Association between the Severity of Nonalcoholic Fatty Liver Disease and the Risk of Coronary Artery Calcification
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Medical History Records and Laboratory Assessments
2.3. Hepatic Ultrasonography
- (1)
- Absent: normal echotexture of the liver was revealed without different echogenicities between liver parenchyma and renal cortex.
- (2)
- Mild: a slight increase in the echogenicity of the liver with normal visualization of intrahepatic vessels and diaphragm, and a mild difference in echogenicity between liver parenchyma and renal cortex.
- (3)
- Moderate: a moderate increase in the echogenicity of the liver with impaired appearance of intrahepatic vessels and diaphragm, and a difference in echogenicity between liver parenchyma and renal cortex.
- (4)
- Severe: a significant increase in the echogenicity of the liver with poor visualization of intrahepatic vessels and diaphragm combined with a significant difference in echogenicity between liver parenchyma and renal cortex.
2.4. Coronary Artery Calcium Score by Cardiac Multidetector Computed Tomography
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Adams, L.A.; Feldstein, A.E. Nonalcoholic steatohepatitis: Risk factors and diagnosis. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 623–635. [Google Scholar]
- Zhou, J.-H.; Cai, J.-J.; She, Z.-G.; Li, H.-L. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J. Gastroenterol. 2019, 25, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Isabela Andronescu, C.; Purcarea, M.R.; Aurel Babes, P. The role of noninvasive tests and liver biopsy in the diagnosis of nonalcoholic fatty liver disease. J. Med. Life 2018, 11, 243–246. [Google Scholar] [CrossRef]
- Bonci, E.; Chiesa, C.; Versacci, P.; Anania, C.; Silvestri, L.; Pacifico, L. Association of nonalcoholic fatty liver disease with subclinical cardiovascular changes: A systematic review and meta-analysis. Biomed. Res. Int. 2015, 2015, 213737. [Google Scholar] [CrossRef]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef]
- Lee, M.-K.; Park, H.-J.; Jeon, W.S.; Park, S.E.; Park, C.-Y.; Lee, W.-Y.; Oh, K.-W.; Park, S.-W.; Rhee, E.-J. Higher association of coronary artery calcification with non-alcoholic fatty liver disease than with abdominal obesity in middle-aged Korean men: The Kangbuk Samsung Health Study. Cardiovasc. Diabetol. 2015, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojas, J.G.; Medina-Urrutia, A.X.; Jorge-Galarza, E.; González-Salazar, C.; Kimura-Hayama, E.; Cardoso-Saldaña, G.; Posadas-Sánchez, R.; Martínez-Alvarado, R.; Posadas-Romero, C. Fatty liver increases the association of metabolic syndrome with diabetes and atherosclerosis. Diabetes Care 2013, 36, 1726–1728. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Soares Monteiro, L.B. Ultrasound-Based Techniques for the Diagnosis of Liver Steatosis. World J. Gastroenterol. 2019, 25, 6053–6062. [Google Scholar] [CrossRef]
- Saverymuttu, S.H.; Joseph, A.E.; Maxwell, J.D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br. Med. J. 1986, 292, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, F.W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Hsu, C.S.; Kao, J.H. Non-alcoholic fatty liver disease: An emerging liver disease in Taiwan. J. Med. Assoc. 2012, 111, 527–535. [Google Scholar]
- Pletcher, M.J.; Tice, J.; Pignone, M.; Browner, W.S. Using the coronary artery calcium score to predict coronary heart disease events: A systematic review and meta-analysis. Arch. Intern. Med. 2004, 164, 1285–1292. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Adelhoefer, S.; Uddin, S.M.I.; Osei, A.D.; Obisesan, O.H.; Blaha, M.J.; Dzaye, O. Coronary artery calcium scoring: New insights into clinical interpretation-lessons from the CAC consortium. Radiol. Cardiothorac. Imaging 2020, 2, e200281. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Evans, C.V.; Johnson, E.; Redmond, N.; Coppola, E.L.; Smith, N. nontraditional risk factors in cardiovascular disease risk assessment: Updated evidence report and systematic review for the us preventive services task force. JAMA 2018, 320, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S.; Mosler, T.P. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Kang, Y.M.; Yoo, J.H.; Lee, J.; Lee, S.E.; Yang, D.H.; Kang, J.-W.; Park, J.-Y.; Jung, C.H.; Kim, H.-K.; et al. The impact of non-alcoholic fatty liver disease and metabolic syndrome on the progression of coronary artery calcification. Sci. Rep. 2018, 8, 12004. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, L.R.; De Matos, R.C.; E Souza, Y.P.D.M.; Vieira-Soares, D.; Muller-Machado, G.; Pollo-Flores, P. Non-alcoholic fatty liver disease and its links with inflammation and atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 7. [Google Scholar]
- Kim, D.; Choi, S.-Y.; Park, E.H.; Lee, W.; Kang, J.H.; Kim, W.R.; Kim, Y.J.; Yoon, J.-H.; Jeong, S.H.; Lee, D.H.; et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012, 56, 605–613. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, H.Y.; Lee, H.S.; Jung, K.S.; Lee, M.H.; Jhee, J.H.; Kim, T.H.; Lee, J.E.; Kim, H.J.; Kim, B.S.; et al. Association between non-alcoholic fatty liver disease and coronary calcification depending on sex and obesity. Sci. Rep. 2020, 10, 1025. [Google Scholar]
- Jaruvongvanich, V.; Wirunsawanya, K.; Sanguankeo, A.; Upala, S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: A systematic review and meta-analysis. Dig. Liver Dis. 2016, 48, 1410–1417. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.Y.; Park, S.E.; Park, C.-Y.; Lee, W.-Y.; Oh, K.-W.; Park, S.-W.; Rhee, E.-J. Increased risk for development of coronary artery calcification in subjects with non-alcoholic fatty liver disease and systemic inflammation. PLoS ONE 2017, 12, e0180118. [Google Scholar]
- Koo, B.K.; Allison, M.A.; Criqui, M.H.; Denenberg, J.O.; Wright, C.M. The association between liver fat and systemic calcified atherosclerosis. J. Vasc. Surg. 2020, 71, 204–211.e4. [Google Scholar]
- Kang, M.K.; Kang, B.H.; Kim, J.H. Nonalcoholic fatty liver disease is associated with the presence and morphology of subclinical coronary atherosclerosis. Yonsei Med. J. 2015, 56, 1288–1295. [Google Scholar] [PubMed]
- Haddad, T.M.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), s209–s216. [Google Scholar] [CrossRef]
- Lahti, S.J.; Feldman, D.I.; Dardari, Z.; Mirbolouk, M.; Orimoloye, O.A.; Osei, A.D.; Graham, G.; Rumberger, J.; Shaw, L.; Budoff, M.J.; et al. The association between left main coronary artery calcium and cardiovascular-specific and total mortality: The coronary artery calcium consortium. Atherosclerosis 2019, 286, 172–178. [Google Scholar] [CrossRef]
- Liu, K.; Wong, V.W.-S.; Lau, K.; Du Liu, S.; Tse, Y.-K.; Yip, T.C.-F.; Kwok, R.; Chan, A.Y.-W.; Chan, H.L.-Y.; Wong, G.L.-H. Prognostic value of controlled attenuation parameter by transient elastography. Am. J. Gastroenterol. 2017, 112, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Bayturan, O.; Tuzcu, E.M.; Lavoie, A.; Hu, T.; Wolski, K.; Schoenhagen, P.; Kapadia, S.; Nissen, S.E.; Nicholls, S.J. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch. Intern. Med. 2010, 170, 478–484. [Google Scholar] [CrossRef]
- Cuspidi, C.; Sala, C.; Provenzano, F.; Tadic, M.; Gherbesi, E.; Grassi, G.; Mancia, G. Metabolic syndrome and subclinical carotid damage: A meta-analysis from population-based studies. J. Hypertens. 2018, 36, 23–30. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Shao, Z.; Li, Y.-L.; Wulasihan, M.; Chen, X.-H. Prevalence of and risk factors for non-alcoholic fatty liver disease in a Chinese population: An 8-year follow-up study. World J. Gastroenterol. 2016, 22, 3663–3669. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kim, H.I.; Lee, Y.-J.; Lee, J.W.; Kim, K.M.; Bae, J.C. Differing associations between fatty liver and dyslipidemia according to the degree of hepatic steatosis in Korea. J. Lipid Atheroscler. 2019, 8, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-C.; Wild, S.H.; Kwag, H.J.; Byrne, C.D. Fatty liver, insulin resistance, and features of metabolic syndrome: Relationships with coronary artery calcium in 10,153 people. Diabetes Care 2012, 35, 2359–2364. [Google Scholar] [CrossRef]
- Toledo, F.G.; Sniderman, A.D.; Kelley, D.E. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care 2006, 29, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Ning, H.; Lewis, C.E.; Shay, C.M.; Wilkins, J.; Carr, J.J.; Terry, J.G.; Lloyd-Jones, D.M.; Jacobs, D.R.; Carnethon, M.R. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The coronary artery risk development in young adults study. Atherosclerosis 2014, 235, 599–605. [Google Scholar] [CrossRef]
- Mills, E.P.; Brown, K.P.D.; Smith, J.D.; Vang, P.W.; Trotta, K. Treating nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A review of efficacy and safety. Adv. Endocrinol. Metab. 2018, 9, 15–28. [Google Scholar] [CrossRef]
- Caldwell, S. NASH Therapy: Omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin. Mol. Hepatol. 2017, 23, 103–108. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wong, V.W.-S.; Peleg, N.; et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef]
- Ballestri, S.; Lonardo, A.; Romagnoli, D.; Carulli, L.; Losi, L.; Day, C.P.; Loria, P. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012, 32, 1242–1252. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Shariatifar, B.; Khodadoostan, M.; Motamedi, N.; Abdolahi, H. Comparison of liver enzymes level and sonographic findings value with liver biopsy findings in nonalcoholic fatty liver disease patients. Adv. Biomed. Res. 2016, 5, 40. [Google Scholar] [CrossRef]
- Nelson, S.M.; Hoskins, J.D.; Lisanti, C.; Chaudhuri, J. Ultrasound fatty liver indicator: A simple tool for differentiating steatosis from nonalcoholic steatohepatitis: Validity in the average obese population. J. Ultrasound Med. 2020, 39, 749–759. [Google Scholar] [CrossRef]
- Tanpowpong, N.; Panichyawat, S. Comparison of sonographic hepatorenal ratio and the degree of hepatic steatosis in magnetic resonance imaging-proton density fat fraction. J. Ultrason. 2020, 20, e169–e175. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Lam, J.; Peterson, M.R.; Middleton, M.; Hamilton, G.; Le, T.-A.; Bettencourt, R.; Changchien, C.; Brenner, D.; Sirlin, C.; et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013, 58, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Pu, K.; Wang, Y.; Bai, S.; Wei, H.; Zhou, Y.; Fan, J.; Qiao, L. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: A systematic review and meta-analysis. BMC Gastroenterol. 2019, 19, 51. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Adams, L.A.; De Lédinghen, V.; Wong, G.L.-H.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH—Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.S.; Cho, Y.K.; Kim, E.H.; Lee, M.J.; Bae, I.Y.; Jung, C.H.; Park, J.-Y.; Kim, H.-K.; Lee, W.J. Association between noninvasive assessment of liver fibrosis and coronary artery calcification progression in patients with nonalcoholic fatty liver disease. Sci. Rep. 2020, 10, 18323. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Mantovani, A.; Baldelli, E.; Lugari, S.; Maurantonio, M.; Nascimbeni, F.; Marrazzo, A.; Romagnoli, D.; Targher, G.; Lonardo, A. Liver FIBROSIS biomarkers accurately exclude advanced fibrosis and are associated with higher cardiovascular risk scores in patients with NAFLD or viral chronic liver disease. Diagnostics 2021, 11, 98. [Google Scholar] [CrossRef]
- Piazzolla, V.A.; Mangia, A. Noninvasive diagnosis of NAFLD and NASH. Cells 2020, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
| Total | Males: 361 (66.2%) | Females: 184 (33.8%) | |
|---|---|---|---|
| Age (years) | 54.63 ± 10.60 | 53.69 ± 10.66 | 56.46 ± 10.26 |
| Hypertension | |||
| Yes | 168 (30.8%) | 127 (35.2%) | 41 (22.3%) |
| No | 377 (69.2%) | 234 (64.8%) | 143 (77.7%) |
| Diabetes | |||
| Yes | 64 (11.7%) | 47 (13%) | 17 (9.2%) |
| No | 481 (88.3%) | 314 (87%) | 167 (90.8%) |
| Hyperlipidemia | |||
| Yes | 111 (20.4%) | 79 (21.9%) | 32 (17.4%) |
| No | 434 (79.6%) | 282 (78.1%) | 152 (82.6%) |
| Height (cm) | 164.90 ± 8.45 | 169.10 ± 6.33 | 156.68 ± 5.53 |
| Weight (kg) | 68.49 ± 13.32 | 73.60 ± 12.10 | 58.47 ± 9.30 |
| BMI (kg/m2) | 25.08 ± 3.73 | 25.72 ± 3.64 | 23.83 ± 3.60 |
| SBP (mmHg) | 128.15 ± 16.62 | 128.72 ± 3.64 | 127.03 ± 17.87 |
| DBP (mmHg) | 76.52 ± 12.06 | 79.92 ± 10.89 | 69.85 ± 11.47 |
| AST (U/L) | 23.26 ± 11.17 | 24.28 ± 11.25 | 21.25 ± 10.75 |
| ALT (IU/L) | 26.85 ± 19.07 | 30.80 ± 20.97 | 19.09 ± 11.19 |
| HbA1c (%) | 6.07 ± 1.06 | 6.16 ± 1.17 | 5.87 ± 0.79 |
| Total cholesterol (mg/dL) | 193.67 ± 40.28 | 192.2 ± 39.38 | 196.55 ± 41.96 |
| HDL (mg/dL) | 48.83 ± 25.48 | 45.54 ± 29.49 | 55.29 ± 12.50 |
| LDL (mg/dL) | 118.86 ± 35.37 | 119.43 ± 35.39 | 117.75 ± 35.42 |
| TG (mg/dL) | 152.80 ± 106.82 | 170.85 ± 120.57 | 117.40 ± 58.49 |
| UA (mg/dL) | 5.92 ± 1.47 | 6.46 ± 1.33 | 4.87 ± 1.14 |
| NAFLD staging | |||
| Normal | 108 (19.8%) | 54 (15%) | 54 (29.3%) |
| Mild | 194 (35.6%) | 117 (32.4%) | 77 (41.8%) |
| Moderate | 202 (37.1%) | 153 (42.4%) | 49 (26.6%) |
| Severe | 41 (7.5%) | 37 (10.2%) | 4 (2.2%) |
| CAC score | |||
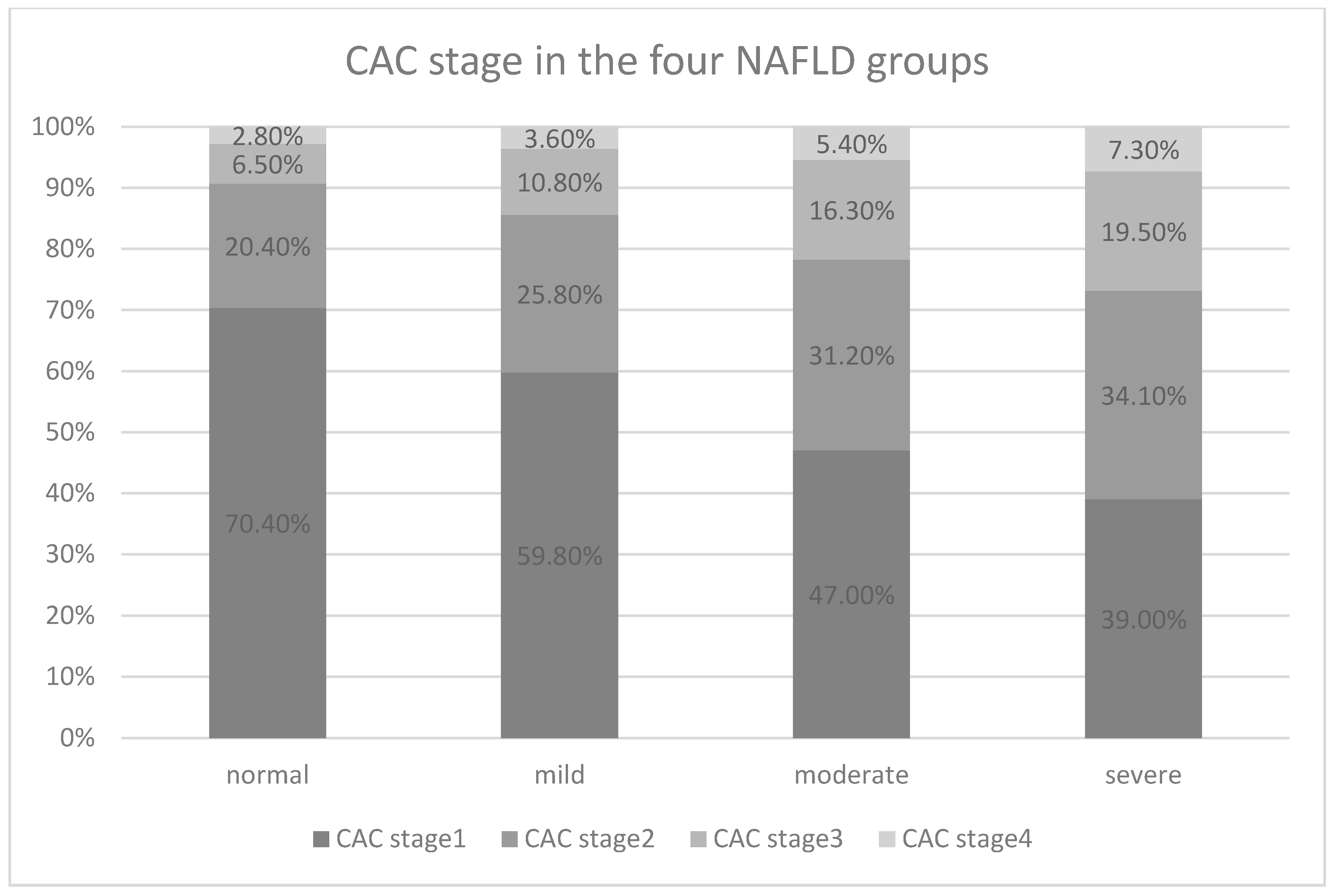
| 1 | 303 (55.6%) | 167 (46.3%) | 136 (73.9%) |
| 2 | 149 (27.3%) | 116 (32.1%) | 33 (17.9%) |
| 3 | 69 (12.7%) | 59 (16.3%) | 10 (5.4%) |
| 4 | 24 (4.4%) | 19 (5.3%) | 5 (2.7%) |
| Non-NAFLD (n = 108) | Mild NAFLD (n = 194) | Moderate NAFLD (n = 202) | Severe NAFLD (n = 41) | p-Value | |
|---|---|---|---|---|---|
| Sex | <0.001 | ||||
| Male | 54 (50%) | 177 (60.3%) | 153 (75.7%) | 37 (90.2%) | |
| Female | 54 (50%) | 77 (39.7%) | 49 (24.3%) | 7 (9.8%) | |
| Age (years) | 52.13 ± 11.39 | 55.64 ± 10.01 | 55.54 ± 10.57 | 51.88 ± 10.14 | 0.007 |
| Weight (kg) | 58.94 ± 9.43 | 65.62 ± 11.57 | 72.60 ± 10.37 | 87.05 ± 16.20 | <0.001 |
| Body mass index (kg/m2) | 22.27 ± 2.87 | 24.29 ± 3.16 | 26.34 ± 3.03 | 30.04 ± 3.91 | <0.001 |
| Hypertension | 0.003 | ||||
| Yes | 24 (22.2%) | 50 (25.8%) | 76 (37.6%) | 18 (43.9%) | |
| No | 84 (77.8%) | 144 (74.2%) | 126 (62.4%) | 23 (56.1%) | |
| Diabetes mellitus | 0.001 | ||||
| Yes | 7 (6.5%) | 15 (7.7%) | 32 (15.8%) | 10 (24.4%) | |
| No | 101 (93.5%) | 179 (92.3%) | 170 (84.2%) | 31 (75.6%) | |
| Hyperlipidemia | <0.001 | ||||
| Yes | 11 (10.2%) | 34 (17.5%) | 59 (29.2%) | 7 (17.1%) | |
| No | 97 (89.8%) | 160 (82.5%) | 143 (70.8%) | 34 (82.9%) | |
| Systolic blood pressure (mmHg) | 123.3 ± 18.44 | 127.92 ± 16.11 | 130.41 ± 15.74 | 130.93 ± 15.84 | 0.003 |
| Diastolic blood pressure (mmHg) | 72.21 ± 12.57 | 75.42 ± 11.68 | 78.66 ± 11.16 | 82.49 ± 12.45 | <0.001 |
| HbA1c (%) | 5.78 ± 0.91 | 5.87 ± 0.80 | 6.25 ± 1.06 | 6.85 ± 1.06 | <0.001 |
| Total cholesterol (mg/dL) | 191.35 ± 37.47 | 195.02 ± 41.59 | 195.10 ± 39.35 | 185.51 ± 45.62 | 0.691 |
| Triglycerides (mg/dL) | 101.47 ± 50.01 | 142.68 ± 88.64 | 175.61 ± 117.75 | 223.54 ± 159.81 | <0.001 |
| HDL-C (mg/dL) | 54.80 ± 13.29 | 48.79 ± 12.03 | 47.26 ± 38.32 | 41.07 ± 10.47 | 0.014 |
| LDL-C (mg/dL) | 116.43 ± 32.63 | 118.76 ± 36.43 | 122.07 ± 34.95 | 109.98 ± 38.44 | 0.19 |
| Uric acid (mg/dL) | 5.32 ± 1.47 | 5.60 ± 1.44 | 6.42 ± 1.31 | 6.54 ± 1.40 | <0.001 |
| AST (U/L) | 21.06 ± 8.04 | 21.23 ± 9.09 | 24.65 ± 12.29 | 31.73 ± 15.78 | <0.001 |
| ALT (IU/L) | 19.34 ± 13.15 | 22.27 ± 17.20 | 30.91 ± 18.44 | 48.29 ± 23.30 | <0.001 |
| CAC score > 0 | <0.001 | ||||
| Yes | 32 (29.6%) | 78 (40.2%) | 107 (53%) | 25 (61%) | |
| No | 76 (70.4%) | 116 (59.8%) | 95 (47%) | 16 (39%) | |
| LM involved | <0.001 | ||||
| Yes | 4 (3.7%) | 16 (8.2%) | 30 (14.9%) | 10 (24.4%) | |
| No | 104 (96.3%) | 178 (91.8%) | 172 (85.1%) | 31 (75.6%) | |
| LAD involved | 0.001 | ||||
| Yes | 26 (24.1%) | 66 (34%) | 90 (44.6%) | 21 (51.2%) | |
| No | 82 (75.9%) | 128 (66%) | 112 (55.4%) | 20 (48.8%) | |
| LCX involved | 0.01 | ||||
| Yes | 10 (9.3%) | 24 (12.4%) | 42 (20.8%) | 10 (24.4%) | |
| No | 98 (90.7%) | 170 (87.6%) | 160 (79.2%) | 31 (75.6%) | |
| RCA involved | 0.001 | ||||
| Yes | 19 (17.6%) | 39 (20.1%) | 55 (27.2%) | 19 (46.3%) | |
| No | 89 (82.4%) | 155 (79.9%) | 147 (72.8%) | 22 (53.7%) |
| Model 1 Odds Ration (95% CI) | p-Value | Model 2 Odds Ration (95% CI) | p-Value | Model 3 Odds Ration (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| CAC > 0 | 1.41 (1.13–1.77) | 0.003 | 1.39 (1.09–1.77) | 0.007 | 1.36 (1.07–1.77) | 0.016 |
| LM involved | 1.98 (1.37–2.87) | <0.001 | 1.85 (1.26–2.7) | 0.002 | 1.71 (1.16–2.54) | 0.007 |
| LAD involved | 1.38 (1.10–1.73) | 0.006 | 1.39 (1.09–1.77) | 0.007 | 1.34 (1.04–1.72) | 0.025 |
| RCA involved | 1.36 (1.06–1.74) | 0.017 | 1.30 (1.00–1.69) | 0.051 | 1.39 (1.05–1.84) | 0.021 |
| LCX involved | 1.46 (1.09–1.97) | 0.013 | 1.41 (1.03–1.93) | 0.032 | 1.41 (1.02–1.95) | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Hsu, W.-C.; Wu, H.-M.; Wang, J.-Y.; Yang, P.-Y.; Lin, I.-C. Association between the Severity of Nonalcoholic Fatty Liver Disease and the Risk of Coronary Artery Calcification. Medicina 2021, 57, 807. https://doi.org/10.3390/medicina57080807
Chen C-C, Hsu W-C, Wu H-M, Wang J-Y, Yang P-Y, Lin I-C. Association between the Severity of Nonalcoholic Fatty Liver Disease and the Risk of Coronary Artery Calcification. Medicina. 2021; 57(8):807. https://doi.org/10.3390/medicina57080807
Chicago/Turabian StyleChen, Chien-Chih, Wei-Chien Hsu, Han-Ming Wu, Jiun-Yi Wang, Pei-Yu Yang, and I-Ching Lin. 2021. "Association between the Severity of Nonalcoholic Fatty Liver Disease and the Risk of Coronary Artery Calcification" Medicina 57, no. 8: 807. https://doi.org/10.3390/medicina57080807
APA StyleChen, C.-C., Hsu, W.-C., Wu, H.-M., Wang, J.-Y., Yang, P.-Y., & Lin, I.-C. (2021). Association between the Severity of Nonalcoholic Fatty Liver Disease and the Risk of Coronary Artery Calcification. Medicina, 57(8), 807. https://doi.org/10.3390/medicina57080807







