Biliary Tree Diagnostics: Advances in Endoscopic Imaging and Tissue Sampling
Abstract
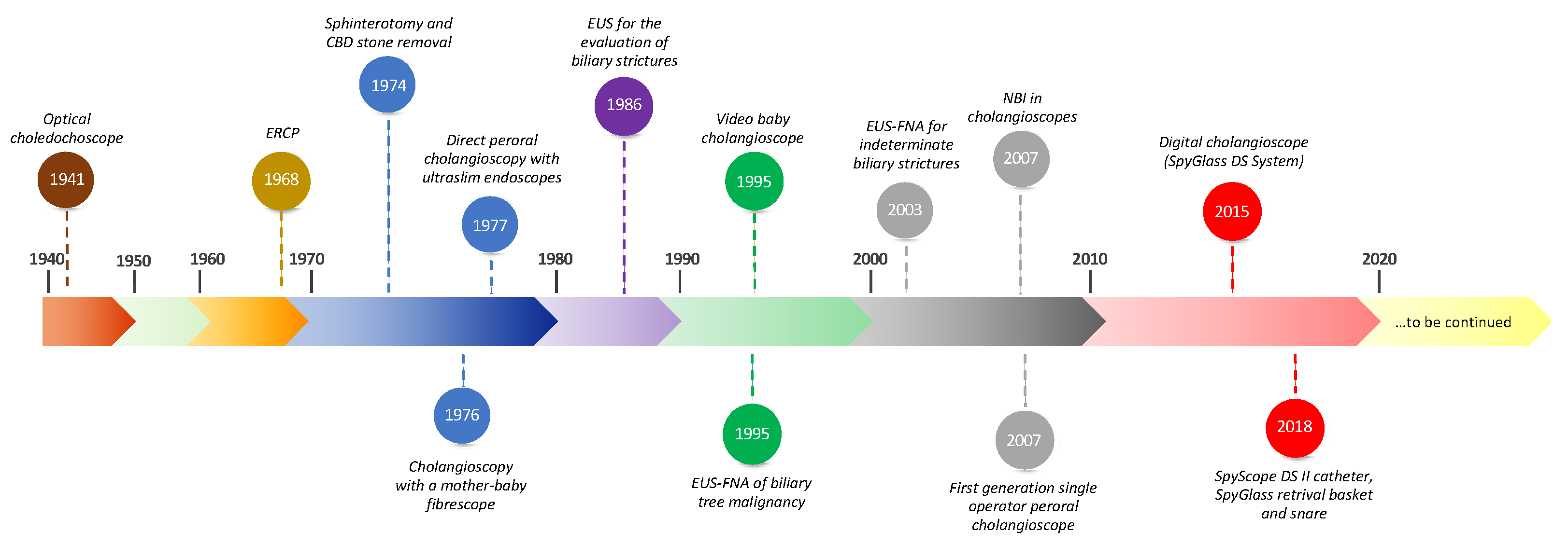
1. Introduction
2. Techniques and Methodologies
2.1. Endoscopic Retrograde Cholangiopancreatography
2.2. Cholangioscopy
2.3. Narrow Band Imaging
2.4. Dye Chromoendoscopy
2.5. Endoscopic Ultrasound
2.6. Intraductal Ultrasound
2.7. Confocal Laser Endomicroscopy
2.8. Autofluorescence Imaging
2.9. Optical Coherence Tomography
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, B.; Moon, J.H.; Cai, Q.; Rerknimitr, R.; Ma, S.; Lakhtakia, S.; Ryozawa, S.; Kutsumi, H.; Yasuda, I.; Shiomi, H.; et al. Review article: Asia-Pacific consensus recommendations on endoscopic tissue acquisition for biliary strictures. Aliment. Pharmacol. Ther. 2018, 48, 138–151. [Google Scholar] [CrossRef]
- Bain, V.G.; Abraham, N.; Jhangri, G.S.; Alexander, T.W.; Henning, R.C.; Hoskinson, M.E.; Maguire, C.G.; Lalor, E.A.; Sadowski, D.C. Prospective study of biliary strictures to determine the predictors of malignancy. Can. J. Gastroenterol. 2000, 14, 397–402. [Google Scholar] [CrossRef][Green Version]
- Krishna, N.; Tummala, P.; Reddy, A.V.; Mehra, M.; Agarwal, B. Dilation of both pancreatic duct and the common bile duct on computed tomography and magnetic resonance imaging scans in patients with or without obstructive jaundice. Pancreas 2012, 41, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Guda, N.M. ERCP cannulation: A review of reported techniques. Gastrointest. Endosc. 2005, 61, 112–125. [Google Scholar] [CrossRef]
- Desa, L.A.; Akosa, A.B.; Lazzara, S.; Domizio, P.; Krausz, T.; Benjamin, I.S. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut 1991, 32, 1188–1191. [Google Scholar] [CrossRef]
- Kurzawinski, T.; Deery, A.; Dooley, J.; Dick, R.; Hobbs, K.; Davidson, B. A prospective controlled study comparing brush and bile exfoliative cytology for diagnosing bile duct strictures. Gut 1992, 33, 1675–1677. [Google Scholar] [CrossRef]
- Tanisaka, Y.; Mizuide, M.; Fujita, A.; Ogawa, T.; Suzuki, M.; Katsuda, H.; Saito, Y.; Miyaguchi, K.; Tashima, T.; Mashimo, Y.; et al. Diagnostic Process Using Endoscopy for Biliary Strictures: A Narrative Review. J. Clin. Med. 2021, 10, 1048. Available online: https://www.mdpi.com/2077-0383/10/5/1048/htm (accessed on 4 October 2021). [CrossRef] [PubMed]
- Burnett, A.S.; Calvert, T.J.; Chokshi, R.J. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J. Surg. Res. 2013, 184, 304–311. [Google Scholar] [CrossRef]
- Kocjan, G.; Smith, A.N. Bile duct brushings cytology: Potential pitfalls in diagnosis. Diagn. Cytopathol. 1997, 16, 358–363. [Google Scholar] [CrossRef]
- Logrono, R.; Kurtycz, D.F.; Molina, C.P.; Trivedi, V.A.; Wong, J.Y.; Block, K.P. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: The experience at 2 university hospitals. Arch. Pathol. Lab. Med. 2000, 124, 387–392. [Google Scholar] [CrossRef]
- de Bellis, M.; Fogel, E.L.; Sherman, S.; Watkins, J.L.; Chappo, J.; Younger, C.; Cramer, H.; Lehman, G.A. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest. Endosc. 2003, 58, 176–182. [Google Scholar] [CrossRef]
- Ponchon, T.; Gagnon, P.; Berger, F.; Labadie, M.; Liaras, A.; Chavaillon, A.; Bory, R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest. Endosc. 1995, 42, 565–572. [Google Scholar] [CrossRef]
- Schoefl, R.; Haefner, M.; Wrba, F.; Pfeffel, F.; Stain, C.; Poetzi, R.; Gangl, A. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand. J. Gastroenterol. 1997, 32, 363–368. [Google Scholar] [CrossRef]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.; Lourdusamy, V.; Sanaka, M.R. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: A systematic review and meta-analysis. Gastroenterol. Rep. 2015, 3, 209–215. [Google Scholar] [CrossRef]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef]
- Burak, K.; Angulo, P.; Pasha, T.M.; Egan, K.; Petz, J.; Lindor, K.D. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am. J. Gastroenterol. 2004, 99, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, E.; Schoen, R.E.; Silverman, W.; Martin, J.; Rabinovitz, M.; Weissfeld, J.L.; Abu-Elmaagd, K.; Madariaga, J.R.; Slivka, A. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest. Endosc. 2002, 56, 40–47, Erratum in Gastrointest. Endosc. 2002, 56, 612. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Enders, F.B.; Halling, K.C.; Lindor, K.D. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008, 48, 1106–1117. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Parsi, M.A. Biliary papillomatosis: Diagnosis with direct peroral cholangioscopy. Gastrointest. Endosc. 2015, 81, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Trindade, A.J.; Sejpal, D.V. Bile Duct Mass Determined to Be Eosinophilic Cholangitis by Digital Cholangioscopy. Clin. Gastroenterol. Hepatol. 2017, 15, e173–e174. Available online: http://www.cghjournal.org/article/S154235651730856X/fulltext. (accessed on 26 September 2021). [CrossRef] [PubMed][Green Version]
- Nishikawa, T.; Tsuyuguchi, T.; Sakai, Y.; Sugiyama, H.; Kishimoto, T.; Ohtsuka, M.; Miyazaki, M.; Yokosuka, O. Preoperative assessment of longitudinal extension of cholangiocarcinoma with peroral video-cholangioscopy: A prospective study. Dig. Endosc. 2014, 26, 450–457. [Google Scholar] [CrossRef]
- Nimura, Y.; Shionoya, S.; Hayakawa, N.; Kamiya, J.; Kondo, S.; Yasui, A. Value of percutaneous transhepatic cholangioscopy (PTCS). Surg. Endosc. 1988, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.C.; Lee, S.K.; Lee, T.Y.; Kwon, S.; Lee, S.S.; Seo, D.W.; Kim, M.H. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy 2007, 39, 731–736. [Google Scholar] [CrossRef]
- Moon, J.H.; Ko, B.M.; Choi, H.J.; Hong, S.J.; Cheon, Y.K.; Cho, Y.D.; Lee, J.S.; Lee, M.S.; Shim, C.S. Intraductal balloon-guided direct peroral cholangioscopy with an ultraslim upper endoscope (with videos). Gastrointest. Endosc. 2009, 70, 297–302. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.J.; Zhang, J.C.; Wang, H.Y.; Li, K.; Niu, S.H. A new hybrid anchoring balloon for direct peroral cholangioscopy using an ultraslim upper endoscope. Dig. Endosc. 2018, 30, 364–371. [Google Scholar] [CrossRef]
- Lim, P.; Aggarwal, V.; Craig, P. Role of balloon-assisted cholangioscopy in a multiethnic cohort to assess complex biliary disease (with videos). Gastrointest. Endosc. 2015, 81, 932–942. [Google Scholar] [CrossRef]
- Lenze, F.; Nowacki, T.M.; Beyna, T.; Ullerich, H. Direct peroral cholangioscopy with a new anchoring technique using the guide probe of Kautz—First clinical experiences. Endoscopy 2017, 49, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; Umeda, J.; et al. Free-hand direct insertion ability into a simulated ex vivo model using a prototype multibending peroral direct cholangioscope (with videos). Gastrointest. Endosc. 2012, 76, 454–457. [Google Scholar] [CrossRef]
- Itoi, T.; Nageshwar Reddy, D.; Sofuni, A.; Ramchandani, M.; Itokawa, F.; Gupta, R.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Ikeuchi, N.; et al. Clinical evaluation of a prototype multi-bending peroral direct cholangioscope. Dig. Endosc. 2014, 26, 100–107. [Google Scholar] [CrossRef]
- Beyna, T.; Farnik, H.; Sarrazin, C.; Gerges, C.; Neuhaus, H.; Albert, J.G. Direct retrograde cholangioscopy with a new prototype double-bending cholangioscope. Endoscopy 2016, 48, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Akasaka, Y.; Yamaguchi, K.; Fujimoto, S.; Kawai, K. Direct endoscopic visualization of the bile and pancreatic duct systems by peroral cholangiopancreatoscopy (PCPS). Gastrointest. Endosc. 1978, 24, 141–145. [Google Scholar] [CrossRef]
- Chen, Y.K. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest. Endosc. 2007, 65, 303–311. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Kommaraju, K.; Zhu, X.; Hebert-Magee, S.; Hawes, R.H.; Vargo, J.J.; Varadarajulu, S.; Parsi, M.A. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: A multicenter clinical experience (with video). Gastrointest. Endosc. 2016, 84, 649–655. [Google Scholar] [CrossRef]
- Itoi, T.; Neuhaus, H.; Chen, Y.K. Diagnostic value of image-enhanced video cholangiopancreatoscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Neuhaus, H.; Parsi, M.; Reddy, D.N.; Pleskow, D.K. Randomized study of digital single-operator cholangioscope compared to fiberoptic single-operator cholangioscope in a novel cholangioscopy bench model. Endosc. Int. Open 2018, 6, E851. [Google Scholar] [CrossRef]
- Choi, H.J.; Moon, J.H.; Lee, Y.N. Advanced Imaging Technology in Biliary Tract Diseases:Narrow-Band Imaging of the Bile Duct. Clin. Endosc. 2015, 48, 498–502. Available online: http://www.e-ce.org/journal/view.php?doi=10.5946/ce.2015.48.6.498 (accessed on 26 September 2021). [CrossRef]
- Seo, D.W.; Lee, S.K.; Yoo, K.S.; Kang, G.H.; Kim, M.H.; Suh, D.J.; Min, Y.I. Cholangioscopic findings in bile duct tumors. Gastrointest. Endosc. 2000, 52, 630–634. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tsuyuguchi, T.; Sakai, Y.; Tsuchiya, S.; Saisyo, H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest. Endosc. 2005, 62, 374–382. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614.e2. [Google Scholar] [CrossRef]
- Turowski, F.; Hügle, U.; Dormann, A.; Bechtler, M.; Jakobs, R.; Gottschalk, U.; Nötzel, E.; Hartmann, D.; Lorenz, A.; Kolligs, F.; et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS™: Results of a multicenter retrospective cohort study. Surg. Endosc. 2018, 32, 3981–3988. [Google Scholar] [CrossRef]
- Draganov, P.V.; Chauhan, S.; Wagh, M.S.; Gupte, A.R.; Lin, T.; Hou, W.; Forsmark, C.E. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: A prospective, long-term follow-up study. Gastrointest. Endosc. 2012, 75, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Onoyama, T.; Takeda, Y.; Kawata, S.; Kurumi, H.; Koda, H.; Yamashita, T.; Hamamoto, W.; Sakamoto, Y.; Matsumoto, K.; Isomoto, H. Adequate tissue acquisition rate of peroral cholangioscopy-guided forceps biopsy. Ann. Transl. Med. 2020, 8, 1073. [Google Scholar] [CrossRef] [PubMed]
- Korrapati, P.; Ciolino, J.; Wani, S.; Shah, J.; Watson, R.; Muthusamy, V.R.; Klapman, J.; Komanduri, S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: A systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E263–E275. [Google Scholar] [CrossRef] [PubMed]
- Parsi, M.A.; Stevens, T.; Vargo, J.J. Diagnostic and therapeutic direct peroral cholangioscopy using an intraductal anchoring balloon. World J. Gastroenterol. 2012, 18, 3992. [Google Scholar] [CrossRef]
- Sethi, A.; Chen, Y.K.; Austin, G.L.; Brown, W.R.; Brauer, B.C.; Fukami, N.N.; Khan, A.H.; Shah, R.J. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: A single-center experience. Gastrointest. Endosc. 2011, 73, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Lakhtakia, S.; Santosh, D.; Anuradha, S.; Tandan, M.; Ramchandani, M.; Rao, G.V.; Reddy, D.N. Narrow band imaging cholangioscopy in hilar cholangiocarcinoma. Indian J. Gastroenterol. 2010, 29, 78–80. [Google Scholar] [CrossRef]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Moriyasu, F.; Gotoda, T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest. Endosc. 2007, 66, 730–736. [Google Scholar] [CrossRef]
- Jang, J.W.; Noh, D.H.; Paik, K.H.; Kim, S.H.; Paik, I.H.; Jung, S.H. Effectiveness of cholangioscopy using narrow band imaging for hepatobiliary malignancies. Ann. Surg. Treat. Res. 2017, 93, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Mounzer, R.; Austin, G.L.; Wani, S.; Brauer, B.C.; Fukami, N.; Shah, R.J. Per-oral video cholangiopancreatoscopy with narrow-band imaging for the evaluation of indeterminate pancreaticobiliary disease. Gastrointest. Endosc. 2017, 85, 509–517. [Google Scholar] [CrossRef]
- Azeem, N.; Gostout, C.J.; Knipschield, M.; Baron, T.H. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest. Endosc. 2014, 79, 773–779.e2. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.; Carr-Locke, D. Advances in Endoscopic Imaging of the Biliary Tree. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 187–204. [Google Scholar] [CrossRef]
- Osanai, M.; Itoi, T.; Igarashi, Y.; Tanaka, K.; Kida, M.; Maguchi, H.; Yasuda, K.; Okano, N.; Imaizumi, H.; Itokawa, F. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: A prospective multicenter study. Endoscopy 2013, 45, 635–642. [Google Scholar] [CrossRef]
- Kandiah, K.; Chedgy, F.J.Q.; Subramaniam, S.; Longcroft-Wheaton, G.; Bassett, P.; Repici, A.; Sharma, P.; Pech, O.; Bhandari, P. International development and validation of a classification system for the identification of Barrett’s neoplasia using acetic acid chromoendoscopy: The Portsmouth acetic acid classification (PREDICT). Gut 2018, 67, 2085–2091. [Google Scholar] [CrossRef]
- Flynn, A.D.; Valentine, J.F. Chromoendoscopy for Dysplasia Surveillance in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 1440–1452. [Google Scholar] [CrossRef]
- Maetani, I.; Ogawa, S.; Sato, M.; Igarashi, Y.; Sakai, Y.; Shibuya, K. Lack of methylene blue staining in superficial epithelia as a possible marker for superficial lateral spread of bile duct cancer. Diagn. Ther. Endosc. 1996, 3, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Kiesslich, R.; Bittinger, F.; Galle, P.R.; Neurath, M.F. Methylene blue-aided cholangioscopy in patients with biliary strictures: Feasibility and outcome analysis. Endoscopy 2008, 40, 563–571. [Google Scholar] [CrossRef]
- Brauer, B.C.; Fukami, N.; Chen, Y.K. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos). Gastrointest. Endosc. 2008, 67, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Garrow, D.; Miller, S.; Sinha, D.; Conway, J.; Hoffman, B.J.; Hawes, R.H.; Romagnuolo, J. Endoscopic ultrasound: A meta-analysis of test performance in suspected biliary obstruction. Clin. Gastroenterol. Hepatol. 2007, 5, 616–623. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef]
- Chhoda, A.; Dawod, S.; Grimshaw, A.; Gunderson, C.; Mahadev, S. Evaluation of diagnostic yield of EUS among patients with asymptomatic common bile duct dilation: Systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 890–901.e8. Available online: http://www.giejournal.org/article/S0016510721014814/fulltext (accessed on 26 September 2021). [CrossRef]
- Fritscher-Ravens, A.; Broering, D.C.; Knoefel, W.T.; Rogiers, X.; Swain, P.; Thonke, F.; Bobrowski, C.; Topalidis, T.; Soehendra, N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am. J. Gastroenterol. 2004, 99, 45–51. [Google Scholar] [CrossRef]
- Rösch, T.; Hofrichter, K.; Frimberger, E.; Meining, A.; Born, P.; Weigert, N.; Allescher, H.D.; Classen, M.; Barbur, M.; Schenck, U.; et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest. Endosc. 2004, 60, 390–396. [Google Scholar] [CrossRef]
- DeWitt, J.; Misra, V.L.; Leblanc, J.K.; McHenry, L.; Sherman, S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest. Endosc. 2006, 64, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Weilert, F.; Bhat, Y.M.; Binmoeller, K.F.; Kane, S.; Jaffee, I.M.; Shaw, R.E.; Cameron, R.; Hashimoto, Y.; Shah, J.N. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: Results of a prospective, single-blind, comparative study. Gastrointest. Endosc. 2014, 80, 97–104. [Google Scholar] [CrossRef]
- Onda, S.; Ogura, T.; Kurisu, Y.; Masuda, D.; Sano, T.; Takagi, W.; Fukunishi, S.; Higuchi, K. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Ther. Adv. Gastroenterol. 2016, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- De Moura, D.T.H.; Moura, E.G.H.; Bernardo, W.M.; De Moura, E.T.H.; Baraca, F.I.; Kondo, A.; Matuguma, S.E.; Almeida Artifon, E.L. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc. Ultrasound 2018, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Han, J.P.; Lee, T.H.; Hong, S.J.; Kim, H.K.; Noh, H.M.; Lee, Y.N.; Choi, H.J. EUS-guided FNA and FNB after on-site cytological evaluation in gastric subepithelial tumors. J. Dig. Dis. 2016, 17, 582–587. [Google Scholar] [CrossRef] [PubMed]
- van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef]
- El Chafic, A.H.; Dewitt, J.; Leblanc, J.K.; El Hajj, I.I.; Cote, G.; House, M.G.; Sherman, S.; McHenry, L.; Pitt, H.A.; Johnson, C.; et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy 2013, 45, 883–889. [Google Scholar] [CrossRef]
- Xie, C.; Aloreidi, K.; Patel, B.; Ridgway, T.; Thambi-Pillai, T.; Timmerman, G.; Khan, A.; Atiq, M. Indeterminate biliary strictures: A simplified approach. Expert Rev. Gastroenterol. Hepatol. 2017, 12, 189–199. Available online: https://www.tandfonline.com/doi/abs/10.1080/17474124.2018.1391090 (accessed on 26 September 2021). [CrossRef] [PubMed]
- Meister, T.; Heinzow, H.S.; Woestmeyer, C.; Lenz, P.; Menzel, J.; Kucharzik, T.; Domschke, W.; Domagk, D. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J. Gastroenterol. 2013, 19, 874–881. [Google Scholar] [CrossRef]
- Vazquez-Sequeiros, E.; Baron, T.H.; Clain, J.E.; Gostout, C.J.; Norton, I.D.; Petersen, B.T.; Levy, M.J.; Jondal, M.L.; Wiersema, M.J. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest. Endosc. 2002, 56, 372–379. Available online: http://www.giejournal.org/article/S0016510702700412/fulltext (accessed on 26 September 2021). [CrossRef]
- Farrell, R.J.; Agarwal, B.; Brandwein, S.L.; Underhill, J.; Chuttani, R.; Pleskow, D.K. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest. Endosc. 2002, 56, 681–687. [Google Scholar] [CrossRef]
- Stavropoulos, S.; Larghi, A.; Verna, E.; Battezzati, P.; Stevens, P. Intraductal ultrasound for the evaluation of patients with biliary strictures and no abdominal mass on computed tomography. Endoscopy 2005, 37, 715–721. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Visrodia, K.H.; Levy, M.J.; Gostout, C.J. Advanced endoscopic imaging of indeterminate biliary strictures. World J. Gastrointest. Endosc. 2015, 7, 1268–1278. [Google Scholar] [CrossRef]
- Menzel, J.; Poremba, C.; Dietl, K.H.; Domschke, W. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand. J. Gastroenterol. 2000, 35, 77–82. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Wu, J.C.; Bie, L.; Xia, L.; Gong, B. Diagnostic Utility of Endoscopic Retrograde Cholangiography/Intraductal Ultrasound (ERC/IDUS) in Distinguishing Malignant from Benign Bile Duct Obstruction. Dig. Dis. Sci. 2016, 61, 610–617. [Google Scholar] [CrossRef]
- Naitoh, I.; Nakazawa, T.; Hayashi, K.; Miyabe, K.; Shimizu, S.; Kondo, H.; Nishi, Y.; Yoshida, M.; Umemura, S.; Hori, Y.; et al. Comparison of intraductal ultrasonography findings between primary sclerosing cholangitis and IgG4-related sclerosing cholangitis. J. Gastroenterol. Hepatol. 2015, 30, 1104–1109. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jgh.12894 (accessed on 26 September 2021). [CrossRef]
- Ramchandani, M.; Nageshwar Reddy, D.; Lakhtakia, S.; Gupta, R.; Tandan, M.; Rao, G.V. Role of single-operator per-oral cholangioscopy and intraductal US in assessment of portal biliopathy (with videos). Gastrointest. Endosc. 2014, 79, 1015–1019. [Google Scholar] [CrossRef]
- Bhatia, V.; Shasthry, S.M.; Mukund, A. Intraductal Sonography in Patients with Portal Cavernoma Cholangiopathy. J. Ultrasound Med. 2016, 35, 651–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, S.U.; Park, C.H.; Kee, W.J.; Lee, J.H.; Rew, S.J.; Park, S.Y.; Kim, H.S.; Choi, S.K.; Rew, J.S. Intraductal Ultrasonography without Radiocontrast Cholangiogramin Patients with Extrahepatic Biliary Disease. Gut Liver 2015, 9, 540–546. [Google Scholar] [CrossRef]
- Tanisaka, Y.; Ryozawa, S.; Nonaka, K.; Yasuda, M.; Fujita, A.; Ogawa, T.; Mizuide, M.; Tashima, T.; Araki, R. Diagnosis of Biliary Strictures Using Probe-Based Confocal Laser Endomicroscopy under the Direct View of Peroral Cholangioscopy: Results of a Prospective Study (with Video). Gastroenterol. Res. Pract. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Meining, A.; Chen, Y.K.; Pleskow, D.; Stevens, P.; Shah, R.J.; Chuttani, R.; Michalek, J.; Slivka, A. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: A multicenter experience. Gastrointest. Endosc. 2011, 74, 961–968. [Google Scholar] [CrossRef]
- Meining, A.; Shah, R.J.; Slivka, A.; Pleskow, D.; Chuttani, R.; Stevens, P.D.; Becker, V.; Chen, Y.K. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy 2012, 44, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Caillol, F.; Filoche, B.; Gaidhane, M.; Kahaleh, M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: The Paris Classification. Dig. Dis. Sci. 2013, 58, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Slivka, A.; Gan, I.; Jamidar, P.; Costamagna, G.; Cesaro, P.; Giovannini, M.; Caillol, F.; Kahaleh, M. Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy for the characterization of indeterminate biliary strictures: Results of a prospective multicenter international study. Gastrointest. Endosc. 2015, 81, 282–290. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Sun, B.; Zhang, W.M.; Zhang, Z.Z.; He, Y.P.; Yang, X.J. Probe-based confocal laser endomicroscopy for the diagnosis of undetermined biliary stenoses: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 666–673. [Google Scholar] [CrossRef]
- Wang, K.K.; Carr-Locke, D.L.; Singh, S.K.; Neumann, H.; Bertani, H.; Galmiche, J.-P.; Arsenescu, R.I.; Caillol, F.; Chang, K.J.; Chaussade, S.; et al. Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence. United Eur. Gastroenterol. J. 2015, 3, 230–254. Available online: https://mayoclinic.pure.elsevier.com/en/publications/use-of-probe-based-confocal-laser-endomicroscopy-pcle-in-gastroin (accessed on 26 September 2021). [CrossRef]
- American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee; Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.R.; et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest. Endosc. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Uedo, N.; Iishi, H.; Tatsuta, M.; Yamada, T.; Ogiyama, H.; Imanaka, K.; Sugimoto, N.; Higashino, K.; Ishihara, R.; Narahara, H.; et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest. Endosc. 2005, 62, 521–528. [Google Scholar] [CrossRef]
- Itoi, T.; Shinohara, Y.; Takeda, K.; Nakamura, K.; Takei, K. Improvement of Choledochoscopy: Chromoendocholedochoscopy, Autofluorescence Imaging, or Narrow-Band Imaging. Dig. Endosc. 2007, 19, S95–S104. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1443-1661.2007.00713.x (accessed on 26 September 2021). [CrossRef]
- Huynh, R.; Owers, C.; Pinto, C.; Nguyen, T.-M.; Kwok, T. Endoscopic Evaluation of Biliary Strictures: Current and Emerging Techniques. Clin. Endosc. 2021, 54, 825–832. Available online: http://www.e-ce.org/journal/view.php?doi=10.5946/ce.2021.048 (accessed on 26 September 2021). [CrossRef]
- Seitz, U.; Freund, J.; Jaeckle, S.; Feldchtein, F.; Bohnacker, S.; Thonke, F.; Gladkova, N.; Brand, B.; Schröder, S.; Soehendra, N. First in vivo optical coherence tomography in the human bile duct. Endoscopy 2001, 33, 1018–1021. [Google Scholar] [CrossRef]
- de Boer, J.F.; Wojtkowski, M.; Leitgeb, R. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT [Invited]. Biomed. Opt. Express 2017, 8, 3248–3280. Available online: https://www.osapublishing.org/viewmedia.cfm?uri=boe-8-7-3248&seq=0&html=true (accessed on 26 September 2021). [CrossRef]
- Mahmud, M.S.; May, G.R.; Kamal, M.M.; Khwaja, A.S.; Sun, C.; Vitkin, A.; Yang, V.X. Imaging pancreatobiliary ductal system with optical coherence tomography: A review. World J. Gastrointest. Endosc. 2013, 5, 540–550. [Google Scholar] [CrossRef]
- Testoni, P.A.; Mangiavillano, B. Optical coherence tomography for bile and pancreatic duct imaging. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 637–653. [Google Scholar] [CrossRef]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitris, C.; Southern, J.F.; Fujimoto, J.G. In vivo endoscopic optical biopsy with optical coherence tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef]
- Pfau, P.R.; Sivak, M.V., Jr.; Chak, A.; Kinnard, M.; Wong, R.C.; Isenberg, G.A.; Izatt, J.A.; Rollins, A.; Westphal, V. Criteria for the diagnosis of dysplasia by endoscopic optical coherence tomography. Gastrointest. Endosc. 2003, 58, 196–202. [Google Scholar] [CrossRef]
- Isenberg, G.; Sivak, M.V., Jr.; Chak, A.; Wong, R.C.; Willis, J.E.; Wolf, B.; Rowland, D.Y.; Das, A.; Rollins, A. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: A prospective, double-blinded study. Gastrointest. Endosc. 2005, 62, 825–831. [Google Scholar] [CrossRef]
- Testoni, P.A.; Mangiavillano, B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J. Gastroenterol. 2008, 14, 6444. [Google Scholar] [CrossRef]
- Arvanitakis, M.; Hookey, L.; Tessier, G.; Demetter, P.; Nagy, N.; Stellke, A.; De Maertelaer, V.; Devière, J.; Le Moine, O. Intraductal optical coherence tomography during endoscopic retrograde cholangiopancreatography for investigation of biliary strictures. Endoscopy 2009, 41, 696–701. [Google Scholar] [CrossRef]
- Testoni, P.A.; Mariani, A.; Mangiavillano, B.; Arcidiacono, P.G.; Di Pietro, S.; Masci, E. Intraductal optical coherence tomography for investigating main pancreatic duct strictures. Am. J. Gastroenterol. 2007, 102, 269–274. [Google Scholar] [CrossRef]

| Biliary Tree Disorders | |
|---|---|
| Biliary anatomical abnormalities | Choledochal Cyst, Biliary atresia, Caroli disease |
| Cholangitis | Infective, Primary sclerosing cholangitis (PSC), Primary biliary cholangitis (PBC), IgG4-related cholangitis, Secondary sclerosing cholangitis |
| Benign Tumors of the Bile Ducts | Biliary hamartoma, Intraductal papillary neoplasm of the bile duct, Biliary Mucinous Cystic Neoplasms |
| Malignant Tumors of the Bile Ducts | Cholangiocarcinoma |
| Choledocholithiasis | |
| Post inflammatory biliary strictures | |
| Disorders of gallbladder | Cholecystitis, Cholelithiasis, Mirizzi’s syndrome |
| Disorders of the ampulla of Vater | Ampullary tumours (adenoma and adenocarcinoma) |
| Iatrogenic disorders | Accidental surgical ligation |
| Others | AIDS cholangiopathy, parasites |
| Benign | Iatrogenic | Post-surgery (e.g., cholecystectomy-related, anastomotic, ischemic) | ||
| Chemotherapy, radiotherapy, traumatic injury | ||||
| Inflammatory or Autoimmune | IgG4-associated cholangitis PSC | Eosinophilic cholangitis Sarcoidosis | ||
| Intraluminal Obstruction or Extraluminal Compression | Choledocholitiasis Chronic pancreatitis | Mirizzi syndrome Groove pancreatitis | Inflammatory pseudotumors | |
| Vascular | Vasculitis Ischemic cholangiopathy | Portal hypertensive biliopathy | ||
| Infectious | Bacterial (recurrent pyogenic cholangitis) Parasite | Human Immunodeficiency Virus (HIV) cholangiopathy | ||
| Malignant | Pancreatic Adenocarcinoma Cholangiocarcinoma | Ampullary adenocarcinoma Gallbladder cancer | Hepatocellular carcinoma (HCC) Lymphoma | Metastatic adenocarcinoma Compressive lymphadenopathy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghisa, M.; Bellumat, A.; De Bona, M.; Valiante, F.; Tollardo, M.; Riguccio, G.; Iacobellis, A.; Savarino, E.; Buda, A. Biliary Tree Diagnostics: Advances in Endoscopic Imaging and Tissue Sampling. Medicina 2022, 58, 135. https://doi.org/10.3390/medicina58010135
Ghisa M, Bellumat A, De Bona M, Valiante F, Tollardo M, Riguccio G, Iacobellis A, Savarino E, Buda A. Biliary Tree Diagnostics: Advances in Endoscopic Imaging and Tissue Sampling. Medicina. 2022; 58(1):135. https://doi.org/10.3390/medicina58010135
Chicago/Turabian StyleGhisa, Matteo, Angelo Bellumat, Manuela De Bona, Flavio Valiante, Marco Tollardo, Gaia Riguccio, Angelo Iacobellis, Edoardo Savarino, and Andrea Buda. 2022. "Biliary Tree Diagnostics: Advances in Endoscopic Imaging and Tissue Sampling" Medicina 58, no. 1: 135. https://doi.org/10.3390/medicina58010135
APA StyleGhisa, M., Bellumat, A., De Bona, M., Valiante, F., Tollardo, M., Riguccio, G., Iacobellis, A., Savarino, E., & Buda, A. (2022). Biliary Tree Diagnostics: Advances in Endoscopic Imaging and Tissue Sampling. Medicina, 58(1), 135. https://doi.org/10.3390/medicina58010135






