Appraisal of Experimental Methods to Manage Menopause and Infertility: Intraovarian Platelet-Rich Plasma vs. Condensed Platelet-Derived Cytokines
Abstract
1. Introduction
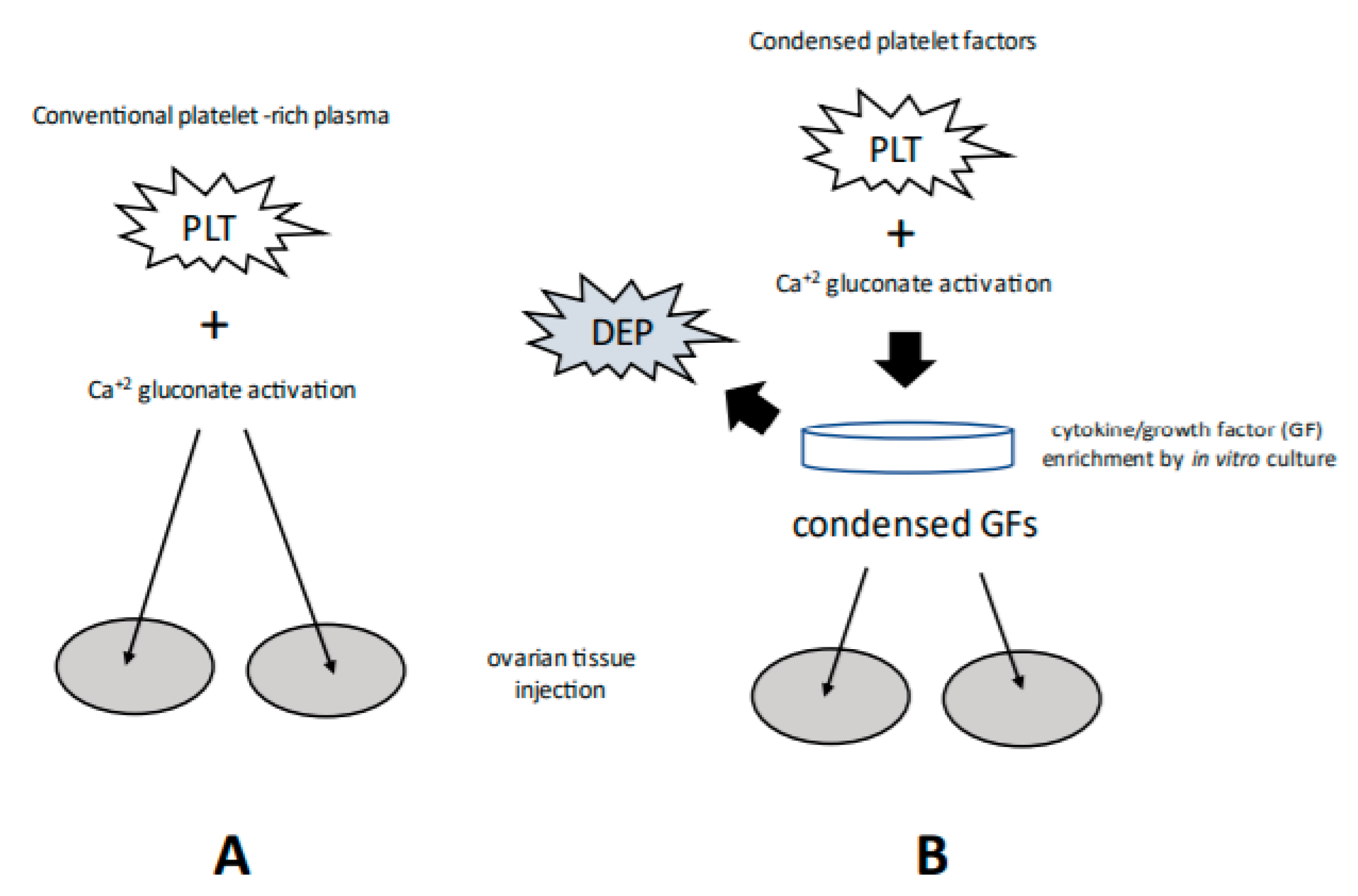
2. Therapeutic Rationale
3. Ovarian PRP: Veterinary and Human Research
4. Patient and Protocol Differences
5. Considerations and Contraindications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sfakianoudis, K.; Simopoulou, M.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Rapani, A.; Giannelou, P.; Nitsos, N.; Kokkali, G.; et al. Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. J. Clin. Med. 2020, 9, 1809. [Google Scholar] [CrossRef]
- Sills, E.S.; Rickers, N.S.; Svid, C.; Rickers, J.M.; Wood, S.H. Normalized Ploidy Following 20 Consecutive Blastocysts with Chromosomal Error: Healthy 46,XY Pregnancy with IVF after Intraovarian Injection of Autologous Enriched Platelet-derived Growth Factors. Int. J. Mol. Cell. Med. 2019, 8, 84–89. [Google Scholar]
- Pantos, K.; Nitsos, N.; Kokkali, G.; Vaxevanoglou, T.; Markomichali, C.; Pantou, A.; Grammatis, M.; Lazaros, L.; Sfakianoudis, K. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet rich plasma treatment. In Proceedings of the ESHRE 32nd Annual Meeting, Helsinki, Finland, 3–6 July 2016. [abstract] Hum Reprod 2016; Suppl. 1, p. i301. [Google Scholar]
- Yao, D.; Feng, G.; Zhao, F.; Hao, D. Effects of platelet-rich plasma on the healing of sternal wounds: A meta-analysis. Wound Repair Regen. 2021, 29, 153–167. [Google Scholar] [CrossRef]
- Dervishi, G.; Liu, H.; Peternel, S.; Labeit, A.; Peinemann, F. Autologous platelet-rich plasma therapy for pattern hair loss: A systematic review. J. Cosmet. Dermatol. 2020, 19, 827–835. [Google Scholar] [CrossRef]
- Dunn, A.; Long, T.; Kleinfelder, R.E.; Zarraga, M.B. The Adjunct Use of Platelet-Rich Plasma in Split-Thickness Skin Grafts: A Systematic Review. Adv. Ski. Wound Care 2021, 34, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, U.S.; Puri, N.; More, C.B.; Gupta, R.; Gupta, D. Comparative evaluation of effectiveness of autologous platelet rich plasma and intralesional corticosteroids in the management of erosive oral Lichen planus-a clinical study. J. Oral Biol. Craniofacial Res. 2020, 10, 714–718. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review—The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef]
- Evers, J. Do we need an RCT for everything? Hum. Reprod. 2017, 32, 483–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wood, S.H.; Sills, E.S. Intraovarian vascular enhancement via stromal injection of platelet-derived growth factors: Exploring subsequent oocyte chromosomal status and in vitro fertilization outcomes. Clin. Exp. Reprod. Med. 2020, 47, 94–100. [Google Scholar] [CrossRef]
- Bos-Mikich, A.; Ferreira, M.O.; De Oliveira, R.; Frantz, N. Platelet-rich plasma or blood-derived products to improve endometrial receptivity? J. Assist. Reprod. Genet. 2019, 36, 613–620. [Google Scholar] [CrossRef]
- Kamath, M.S.; Mascarenhas, M.; Franik, S.; Liu, E.; Sunkara, S.K. Clinical adjuncts in in vitro fertilization: A growing list. Fertil. Steril. 2019, 112, 978–986. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Rapani, A.; Pantou, A.; Vaxevanoglou, T.; Kokkali, G.; Koutsilieris, M.; Pantos, K. A Case Series on Platelet-Rich Plasma Revolutionary Management of Poor Responder Patients. Gynecol. Obstet. Investig. 2018, 84, 99–106. [Google Scholar] [CrossRef]
- Farimani, M.; Heshmati, S.; Poorolajal, J.; Bahmanzadeh, M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol. Biol. Rep. 2019, 46, 1611–1616. [Google Scholar] [CrossRef]
- Sills, E.S.; Rickers, N.S.; Wood, S.H. Intraovarian insertion of autologous platelet growth factors as cell-free concentrate: Fertility recovery and first unassisted conception with term delivery at age over 40. Int. J. Reprod. Biomed. 2020, 18, 1081–1086. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Retsina, D.; Maziotis, E.; Tsioulou, P.; Giannelou, P.; Pantos, K.; Koutsilieris, M.; Vlahos, N.; et al. Novel Approaches in Addressing Ovarian Insufficiency in 2019: Are We There Yet? Cell Transplant. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Sheshpari, S.; Pazhang, M.; Miranda-Bedate, A.; Beheshti, R.; Abbasi, M.M.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reprod. Biol. Endocrinol. 2020, 18, 78. [Google Scholar] [CrossRef]
- Anvari, S.S.; Dehgan, G.; Razi, M. Preliminary Findings of Platelet-Rich Plasma-Induced Ameliorative Effect on Polycystic Ovarian Syndrome. Cell J. 2019, 21, 243–252. [Google Scholar]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Anna, L.-C. Improvement of Embryo Recovery in Holstein Cows Treated by Intra-Ovarian Platelet Rich Plasma before Superovulation. Vet. Sci. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, P.; Takmaz, T.; Tok, O.E.; Islek, S.; Yiğit, E.N.; Ficicioglu, C. The protective effect of platelet-rich plasma administrated on ovarian function in female rats with Cy-induced ovarian damage. J. Assist. Reprod. Genet. 2020, 37, 865–873. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth Factor and Catabolic Cytokine Concentrations Are Influenced by the Cellular Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef]
- Sills, E.S.; Petersen, J.L.; Rickers, N.S.; Wood, S.H.; Li, X. Regenerative Effect of Intraovarian Injection of Activated Autologous Platelet Rich Plasma: Serum Anti-Mullerian Hormone Levels Measured Among Poor-Prognosis In Vitro Fertilization Patients. Int. J. Regen. Med. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Impact of incubation method on the release of growth factors in non-Ca2+-activated PRP, Ca2+-activated PRP, PRF and A-PRF. J. Cranio-Maxillofac. Surg. 2019, 47, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.J.D.; Matsiko, A.; Tomazette, M.R.; Rocha, W.K.; Cordeiro-Spinetti, E.; Levingstone, T.; Farina, M.; O’Brien, F.J.; El-Cheikh, M.C.; Balduino, A. Platelet-rich plasma releasate differently stimulates cellular commitment toward the chondrogenic lineage according to concentration. J. Tissue Eng. 2015, 6. [Google Scholar] [CrossRef]
- Machado, E.S.; Leite, R.; Dos Santos, C.C.; Artuso, G.L.; Gluszczak, F.; De Jesus, L.G.; Caldas, J.M.P.; Bredemeier, M. Turn down-turn up: A simple and low-cost protocol for preparing platelet-rich plasma. Clinics 2019, 74, e1132. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; da Cruz Pacheco, Í.; Amaral, R.J.F.C.D.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Petryk, N.; Petryk, M. Ovarian Rejuvenation Through Platelet-Rich Autologous Plasma (PRP)—A Chance to Have a Baby Without Donor Eggs, Improving the Life Quality of Women Suffering from Early Menopause Without Synthetic Hormonal Treatment. Reprod. Sci. 2020, 27, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Sills, E.S.; Li, X.; Rickers, N.S.; Wood, S.H.; Palermo, G.D. Metabolic and neurobehavioral response following intraovarian administration of autologous activated platelet rich plasma: First qualitative data. Neuro Endocrinol. Lett. 2019, 39, 427–433. [Google Scholar]
- Stojkovska, S.; Dimitrov, G.; Stamenkovska, N.; Hadzi-Lega, M.; Petanovski, Z. Live Birth Rates in Poor Responders’ Group after Previous Treatment with Autologous Platelet-Rich Plasma and Low Dose Ovarian Stimulation Compared with Poor Responders Used Only Low Dose Ovarian Stimulation Before in Vitro Fertilization. Open Access Maced. J. Med Sci. 2019, 7, 3184–3188. [Google Scholar] [CrossRef]
- Tinjiæ, S.; Abazoviæ, D.; Ljubiæ, D.; Vujoviæ, S.; Vojvodiæ, D.; Božanoviæ, T.; Ljubiæ, A. Ovarian Rejuvenation. Donald Sch. J. Ultrasound Obstet. Gynecol. 2019, 13, 64–68. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Autologous Platelet-Rich Plasma (PRP) Infusions and Biomarkers of Ovarian Rejuvenation and Ageing Mitigation. NCT03178695. Available online: https://clinicaltrials.gov/ct2/show/NCT03178695 (accessed on 7 March 2017).
- Schrör, K. Aspirin and Platelets: The Antiplatelet Action of Aspirin and Its Role in Thrombosis Treatment and Prophylaxis. Semin. Thromb. Hemost. 1997, 23, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef]
- Wu, J.; Zern, M.A. Hepatic stellate cells: A target for the treatment of liver fibrosis. J. Gastroenterol. 2000, 35, 665–672. [Google Scholar] [CrossRef]
- Tong, Z.; Dai, H.; Chen, B.; Abdoh, Z.; Guzman, J.; Costabel, U. Inhibition of Cytokine Release From Alveolar Macrophages in Pulmonary Sarcoidosis by Pentoxifylline: Comparison with dexamethasone. Chest 2003, 124, 1526–1532. [Google Scholar] [CrossRef]
- Urman, B.; Boza, A. Reply: Every established treatment had been experimental at the beginning. Hum. Reprod. 2020, 35, 1720–1721. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Barad, D.H.; Gleicher, N. Defining assisted reproductive technology success. Fertil. Steril. 2013, 100, e30. [Google Scholar] [CrossRef]
- Gunderson, S.; Jungheim, E.S.; Kallen, C.B.; Omurtag, K. Public reporting of IVF outcomes influences medical decision-making and physician training. Fertil. Res. Pract. 2020, 6, 1. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M.S. Principles and methods of preparation of platelet-rich plasma: A review and author′s perspective. J. Cutan. Aesthetic Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, C. Anti-Müllerian hormone and antral follicle count differ in their ability to predict cumulative treatment outcomes of the first complete ovarian stimulation cycle in patients from POSEIDON groups 3 and 4. J. Obstet. Gynaecol. Res. 2020, 46, 1801–1808. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Klassen, M.P.; Hua, Y.; Barres, B.A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 2002, 296, 1860–1864. [Google Scholar] [CrossRef]
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Laner-Plamberger, S.; Oeller, M.; Mrazek, C.; Hartl, A.; Sonderegger, A.; Rohde, E.; Strunk, D.; Schallmoser, K. Upregulation of mitotic bookmarking factors during enhanced proliferation of human stromal cells in human platelet lysate. J. Transl. Med. 2019, 17, 432. [Google Scholar] [CrossRef]
- Cho, J.; Kim, T.-H.; Seok, J.; Jun, J.H.; Park, H.; Kweon, M.; Lim, J.-Y.; Kim, G.J. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab. Investig. 2021, 101, 304–317. [Google Scholar] [CrossRef]
- Harper, J.; Jackson, E.; Sermon, K.; Aitken, R.J.; Harbottle, S.; Mocanu, E.; Hardarson, T.; Mathur, R.; Viville, S.; Vail, A.; et al. Adjuncts in the IVF laboratory: Where is the evidence for ‘add-on’ interventions? Hum. Reprod. 2017, 32, 485–491. [Google Scholar] [CrossRef]
- Urman, B.; Boza, A.; Balaban, B. Platelet-rich plasma another add-on treatment getting out of hand? How can clinicians preserve the best interest of their patients? Hum. Reprod. 2019, 34, 2099–2103. [Google Scholar] [CrossRef]
- Sills, E. The Scientific and Cultural Journey to Ovarian Rejuvenation: Background, Barriers, and Beyond the Biological Clock. Medicines 2021, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.B.; Hennebold, J. Ovarian germline stem cells: An unlimited source of oocytes? Fertil. Steril. 2014, 101, 20–30. [Google Scholar] [CrossRef][Green Version]
- Dunlop, C.E.; Telfer, E.E.; Anderson, R.A. Ovarian stem cells—Potential roles in infertility treatment and fertility preservation. Maturitas 2013, 76, 279–283. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, D.; Wang, L.; Liu, M.; Mao, J.; Yin, Y.; Ye, X.; Liu, N.; Han, J.; Gao, Y.; et al. No evidence for neo-oogenesis may link to ovarian senescence in adult monkey. Stem Cells 2013, 31, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Mitoh, S.; Yusa, Y. Extreme autotomy and whole-body regeneration in photosynthetic sea slugs. Curr. Biol. 2021, 31, R233–R234. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sills, E.S.; Wood, S.H. Appraisal of Experimental Methods to Manage Menopause and Infertility: Intraovarian Platelet-Rich Plasma vs. Condensed Platelet-Derived Cytokines. Medicina 2022, 58, 3. https://doi.org/10.3390/medicina58010003
Sills ES, Wood SH. Appraisal of Experimental Methods to Manage Menopause and Infertility: Intraovarian Platelet-Rich Plasma vs. Condensed Platelet-Derived Cytokines. Medicina. 2022; 58(1):3. https://doi.org/10.3390/medicina58010003
Chicago/Turabian StyleSills, E. Scott, and Samuel H. Wood. 2022. "Appraisal of Experimental Methods to Manage Menopause and Infertility: Intraovarian Platelet-Rich Plasma vs. Condensed Platelet-Derived Cytokines" Medicina 58, no. 1: 3. https://doi.org/10.3390/medicina58010003
APA StyleSills, E. S., & Wood, S. H. (2022). Appraisal of Experimental Methods to Manage Menopause and Infertility: Intraovarian Platelet-Rich Plasma vs. Condensed Platelet-Derived Cytokines. Medicina, 58(1), 3. https://doi.org/10.3390/medicina58010003






