Usefulness of Serial Multiorgan Point-of-Care Ultrasound in Acute Heart Failure: Results from a Prospective Observational Cohort
Abstract
:1. Introduction
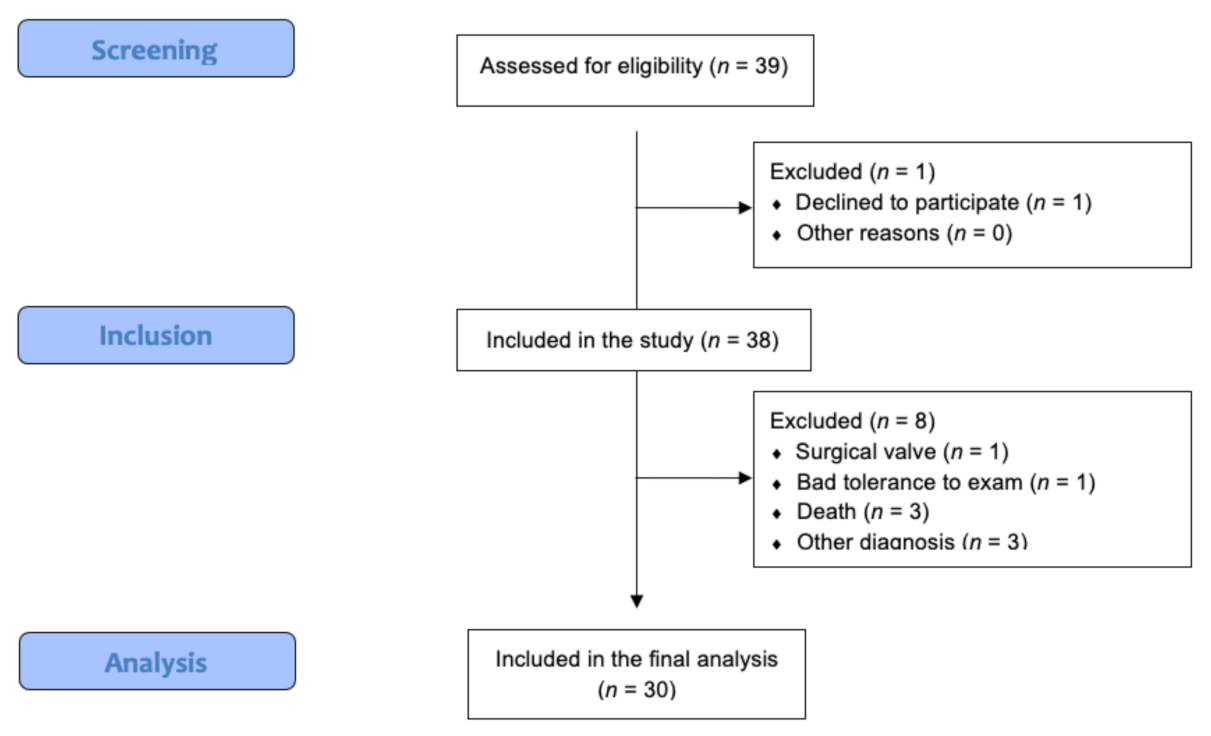
2. Materials and Methods
2.1. Patient Selection
2.2. Initial Patient Assessment
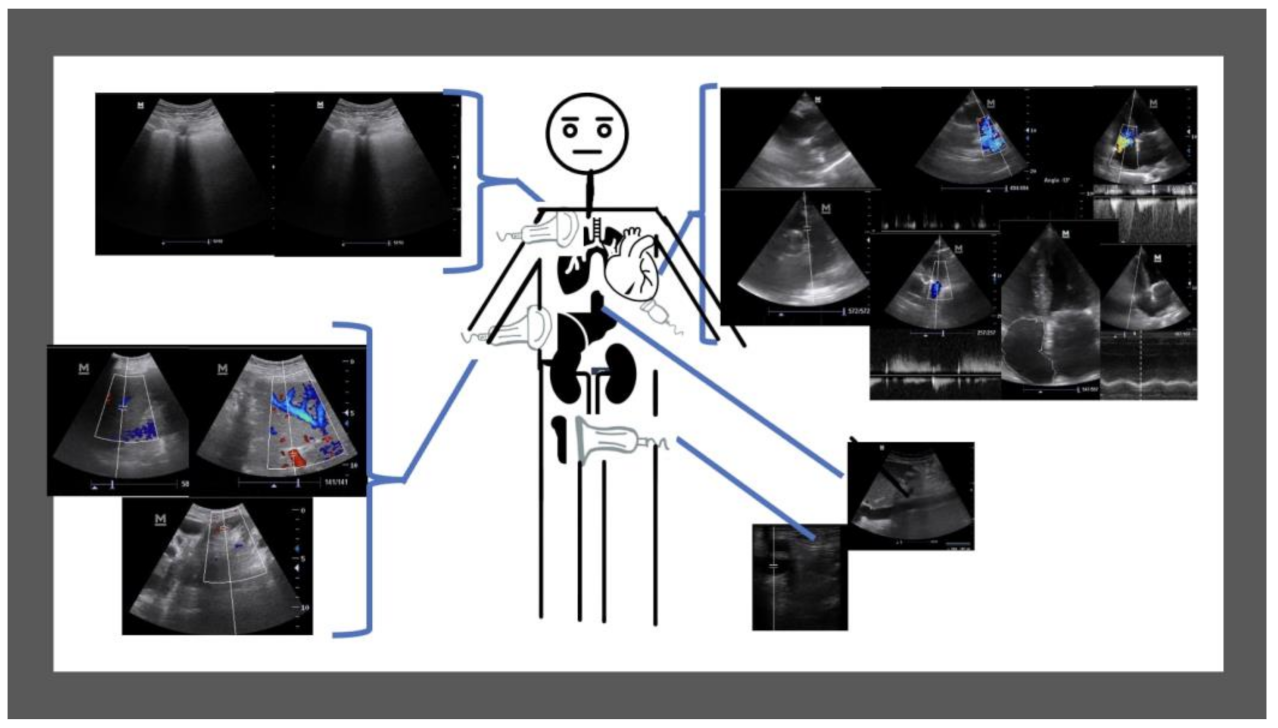
2.3. Ultrasound Data Collection
2.4. Outcome Measures and Definitions
2.5. Statistical Analysis
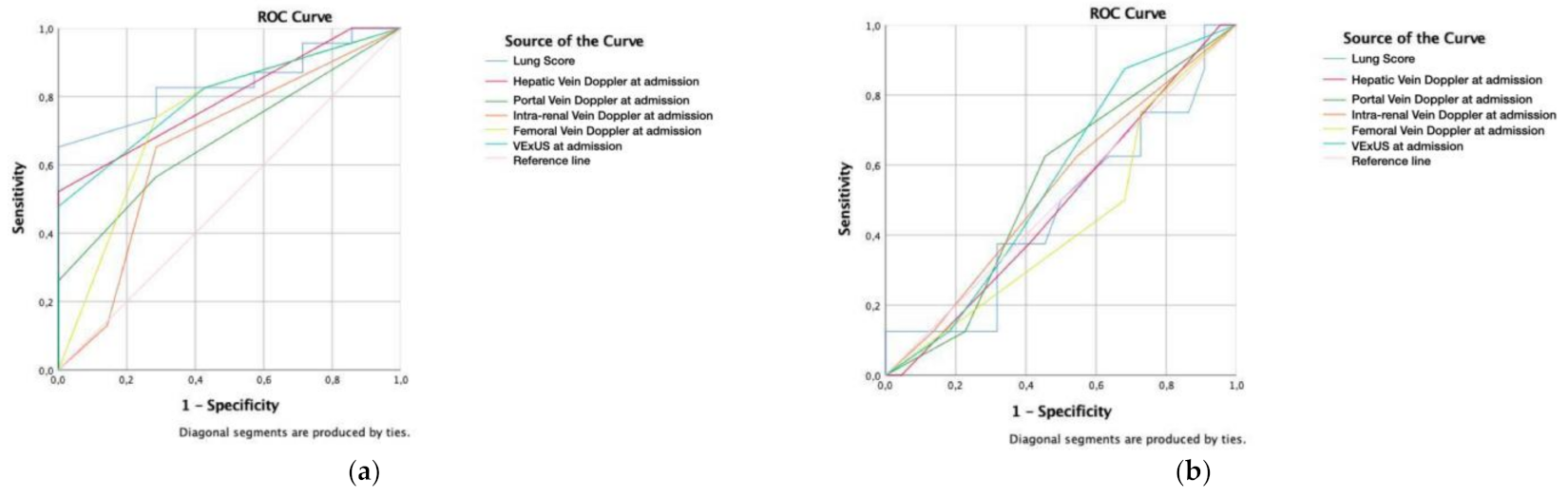
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Rev. Esp. Cardiol. 2016, 69, e1–e62. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Veldhuisen, D.J.; Bauersachs, J.; Borlaug, B.A.; Celutkiene, J.; Coats, A.J.S.; Crespo-Leiro, M.G.; Guazzi, M.; Harjola, V.-P.; Heyans, S.; et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology: Right ventricular function in HFpEF. Eur. J. Heart Fail. 2018, 20, 16–37. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Howard, L.S.; Gomberg-Maitland, M.; Hoeper, M.M. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation 2020, 141, 678–693. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ishii, S.; Yazaki, M.; Fujita, T.; Iida, Y.; Kaida, T.; Nabeta, T.; Nakatani, E.; Maekawa, E.; Yanagisawa, T.; et al. Portal congestion and intestinal edema in hospitalized patients with heart failure. Heart Vessel. 2018, 33, 740–751. [Google Scholar] [CrossRef]
- Brunkhorst, F.M. Endotoxins in chronic heart failure. Lancet 1999, 354, 599. [Google Scholar] [CrossRef]
- Benkreira, A.; Beaubien-Souligny, W.; Mailhot, T.; Bouabdallaoui, N.; Robillard, P.; Desjardins, G.; Lamarche, Y.; Cossette, S.; Denault, A. Portal hypertension is associated with congestive encephalopathy and delirium after cardiac surgery. Can. J. Cardiol. 2019, 35, 1134–1141. [Google Scholar] [CrossRef]
- Alprecht-Quiroz, P.; Zúñiga-Pineda, B.; Lara-Terán, J.J.; Cáceres-Vinueza, S.V.; Duarte-Vera, Y.C. Síndrome cardiorrenal: Aspectos clínicos y ecocardiográficos. Arch. Cardiol. 2020, 90, 5088. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with point-of-care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Kircher, B.J.; Himelman, R.B.; Schiller, N.B. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am. J. Cardiol. 1990, 66, 493–496. [Google Scholar] [CrossRef]
- Simonson, J.S.; Schiller, N.B. Sonospirometry: A new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J. Am. Coll. Cardiol. 1988, 11, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Pellicori, P.; Carubelli, V.; Zhang, J.; Castiello, T.; Sherwi, N.; Clark, A.L.; Cleland, J.G. IVC diameter in patients with chronic heart failure: Relationships and prognostic significance. JACC Cardiovasc. Imaging 2013, 6, 16–28. [Google Scholar] [CrossRef]
- Via, G.; Tavazzi, G.; Price, S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: A physiologically based point of view. Intensive Care Med. 2016, 42, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koratala, A. Utility of Doppler ultrasound derived hepatic and portal venous waveforms in the management of heart failure exacerbation. Clin. Case Rep. 2020, 8, 1489–1493. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Castelli, A.; Mattioli, G. Relationship between mean right atrial pressure and Doppler parameters in patients with right ventricular infarction. Clin. Cardiol. 2000, 23, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Baikpour, M.; Ozturk, A.; Dhyani, M.; Mercaldo, N.D.; Pierce, T.T.; Grajo, J.R.; Samir, A.E. Portal venous pulsatility index: A novel biomarker for diagnosis of high-risk nonalcoholic fatty liver disease. AJR Am. J. Roentgenol. 2020, 214, 786–791. [Google Scholar] [CrossRef]
- Singh, N.G.; Kumar, K.N.; Nagaraja, P.S.; Manjunatha, N. Portal venous pulsatility fraction, a novel transesophageal echocardiographic marker for right ventricular dysfunction in cardiac surgical patients. Ann. Card. Anaesth. 2020, 23, 39–42. [Google Scholar]
- Rengo, C.; Brevetti, G.; Sorrentino, G.; D’Amato, T.; Imparato, M.; Vitale, D.F.; Acanfora, D.; Rengo, F. Portal vein pulsatility ratio provides a measure of right heart function in chronic heart failure. Ultrasound Med. Biol. 1998, 24, 327–332. [Google Scholar] [CrossRef]
- Catalano, D.; Caruso, G.; DiFazzio, S.; Carpinteri, G.; Scalisi, N.; Trovato, G.M. Portal vein pulsatility ratio and heart failure. J. Clin. Ultrasound 1998, 26, 27–31. [Google Scholar] [CrossRef]
- Hu, J.-T.; Yang, S.-S.; Lai, Y.-C.; Shih, C.-Y.; Chang, C.-W. Percentage of peak-to-peak pulsatility of portal blood flow can predict right-sided congestive heart failure. World J. Gastroenterol. 2003, 9, 1828–1831. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Beaubien-Souligny, W.; Denault, A.Y.; Rouleau, J.L. Impacts of right ventricular function and venous congestion on renal response during depletion in acute heart failure. ESC Heart Fail. 2020, 7, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Eljaiek, R.; Cavayas, Y.A.; Rodrigue, E.; Desjardins, G.; Lamarche, Y.; Toupin, F.; Denault, A.; Beaubien-Souligny, W. High postoperative portal venous flow pulsatility indicates right ventricular dysfunction and predicts complications in cardiac surgery patients. Br. J. Anaesth. 2019, 122, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, K.; Nakamura, K.; Satomi, G.; Kondo, M.; Hirosawa, K. Evaluation of tricuspid regurgitation by blood flow pattern in the hepatic vein using pulsed Doppler technique. Am. Heart J. 1984, 108, 516–523. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Beaubien-Souligny, W.; Oussaïd, E.; Henri, C.; Racine, N.; Denault, A.Y.; Rouleau, J.L. Assessing splanchnic compartment using portal venous Doppler and impact of adding it to the EVEREST score for risk assessment in heart failure. CJC Open 2020, 2, 311–320. [Google Scholar] [CrossRef]
- De la Espriella-Juan, R.; Núñez, E.; Miñana, G.; Sanchis, J.; Bayés-Genís, A.; González, J.; Chorro, J.; Nunez, J. Intrarenal venous flow in cardiorenal syndrome: A shining light into the darkness. ESC Heart Fail. 2018, 5, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Nijst, P.; Martens, P.; Dupont, M.; Tang, W.H.W.; Mullens, W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 2017, 5, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Watanabe, K.; Sato, Y.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Ichijo, Y.; Yokokawa, T.; Misaka, T.; Oikawa, M.; et al. Intrarenal Doppler ultrasonography reflects hemodynamics and predicts prognosis in patients with heart failure. Sci. Rep. 2020, 10, 22257. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Husain-Syed, F.; Birk, H.; Ronco, C.; Schörmann, T.; Tello, K.; Richter, M.J.; Wilhelm, J.; Sommer, N.; Steyerberg, E.; Bauer, P.; et al. Doppler-derived renal venous stasis index in the prognosis of right heart failure. J. Am. Heart Assoc. 2019, 8, e013584. [Google Scholar] [CrossRef]
- Wiersema, R.; Kaufmann, T.; van der Veen, H.N.; de Haas, R.J.; Franssen, C.F.M.; Koeze, J.; Van Der Horst, I.C.; Keus, F. Diagnostic accuracy of arterial and venous renal Doppler assessment for acute kidney injury in critically ill patients: A prospective study. J. Crit. Care 2020, 59, 57–62. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VE × US) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur. J. Heart Fail. 2019, 21, 754–766. [Google Scholar] [CrossRef] [Green Version]
- Fadel, B.M.; Mohty, D.; Husain, A.; Alassas, K.; Echahidi, N.; Dahdouh, Z.; Di Salvo, G. Spectral Doppler of the hepatic veins in rate, rhythm, and conduction disorders. Echocardiography 2016, 33, 136–140. [Google Scholar] [CrossRef]
- Goncalvesova, E.; Lesny, P.; Luknar, M.; Solik, P.; Varga, I. Changes of portal flow in heart failure patients with liver congestion. Bratisl. Lek. Listy 2010, 111, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Cuganesan, R. Renal vein Doppler sonography of obstructive uropathy. AJR Am. J. Roentgenol. 2002, 178, 921–925. [Google Scholar] [CrossRef]
- Denault, A.Y.; Aldred, M.P.; Hammoud, A.; Zeng, Y.H.; Beaubien-Souligny, W.; Couture, E.J.; Jarry, S.; Gebhard, C.E.; Langevin, S.; Lamarche, Y.; et al. Doppler interrogation of the femoral vein in the critically Ill patient: The fastest potential acoustic window to diagnose right ventricular dysfunction? Crit. Care Explor. 2020, 2, e0209. [Google Scholar] [CrossRef] [PubMed]
- Abu-Yousef, M.M.; Kakish, M.E.; Mufid, M. Pulsatile venous Doppler flow in lower limbs: Highly indicative of elevated right atrium pressure. Am. J. Roentgenol. 1996, 167, 977–980. [Google Scholar] [CrossRef]
- Alimoğlu, E.; Erden, A.; Gursel, K.; Olcer, T. Correlation of right atrial pressure and blood flow velocities in the common femoral vein obtained by duplex Doppler sonography. J. Clin. Ultrasound. 2001, 29, 87–91. [Google Scholar] [CrossRef]
- Taute, B.M.; Schmidt, H.; Bach, A.; Fischer, R.; Le, C.; Tran, H.; Amoury, M.; Melnyk, H. Spectral Doppler Waveform Analysis of Common Femoral Veins for the Detection of Right Ventricular Dysfunction in Acute Pulmonary Embolism. J. Cardiovasc. Dis. Diagn 2015, 3. [Google Scholar] [CrossRef] [Green Version]
| Demographics | n (%) |
|---|---|
| Gender (female)–n (%) | 15 (50.0) |
| Age (years)–mean (SD) | 79 (13.4) |
| Previolus Diseases | n (%) |
| Hypertension–n (%) | 25 (83.3) |
| Dyslipidemia–n (%) | 12 (40.0) |
| Diabetes mellitus–n (%) | 8 (26.7) |
| Chronic kidney disease (stage 3 or later)–n (%) | 8 (26.7) |
| Previous recent hospitalization–n (%) | 5 (6.7) |
| Cardiovascular disease–n (%) | 19 (63.3) |
| Atrial fibrillation–n (%) | 8 (26.7) |
| Reduced ejection fraction–n (%) | 3 (10.0) |
| Pulmonary disease–n (%) | 9 (30.0) |
| Physical Exam | mean (SD) |
| Weight (kg) at admission–mean (SD) | 81.1 (16.7) |
| Weight (kg) at discharge–mean (SD) | 65.6 (19.2) |
| Laboratory Results | |
| NT-proBNP at admission pg/L–mean (SD) | 10,846.7 (11,693.8) |
| Urea at admission mg/dL–mean (SD) | 58.3 (29.2) |
| Sodium at admission mg/dL–mean (SD) | 137.5 (6.5) |
| Creatinine at admission–mg/dL–mean (SD) | 1.13 (0.5) |
| Hemoglobin at admission–g/dL–mean (SD) | 12.9 (2.4) |
| NT-proBNP at discharge pg/L–mean (SD) | 6987.3 (8999.1) |
| Urea at discharge mg/dL–mean (SD) | 88.7 (41.1) |
| Sodium at discharge mg/dL–mean (SD) | 141.1 (3.9) |
| Creatinine at discharge–mg/dL–mean (SD) | 1.3 (0.5) |
| Hemoglobin at discharge–g/dL–mean (SD) | 12.9 (2.2) |
| Change in the NT-proBNP pg/L–mean (SD) | −3859.4 (−7700.3) |
| Ultrasound Exam–At Admission | n (%) |
| Heart rhythm during ultrasound exam | |
| Sinusal rhythm | 10 (33.3) |
| Atrial fibrillation | 18 (60) |
| Atrial flutter | 2 (6.7) |
| Inferior vena cava of >2.1 cm and < 50% of collapsability | 12 (40.0) |
| Inferior vena cava of <2.1 cm and > 50% of collapsability | 9 (30.0) |
| Lung score at admission–mean (SD) | 16.5 (9.2) |
| Tricuspid regurgitation | |
| Moderate tricuspid regurgitation (>2.8 m/s and <3.4 m/s)–n (%) | 6 (20.0) |
| Severe tricuspid regurgitation (>3.4 m/s)–n (%) | 9 (30.0) |
| Pericardial effusion–n (%) | 4 (13.3) |
| Low TAPSE (<16 mm)–n (%) | 11 (36.7) |
| Mildly reduced ejection fraction (40–49%)–n (%) | 5 (16.7) |
| Reduced ejection fraction (<40%)–n (%) | 5 (16.7) |
| Probability of pulmonary hypertension * | |
| Low | 7 (23.3) |
| Intermediate | 12 (40.0) |
| High | 11 (36.7) |
| Hepatic vein at admission | |
| S > D | 1 (3.3) |
| S < D | 17 (56.7) |
| S Reversal | 11 (36.7) |
| Not measurable | 1 (3.3) |
| Portal vein at admission | |
| Pulsatility < 30% | 15 (23.3) |
| Pulsatility 30–50% | 9 (40.0) |
| Pulsatility > 50% | 6 (36.7) |
| Intra-renal vein at admission | |
| Continuous monophasic | 13 (33.3) |
| Biphasic flow | 13 (43.3) |
| Discontinuous monophasic | 4 (13.3) |
| Femoral vein at admission | |
| Pulsatility < 30% | 8 (26.7) |
| Pulsatility 30–50% | 3 (10.0) |
| Pulsatility > 50% | 19 (63.3) |
| Ultrasound Exam–At Discharge | n (%) |
| Inferior vena cava of >2.1 cm and < 50% of collapsability | 12 (40.0) |
| Inferior vena cava of <2.1 cm and > 50% of collapsability | 9 (30.0) |
| Lung score at discharge–mean (SD) | 9.3 (8.1) |
| Change in lung score–mean (SD) | 6.7 (10.4) |
| Inferior vena cava of >2.1 cm and <50% of collapsability | 8 (26.7) |
| Inferior vena cava of <2.1 cm and >50% of collapsability | 10 (33.3) |
| Improve in inferior vena cava–n (%) | 14 (46.7) |
| Hepatic vein at discharge | |
| S > D | 9 (30.0) |
| S < D | 11 (36.7) |
| S reversal | 9 (30.0) |
| Not measurable | 1 (3.3) |
| Improve in hepatic vein profile–n (%) | 22 (73.3) |
| Portal vein at discharge | |
| Pulsatility < 30% | 23 (76.3) |
| Pulsatility 30–50% | 3 (10.0) |
| Pulsatility > 50% | 4 (13.3) |
| Improve in portal vein profile–n (%) | 9 (30.0) |
| Worsening in portal vein profile–n (%) | 2 (6.7) |
| Intra-renal vein at discharge | |
| Continuous monophasic | 17 (56.7) |
| Biphasic flow | 9 (30.0) |
| Discontinuous monophasic | 4 (13.3) |
| Improve in intra-renal vein profile–n (%) | 6 (20.0) |
| Worsening in intra-renal vein profile–n (%) | 4 (13.3) |
| Femoral vein at discharge | |
| Pulsatility < 30% | 13 (43.3) |
| Pulsatility 30–50% | 1 (3.3) |
| Pulsatility > 50% | 16 (53.3) |
| Improve in femoral vein profile–n (%) | 9 (30.0) |
| Worsening in femoral vein profile–n (%) | 3 (10.0) |
| Improve in VE × US score–n (%) | 9 (30.0) |
| Worsening in VE × US score–n (%) | 7 (23.3) |
| VE × US score unchanged–n (%) | 14 (46.7) |
| Follow-Up | |
| Length of stay–days (SD)EVEREST score at admission | 9.1 (4.3) 10.1 (3.1) |
| EVEREST score at discharge | 0.7 (0.8) |
| NYHA at admission | |
| NYHA I | 1 (3.3) |
| NYHA II | 9 (30.0) |
| NYHA III | 18 (60.0) |
| NYHA IV | 2 (6.7) |
| NYHA at discharge | |
| NYHA I | 21 (70.0) |
| NYHA II | 8 (26.7) |
| NYHA III | 1 (3.3) |
| NYHA IV | 0 (0.0) |
| Ultrasound Exam | At Admission | At Discharge | p-Value |
|---|---|---|---|
| Inferior vena cava of >2.1 cm and <50% of collapsibility–n (%) | 12 (40.0) | 12 (40.0) | 0.132 |
| Inferior vena cava of <2.1 cm and >50% of collapsibility–n (%) | 9 (30.0) | 9 (30.0) | 0.132 |
| Lung score–mean (SD) | 16.5 (9.2) | 9.3 (8.1) | <0.001 |
| Hepatic vein (SD) | 2.4 (0.6) | 2.1 (0.9) | 0.002 |
| S > D–n (%) | 1 (3.3) | 9 (30.0) | |
| S < D–n (%) | 17 (56.7) | 11 (36.7) | |
| S reversal–n (%) | 11 (36.7) | 9 (30.0) | |
| Not measurable–n (%) | 1 (3.3) | 1 (3.3) | |
| Portal vein (SD) | 1.7 (0.8) | 1.4 (0.7) | 0.023 |
| Pulsatility < 30%–n (%) | 15 (23.3) | 23 (76.3) | |
| Pulsatility 30–50%–n (%) | 9 (40.0) | 3 (10.0) | |
| Pulsatility > 50%–n (%) | 6 (36.7) | 4 (13.3) | |
| Intra-renal vein (SD) | 1.7 (0.7) | 1.6 (0.7) | 0.293 |
| Continuous monophasic–n (%) | 13 (33.3) | 17 (56.7) | |
| Biphasic flow–n (%) | 13 (43.3) | 9 (30.0) | |
| Discontinuous monophasic–n (%) | 4 (13.3) | 4 (13.3) | |
| VE × US score (SD) | 1.3 (1.0) | 1.2 (1.1) | 0.501 |
| 0–n (%) | 8 (26.7) | 10 (33.3) | |
| 1–n (%) | 11 (36.7) | 9 (30.0) | |
| 2–n (%) | 6 (20.0) | 7 (23.3) | |
| 3–n (%) | 5 (16.7) | 4 (13.3) | |
| Femoral vein (SD) | 2.4 (0.9) | 2.1 (0.2) | 0.161 |
| Pulsatility < 30%–n (%) | 8 (26.7) | 13 (43.3) | |
| Pulsatility 30–50%–n (%) | 3 (10.0) | 1 (3.3) | |
| Pulsatility > 50%–n (%) | 19 (63.3) | 16 (53.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Arrese, M.; García de Casasola-Sánchez, G.; Méndez-Bailón, M.; Montero-Hernández, E.; Cobo-Marcos, M.; Rivas-Lasarte, M.; Caurcel-Díaz, L.; Rodríguez-Fuertes, P.; Villén-Villegas, T.; Tung-Chen, Y. Usefulness of Serial Multiorgan Point-of-Care Ultrasound in Acute Heart Failure: Results from a Prospective Observational Cohort. Medicina 2022, 58, 124. https://doi.org/10.3390/medicina58010124
Torres-Arrese M, García de Casasola-Sánchez G, Méndez-Bailón M, Montero-Hernández E, Cobo-Marcos M, Rivas-Lasarte M, Caurcel-Díaz L, Rodríguez-Fuertes P, Villén-Villegas T, Tung-Chen Y. Usefulness of Serial Multiorgan Point-of-Care Ultrasound in Acute Heart Failure: Results from a Prospective Observational Cohort. Medicina. 2022; 58(1):124. https://doi.org/10.3390/medicina58010124
Chicago/Turabian StyleTorres-Arrese, Marta, Gonzalo García de Casasola-Sánchez, Manuel Méndez-Bailón, Esther Montero-Hernández, Marta Cobo-Marcos, Mercedes Rivas-Lasarte, Luis Caurcel-Díaz, Pablo Rodríguez-Fuertes, Tomas Villén-Villegas, and Yale Tung-Chen. 2022. "Usefulness of Serial Multiorgan Point-of-Care Ultrasound in Acute Heart Failure: Results from a Prospective Observational Cohort" Medicina 58, no. 1: 124. https://doi.org/10.3390/medicina58010124
APA StyleTorres-Arrese, M., García de Casasola-Sánchez, G., Méndez-Bailón, M., Montero-Hernández, E., Cobo-Marcos, M., Rivas-Lasarte, M., Caurcel-Díaz, L., Rodríguez-Fuertes, P., Villén-Villegas, T., & Tung-Chen, Y. (2022). Usefulness of Serial Multiorgan Point-of-Care Ultrasound in Acute Heart Failure: Results from a Prospective Observational Cohort. Medicina, 58(1), 124. https://doi.org/10.3390/medicina58010124








