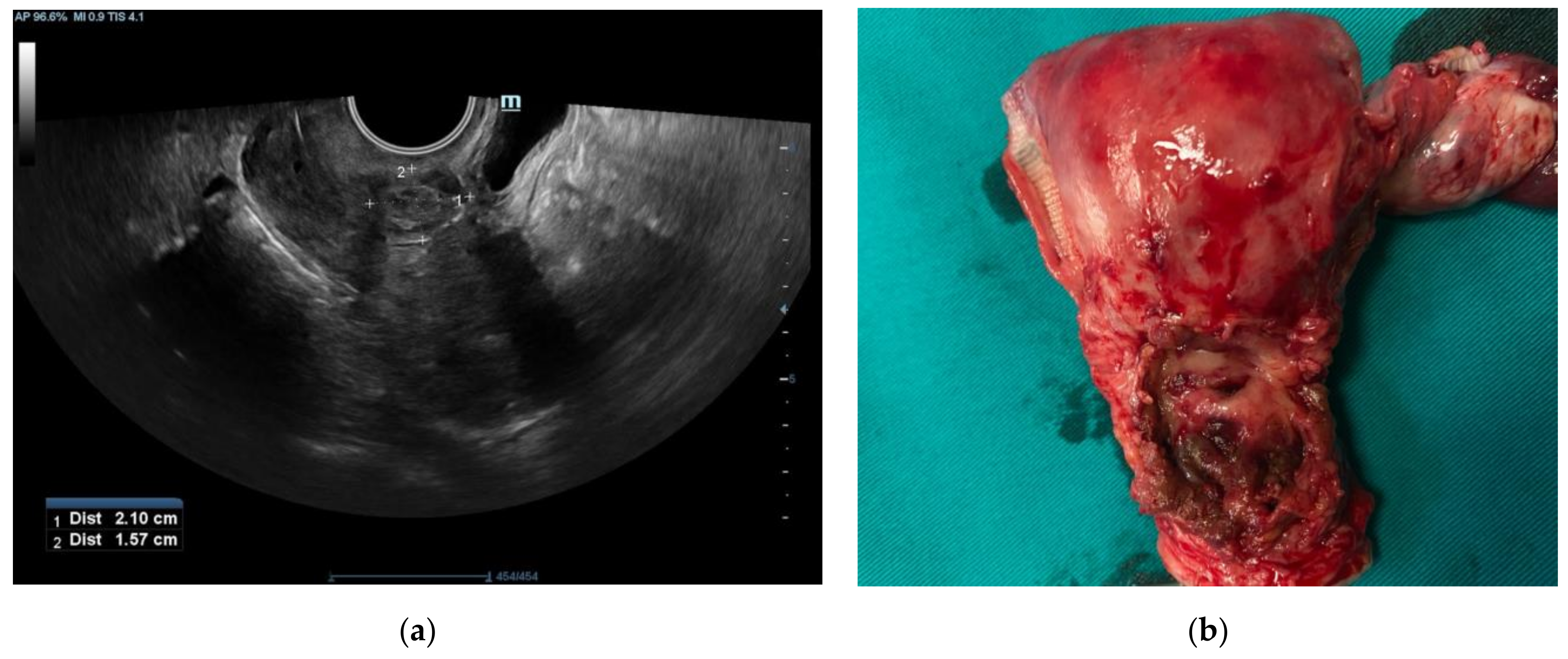
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genest, D.R.B.R.; Fisher, R.A. Gestational Trophoblastic Disease. In WHO Classification of Tumours Pathology and Genetics of Tumours of the Breast and Female Genital Organs; Tavassoli, F.A., Ed.; IARC Press: Lyon, France, 2003; pp. 250–254. [Google Scholar]
- Shih, I.-M.; Kurman, R.J. Epithelioid Trophoblastic Tumor: A Neoplasm Distinct From Choriocarcinoma and Placental Site Trophoblastic Tumor Simulating Carcinoma. Am. J. Surg. Pathol. 1998, 22, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zong, L.; Wang, J.; Wan, X.; Feng, F.; Xiang, Y. Epithelioid Trophoblastic Tumors: Treatments, Outcomes, and Potential Therapeutic Targets. J. Cancer 2019, 10, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Carinelli, S.; Guerrieri, M.E.; Aletti, G.D. Placental Site Trophoblastic Tumor and Epithelioid Trophoblastic Tumor: Clinical and Pathological Features, Prognostic Variables and Treatment Strategy. Gynecol. Oncol. 2019, 153, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lü, W.; Lü, B. Epithelioid Trophoblastic Tumor: An Outcome-Based Literature Review of 78 Reported Cases. Int. J. Gynecol. Cancer 2013, 23. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Simone, K.; Hirt-Walsh, C.; Sabourin, J. Epithelioid Trophoblastic Tumor Presenting as a Caesarean Scar Defect: A Case Report. Gynecol. Oncol. Rep. 2021, 36, 100715. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, J.; Zhang, Y.; Xiong, H.; Sheng, X. Epithelioid Trophoblastic Tumor Coexisting with Choriocarcinoma around an Abdominal Wall Cesarean Scar: A Case Report and Review of the Literature. J. Med. Case Rep. 2020, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Hsiue, E.H.-C.; Hsu, C.; Tseng, L.-H.; Lu, T.-P.; Kuo, K.-T. Epithelioid Trophoblastic Tumor Around an Abdominal Cesarean Scar: A Pathologic and Molecular Genetic Analysis. Int. J. Gynecol. Pathol. 2017, 36, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, K.; Shi, H.; Qin, J.; Lu, B.; Huang, L. Atypical Postcesarean Epithelioid Trophoblastic Lesion with Cyst Formation: A Case Report and Literature Review. Hum. Pathol. 2015, 46, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Rezai, S.; Hughes, A.C.; Henderson, C.E.; Liu, J. Synchronous Choriocarcinoma and Epithelioid Trophoblastic Tumor Concurring at the Cesarean Scar: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2019, 2019, e5093938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson, L.E.; Kong, C.S.; Zaloudek, C. Epithelioid Trophoblastic Tumor of the Uterus in a Postmenopausal Woman: A Case Report and Review of the Literature. Am. J. Surg. Pathol. 2000, 24, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Frijstein, M.M.; Lok, C. a. R.; Trommel, N.E. van; Kate-Booij, M.J. ten; Massuger, L.F. a. G.; Werkhoven, E. van; Kaur, B.; Tidy, J.A.; Sarwar, N.; Golfier, F.; et al. Management and Prognostic Factors of Epithelioid Trophoblastic Tumors: Results from the International Society for the Study of Trophoblastic Diseases Database. Gynecol. Oncol. 2019, 152, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Aniţei, M.-G.; Lazăr, D.-E.; Pleşca, R.A.; Terinte, C.; Iulian, R.; Viorel, S. Uterine Epithelioid Trophoblastic Tumor in a 44-Year-Old Woman: A Diagnostic Dilemma. Clin. Pract. 2021, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.W.; Tidy, J. Placental Site Trophoblastic Tumour and Epithelioid Trophoblastic Tumour. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 74, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ying, W.; Cheng, X.; Wu, X.; Lu, B.; Liang, Y.; Wang, X.; Wan, X.; Xie, X.; Lu, W. A Well-Circumscribed Border with Peripheral Doppler Signal in Sonographic Image Distinguishes Epithelioid Trophoblastic Tumor from Other Gestational Trophoblastic Neoplasms. PLoS ONE 2014, 9, e112618. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.S.; Goldstein, D.P.; Berkowitz, R.S. Placental Site Trophoblastic Tumors and Epithelioid Trophoblastic Tumors: Biology, Natural History, and Treatment Modalities. Gynecol. Oncol. 2017, 144, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B. Pathology of Gestational Trophoblastic Disease (GTD). Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 74, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Hui, P. Gestational Trophoblastic Tumors: A Timely Review of Diagnostic Pathology. Arch. Pathol. Lab. Med. 2018, 143, 65–74. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aničić, R.; Rakić, A.; Maglić, R.; Sretenović, D.; Ristić, A.; Đaković, E.; Nejković, L. A Rare Case of Epithelioid Trophoblastic Tumor Presenting as Hematoma of a Caesarean Scar in the Lower Uterine Segment. Medicina 2022, 58, 34. https://doi.org/10.3390/medicina58010034
Aničić R, Rakić A, Maglić R, Sretenović D, Ristić A, Đaković E, Nejković L. A Rare Case of Epithelioid Trophoblastic Tumor Presenting as Hematoma of a Caesarean Scar in the Lower Uterine Segment. Medicina. 2022; 58(1):34. https://doi.org/10.3390/medicina58010034
Chicago/Turabian StyleAničić, Radomir, Aleksandar Rakić, Rastko Maglić, Dragutin Sretenović, Aleksandar Ristić, Elena Đaković, and Lazar Nejković. 2022. "A Rare Case of Epithelioid Trophoblastic Tumor Presenting as Hematoma of a Caesarean Scar in the Lower Uterine Segment" Medicina 58, no. 1: 34. https://doi.org/10.3390/medicina58010034
APA StyleAničić, R., Rakić, A., Maglić, R., Sretenović, D., Ristić, A., Đaković, E., & Nejković, L. (2022). A Rare Case of Epithelioid Trophoblastic Tumor Presenting as Hematoma of a Caesarean Scar in the Lower Uterine Segment. Medicina, 58(1), 34. https://doi.org/10.3390/medicina58010034





