Abstract
Background and Objectives: Osteoprotegerin (OPG), a potent osteoclast activation inhibitor, decreases bone resorption and plays a role in mediating bone mineral density (BMD). Our aim was to evaluate the relationship between BMD and serum OPG in maintenance hemodialysis (MHD) patients. Materials and Methods: Fasting blood samples were obtained from 75 MHD patients. BMD was measured by dual-energy X-ray absorptiometry in lumbar vertebrae (L2–L4). The WHO classification criteria were applied to define osteopenia and osteoporosis. A commercial enzyme-linked immunosorbent assay was used to measure serum OPG values. Results: Among all MHD patients, seven (9.3%) and 20 patients (26.7%) were defined as osteoporosis and osteopenia, respectively. Female patients had lower lumbar BMD than males (p = 0.002). Older age (p = 0.023), increased serum OPG (p < 0.001) urea reduction rate (p = 0.021), Kt/V (p = 0.027), and decreased body mass index (p = 0.006) and triglycerides (p = 0.020) were significantly different between the normal, osteopenia, and osteoporosis groups. Lumbar spine BMD was positively correlated with body mass index (BMI) (p < 0.001) but negatively correlated with OPG (p < 0.001) and age (p = 0.003). After grouping patients into T scores < −1 and < −2.5, female sex and OPG (adjusted odds ratio [aOR] 1.022, 95% confidence interval [C.I.] 1.011–1.034, p < 0.001) were predictors of T scores < −1, whereas only OPG was predictive of T scores < −2.5 (aOR 1.015, 95% C.I. 1.005–1.026, p = 0.004) by multivariate stepwise logistic regression analysis. The areas under the curve for predicting T scores < −1 or < −2.5 were 0.920 (95% C.I. 0.834–0.970, p < 0.001) and 0.958 (95% C.I. 0.885–0.991, p < 0.001), respectively. Conclusions: Increased serum OPG negatively correlated with lumbar BMD and could be a potential biomarker predictive of osteoporosis in MHD patients.
1. Introduction
Chronic kidney disease (CKD) is a health burden affecting nearly 700 million people worldwide that has a higher risk of cardiovascular disease (CVD) []. CKD is also a prominent risk factor of fractures related to dysregulated bone metabolism, which is known as CKD-related mineral bone disease, and the risk is higher than the risk in the general population [,,]. Mounting evidence has shown that the prevalence of fractures is higher in CKD patients on dialysis than in pre-dialysis CKD patients []. In maintenance hemodialysis (MHD) patients, abnormal low bone mass and density are common, and the examined bone mineral density (BMD) has shown that the prevalence of osteoporosis and osteopenia were 9.5–23% and 16.7–45%, respectively [,]. CKD patients with osteoporosis and osteopenia have a substantially increased risk of fractures leading to a huge public health burden worldwide [].
Multiple risk factors have been reported to be associated with osteoporosis in CKD, including poor nutrition, vitamin-D deficiency, hyperparathyroidism, metabolic acidosis, limited physical activity, and CKD-related metabolic mineral bone disease []. Osteoprotegerin (OPG), which belongs to the tumor necrosis factor receptor family, is known as a humoral glycoprotein that binds to the receptor activator nuclear factor κ-B ligand (RANKL) that inhibits osteoclastogenesis []. In an in vivo study, mice without OPG exhibited marked osteoporosis as well as decreased bone strength []. Moreover, over-expression of OPG resulted in increased bone density in mice as well as inhibition of osteoclast maturation in a dose-dependent manner []. In patients with CKD and MHD, serum OPG progressively increased as renal function declined and was positively correlated with inflammatory markers and with survival [,,,]. Serum OPG levels of dialysis patients were markedly elevated relative to those of controls independent of their serum PTH levels []. A comparison of bone histomorphometry in MHD patients showed that there was a negative association between OPG level and trabecular bone volume (BV/TV) []. However, the relationship between serum OPG and BMD remained inconclusive. Some studies have shown that there was a positive correlation between BMD and annual percentage changes in BMD and serum OPG levels in MHD patients [,]. Another study revealed that there was a negative correlation between serum OPG level and the BMD of the lumbar spine and total hip of female pre-dialysis CKD patients [] and of the femoral neck of MHD patients [,]. Regarding osteoporosis in MHD patients, advanced age and post-menopause status are well-known risk factors for bone loss, but the potential role of OPG in mediating the process of bone loss through its accumulation is not known. Therefore, we conducted this cross-sectional study to determine the role of OPG in the development of osteoporosis and identify the risk factors for bone loss in MHD patients.
2. Materials and Methods
2.1. Patients
From June 2015 to August 2015 at a single hospital who were >50 years old, were receiving standard weekly 4-h dialysis three sessions using high-flux polysulfone disposable artificial kidneys (FX class dialyzer, Fresenius Medical Care, Bad Homburg, Germany) for ≥3 months were enrolled. Patients were excluded if they were receiving treatments such as bisphosphonates, teriparatide, denosumab or estrogen medications to treat osteoporosis, had a history of lumbar fracture or surgery, acute infection, malignancy, acute cardiovascular disease, or if they declined to provide informed consent. The Research Ethics Committee, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (IRB106-62-B) approved this study.
2.2. Biochemical and Anthropometric Analysis
Before receiving HD therapy, fasting blood samples (approximately 5 mL) were collected and immediately centrifuged at 3000× g for 10 min, stored at 4 °C, and analyzed within 1 h after collection. Serum values of biochemical variables were measured by using an autoanalyzer (Siemens Advia 1800, Siemens Healthcare GmbH, Henkestr, Germany). Kt/V and urea reduction ratio (URR) using a formal and single-compartment dialysis urea kinetic model were used to calculate adequacy of HD. Serum OPG (eBioscience Inc., San Diego, CA, USA) and intact parathyroid hormone (iPTH) levels (Abcam, Cambridge, MA, USA) were measured by using a commercially available enzyme immunoassay or enzyme-linked immunosorbent assay, respectively []. Body mass index (BMI) was calculated as (body weight)/(body height)2 (kg)/(m)2 [].
2.3. Bone Mineral Density Measurements
Patients were arranged to examine bone mineral density measurements before HD. Lumbar vertebrate (L2–L4) BMD was measured by using dual-energy X-ray absorptiometry (QDR 4500, Hologic Inc., Marlborough, MA, USA). BMD was expressed as an absolute value (g/cm2) and as a T score (deviation from peak BMD) []. According to World Health Organization criteria, a lumbar bone T score < −2.5 and −1.0 to −2.5 was used to define osteoporosis and osteopenia, respectively [].
2.4. Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation or as the median and interquartile range according to results of the Klomogorov–Smirnov test. Kruskal–Wallis test or by one-way analysis of variance were used to examine the difference among normal, osteopenia and osteoporosis. Comparisons between male and female patients were analyzed by performing Student’s t-test. Categorical variables were expressed as the number of patients and analyzed by the χ2 test. Correlation between clinical variables and lumbar BMD were evaluated by linear regression analysis. Variables that were significantly associated with osteopenia or osteoporosis were examined by multivariate stepwise logistic regression analysis. The receiver operating characteristic (ROC) curve was used to calculate the area under the curve (AUC) to identify the optimal cutoff values of OPG for predicting osteopenia or osteoporosis in MHD patients. MedCalc® Statistical Software version 19.7.1 (MedCalc Software Ltd., Ostend, Belgium) was used to analyze. A p value < 0.05 was indicative of statistically significant.
3. Results
In this study, there were 48 (64%), 20 (26.7%), and 7 (9.3%) in the normal, osteopenia, and osteoporosis groups, respectively (Table 1). Compared with the normal group, patients in the osteopenia or osteoporosis groups were older (63.94 ± 8.89 vs. 70.25 ± 8.84 vs. 68.43 ± 7.09; p = 0.023) and had more females (35.4% vs. 75% vs. 85.7%, p = 0.002), higher levels of OPG (231.27 ± 82.47 vs. 413.87 ± 118.05 vs. 665.37 ± 191.45, p < 0.001), higher URR (0.73 ± 0.04 vs. 0.75 ± 0.04 vs. 0.76 ± 0.04; p = 0.021), and higher Kt/V (1.30 ± 0.16 vs. 1.41 ± 0.17 vs. 1.44 ± 0.17; p = 0.027) but lower BMI (25.92 ± 5.01 vs. 23.61 ± 3.94 vs. 20.26 ± 2.26, p = 0.006). Values of lumbar BMD and T scores were significantly lower for the female MHD patients than for the males (Figure 1). Serum levels of alkaline phosphatase or iPTH showed no significant differences among these three groups.

Table 1.
Clinical characteristics according to different lumbar T-score cut-off points (normal, osteopenia, and osteoporosis) of the 75 hemodialysis patients.
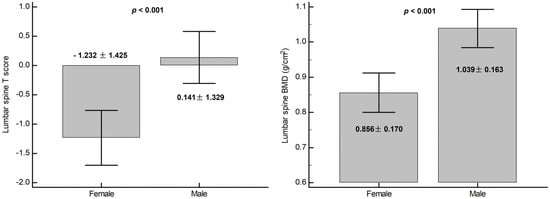
Figure 1.
Different values of lumbar spine BMD and T score between female and male.
Linear correlation analysis showed that BMI (r = 0.40, p < 0.001; r = 0.41, p < 0.001) was positively correlated, whereas age (r = −0.33, p = 0.004; r = −0.34, p = 0.003) and OPG (r = −0.60, p < 0.001, r = −0.58, p < 0.001) was negatively correlated with lumbar T score and BMD, respectively (Figure 2).
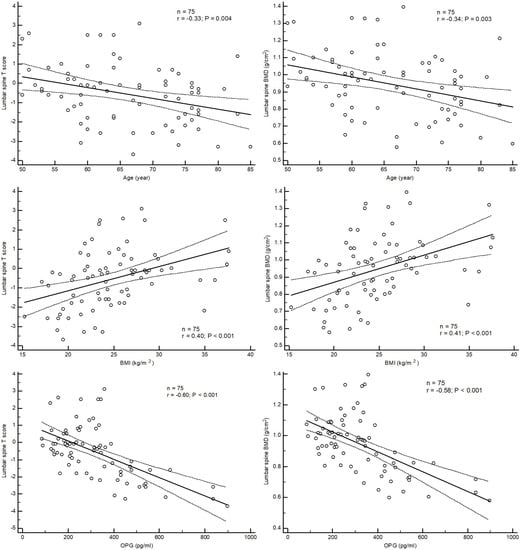
Figure 2.
Correlation between T score and BMD of lumbar spine and age, BMI and OPG.
Patients were then grouped by T score between −1 and −2.5 (osteopenia and osteoporosis, Table 2) and −2.5 (osteoporosis, Table 3). After adjustment for factors significantly associated with T scores < −1 and −2.5 in the univariate logistic regression analysis, the serum OPG level (adjusted odds ratio (aOR) 1.022, 95% confidence interval (C.I. 1.010−1.045, p = 0.002)) and female sex (aOR 13.37; 95% C.I., 2.049−87.30; p = 0.007) were identified as independent predictors of T scores less than −1 (Table 2), whereas serum OPG (aOR 1.015; 95% C.I., 1.005−1.026; p = 0.004) was the single significant predictor for T scores < −2.5 by multivariate stepwise logistic regression analysis (Table 3).

Table 2.
Risk factor for diagnosis of osteopenia and osteoporosis (T score < −1).

Table 3.
Risk factor for diagnosis of osteoporosis (T score < −2.5).
The ROC curve analysis showed that the best serum cutoff values of OPG to predict osteopenia + osteoporosis and osteoporosis alone of MHD patients were 388.38 pg/mL and 394.73 pg/mL, with AUCs of 0.920 (95% C.I., 0.834−0.970; p < 0.001) and 0.958 (95% C.I., 0.885−0.991; p < 0.001), respectively (Figure 3).
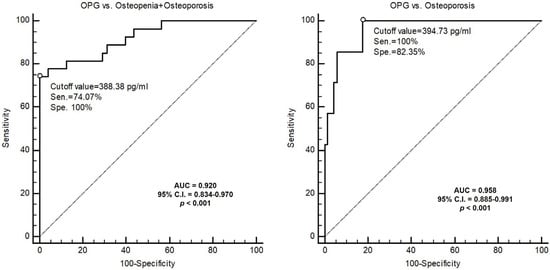
Figure 3.
ROC curve of OPG to predict osteopenia (T score < −1) and osteoporosis (T-score < −2.5) or osteoporosis.
4. Discussion
In this study, we found that female MHD patients had lower BMDs than males and advanced age was positively correlated and serum OPG and BMI were negatively associated with lumbar BMD in MHD patients. Moreover, OPG could be a potential biomarker for the diagnosis of osteoporosis with or without osteopenia of MHD patients.
Among CKD patients, evidence had shown an abnormal quantity and quality of bone metabolism with a high risk of fracture and poor long-term survival [,,,,]. Therefore, the 2017 Kidney Disease: Improving Global Outcomes (KDIGO) guideline recommended dual-exergy X-ray for assessing and managing the risk of fracture in CKD patients []. There are multiple known risk factors related to lower BMD in CKD, especially in dialysis patients. In these studies, female and old age were consistently found to be risk factors related to osteoporosis of advanced CKD and dialysis patients [,,]. Osteo-sarcopenia was a newly accepted concept of a link between osteoporosis and sarcopenia that together could lead to a higher risk of frailty [,]. In a longitudinal study, lower BMD was correlated with low BMI and poor renal outcomes in non-dialysis CKD patients []. Similarly, BMI and especially the skeletal muscle mass index were found to significantly affect the lumbar spine BMD of MHD patients []. Consistent with these studies, we found that MHD had a higher percentage of osteopenia (26.7%) and osteoporosis (9.3%) and that lumbar spine BMD was negatively associated with age and female sex but positively correlated with BMI.
OPG, produced from the CV system and bone, was initially known as osteoclastogenesis inhibitory factor and is a member of the tumor necrosis factor receptor family. OPG has a role in regulating the process of osteoclastogenesis and vascular calcification [,,]. Mice with OPG knockout exhibited increased numbers of osteoclast cells and marked femoral bone loss along with destruction of growth plate, lack of trabecular bone, and abnormal cortical bones as well as dramatically decreased BMD, bone mineral content, stiffness, and strength relative to heterozygous and wild-type controls []. Contrarily, transgenic mice overexpressing OPG exhibited markedly increased bone density of long bones, vertebrae, and pelvis associated with decreased osteoclast-mediated bone resorption []. Moreover, recombinant OPG inhibited osteoclastogenesis in vitro, increased bone density in vivo dose dependently, and protected rats against ovariectomy-associated bone loss []. In addition to exhibiting significantly decreased trabecular and cortical bone density within long bones and vertebrae, OPG-deficient mice showed increased medial arterial calcification of the aorta and renal arteries with endogenous OPG/OPG ligand/RANK expression within the smooth-muscle layer on the other hand [,]. A meta-analysis showed that CKD patients (including those on dialysis) had a 1.04 higher risk of CV mortality associated with an increase of 1 pmol/L in OPG concentration, which indicated a positive relationship between OPG levels and vascular calcification through mediating the process of osteoclastogenesis, osteoblast activation and osteoclast-like formation []. Increased serum OPG level has been associated with a 1.13 higher risk of self-report and/or prevalent vertebral fracture independent of CKD stages and sex, which supposed a compensatory role of OPG with increasing bone loss of CKD []. From these evidence, it seems that increased OPG production and release with increased serum levels of OPG could mediate the process of bone loss but have a role in pathological arterial calcification that results in poor long-term prognosis [,].
Osteoporosis is known as a metabolic bone disease characterized by unequilibrated bone formation and resorption. From a cross-sectional study of about 1000 healthy participants, OPG levels correlated negatively with tartrate-resistant acid phosphatase-5b (a bone resorption marker) but positively with osteocalcin (a bone formation marker), which indicated that OPG tended toward bone formation more than bone resorption []. However, there have been conflicting evidence demonstrating serum OPG levels with regards to BMD in CKD patients. Our findings supported these results such as studies have shown a negative correlation between the serum OPG level and BMD of the lumbar spine and total hip of female pre-dialysis CKD patients [] and of the femoral neck BMD of MHD patients [,]. Furthermore, by observing bone histomorphometry in MHD patients, Barreto et al. demonstrated that independent determinants of osteoporosis determined by trabecular bone volume (BV/TV) were the sRANKL/OPG ratio and length of amenorrhea, which indicated bone loss through the OPG/OPG ligand/RANK signal pathway []. However, in contrast, Nakashima et al. and Moldovan et al. reported a positive correlation between BMD and serum OPG levels in MHD patients [,]; moreover they showed that the annual percentage change in BMD correlated positively with OPG level []. Accordingly, we postulated that OPG might increase with an increase in bone turnover, perhaps as a compensation to prevent bone loss, but the definite mechanism needed further studies. Together with these studies, we found that OPG was independently and negatively associated with lumbar BMD in MHD patients after adjusting the covariates with optimal cutoff values of 388.38 pg/mL and 394.73 pg/mL associated with T scores < −1 (osteopenia and osteoporosis) or −2.5 (osteoporosis), respectively.
There were limitations of this study. First, it was a cross-sectional design. Secondly, the number of MHD patients was limited. Thirdly, we did not measure serum markers of bone formation or resorption. Therefore, to predict bone loss of MHD patients with serum OPG level needed further longitudinal studies to establish a cause and effect.
5. Conclusions
Our study showed that along with old age and female sex, there was a negative relationship between lumbar BMD and serum OPG of MHD patients. These findings indicated that OPG could be a biomarker and a modulator in the regulation of bone loss. These potential characteristics require confirmation in specifically designed studies in MHD patients.
Author Contributions
Conceptualization, C.-W.L. and B.-G.H.; methodology, B.-G.H. and J.-P.T.; formal analysis, B.-G.H. and J.-P.T.; data curation, C.-W.L., and C.-H.W.; writing—original draft preparation, C.-W.L.; writing—review and editing, B.-G.H. and J.-P.T.; supervision, B.-G.H. and J.-P.T.; funding acquisition, C.-H.W. and J.-P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a grant from Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, Grant Numbers TCMF-A 107-01-04.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (IRB106-62-B and approval on 5 June 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hwang, S.J.; Lin, M.Y.; Chen, H.C.; Hwang, S.C.; Yang, W.C.; Hsu, C.C.; Chiu, H.C.; Mau, L.W. Increased risk of mortality in the elderly population with late-stage chronic kidney disease: A cohort study in Taiwan. Nephrol. Dial. Transplant. 2008, 23, 3192–3198. [Google Scholar] [CrossRef]
- Jamal, S.A.; Hayden, J.A.; Beyene, J. Low bone mineral density and fractures in long-term hemodialysis patients: A meta-analysis. Am. J. Kidney Dis. 2007, 49, 674–681. [Google Scholar] [CrossRef]
- Kwon, Y.E.; Choi, H.Y.; Kim, S.; Ryu, D.R.; Oh, H.J.; ESRD Registry Committee of the Korean Society of Nephrology. Fracture risk in chronic kidney disease: A Korean population-based cohort study. Kidney Res. Clin. Pract. 2019, 38, 220–228. [Google Scholar] [CrossRef]
- Naylor, K.L.; McArthur, E.; Leslie, W.D.; Fraser, L.A.; Jamal, S.A.; Cadarette, S.M.; Pouget, J.G.; Lok, C.E.; Hodsman, A.B.; Adachi, J.D.; et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014, 86, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Syed, G.M.; Khan, A.I.; Sirwal, I.A.; Anwar, S.K.; Al-Oufi, A.R.; Balbaid, K.A. Mean bone mineral density and frequency of occurrence of osteopenia and osteoporosis in patients on hemodialysis: A single-center study. Saudi J. Kidney Dis. Transpl. 2014, 25, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Slouma, M.; Sahli, H.; Bahlous, A.; Laadhar, L.; Smaoui, W.; Rekik, S.; Gharsallah, I.; Sallami, M.; Moussa, F.B.; Elleuch, M.; et al. Mineral bone disorder and osteoporosis in hemodialysis patients. Adv. Rheumatol. 2020, 60, 15. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, L.R.; Chen, K.H. Osteoporosis in patients with chronic kidney diseases: A systemic review. Int. J. Mol. Sci. 2020, 21, 6846. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; West, S.L.; Miller, P.D. Fracture risk assessment in patients with chronic kidney disease. Osteoporos. Int. 2012, 23, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef]
- Mizuno, A.; Amizuka, N.; Irie, K.; Murakami, A.; Fujise, N.; Kanno, T.; Sato, Y.; Nakagawa, N.; Yasuda, H.; Mochizuki, S.; et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 1998, 247, 610–615. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Hayashi, S.Y.; Riella, M.C.; Lindholm, B. Elevated levels of plasma osteoprotegerin are associated with all-cause mortality risk and atherosclerosis in patients with stages 3 to 5 chronic kidney disease. Braz. J. Med. Biol. Res. 2014, 47, 995–1002. [Google Scholar] [CrossRef][Green Version]
- Demir, P.; Erdenen, F.; Aral, H.; Emre, T.; Kose, S.; Altunoglu, E.; Dolgun, A.; Inal, B.B.; Turkmen, A. Serum osteoprotegerin levels related with cardiovascular risk factors in chronic kidney disease. J. Clin. Lab. Anal. 2016, 30, 811–817. [Google Scholar] [CrossRef]
- Morena, M.; Terrier, N.; Jaussent, I.; Leray-Moragues, H.; Chalabi, L.; Rivory, J.P.; Maurice, F.; Delcourt, C.; Cristol, J.P.; Canaud, B.; et al. Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2006, 17, 262–270. [Google Scholar] [CrossRef]
- Nakashima, A.; Carrero, J.J.; Qureshi, A.R.; Hirai, T.; Takasugi, N.; Ueno, T.; Taniguchi, Y.; Lindholm, B.; Yorioka, N. Plasma osteoprotegerin, arterial stiffness, and mortality in normoalbuminemic Japanese hemodialysis patients. Osteoporos. Int. 2011, 22, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Avbersek-Luznik, I.; Malesic, I.; Rus, I.; Marc, J. Increased levels of osteoprotegerin in hemodialysis patients. Clin. Chem. Lab. Med. 2002, 40, 1019–1023. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Moyses, R.M.; Neves, C.L.; Jorgetti, V.; Draibe, S.A.; Canziani, M.E.; Carvalho, A.B. Osteoporosis in hemodialysis patients revisited by bone histomorphometry: A new insight into an old problem. Kidney Int. 2006, 69, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yorioka, N.; Doi, S.; Takasugi, N.; Shigemoto, K.; Kohno, N. Osteoprotegerin and bone mineral density in hemodialysis patients. Osteoporos. Int. 2006, 17, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, D.; Rusu, C.; Potra, A.; Moldovan, I.; Patiu, I.M.; Gherman-Caprioara, M.; Kacso, I.M. Osteoprotegerin and uremic osteoporosis in chronic hemodialysis patients. Int. Urol. Nephrol. 2017, 49, 895–901. [Google Scholar] [CrossRef]
- Kim, C.S.; Bae, E.H.; Ma, S.K.; Han, S.H.; Choi, K.H.; Lee, J.; Chae, D.W.; Oh, K.H.; Ahn, C.; Kim, S.W.; et al. Association of serum osteoprotegerin levels with bone loss in chronic kidney disease: Insights from the KNOW-CKD Study. PLoS ONE 2016, 11, e0166792. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lee, R.P.; Wang, C.H.; Fang, T.C.; Lin, N.T.; Chen, I.H.; Hsu, B.G. The association of serum osteoprotegerin and osteoporosis in postmenopausal hemodialysis patients: A pilot study. J. Womens Health 2010, 19, 785–790. [Google Scholar] [CrossRef]
- Doumouchtsis, K.K.; Kostakis, A.I.; Doumouchtsis, S.K.; Tziamalis, M.P.; Stathakis, C.P.; Diamanti-Kandarakis, E.; Dimitroulis, D.; Perrea, D.N. Associations between osteoprotegerin and femoral neck BMD in hemodialysis patients. J. Bone Miner. Res. 2008, 26, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Tsai, J.P.; Lai, Y.H.; Lin, Y.L.; Kuo, C.H.; Wang, C.H.; Hsu, B.G. Serum osteoprotegerin level is positively associated with peripheral artery disease in patients with peritoneal dialysis. Ren. Fail. 2020, 42, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Wang, C.H.; Lin, Y.L.; Kuo, C.H.; Lai, Y.H.; Hsu, B.G.; Tsai, J.P. Serum irisin level is positively associated with bone mineral density in patients on maintenance hemodialysis. Int. J. Endocrinol. 2021, 2021, 8890042. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- West, S.L.; Lok, C.E.; Langsetmo, L.; Cheung, A.M.; Szabo, E.; Pearce, D.; Fusaro, M.; Wald, R.; Weinstein, J.; Jamal, S.A. Bone mineral density predicts fractures in chronic kidney disease. J. Bone Miner. Res. 2015, 30, 913–919. [Google Scholar] [CrossRef]
- Hyun, Y.Y.; Lee, K.B.; Han, S.H.; Choi, K.H.; Park, H.C.; Oh, Y.K.; Park, S.K.; Oh, K.H.; Ahn, C.; KoreaN cohort study for Outcome in patients With CKD (KNOW-CKD) Study Group. Risk factors and renal outcomes of low bone mineral density in patients with non-dialysis chronic kidney disease. Osteoporos. Int. 2020, 31, 2373–2382. [Google Scholar] [CrossRef]
- Fidan, N.; Inci, A.; Coban, M.; Ulman, C.; Kursat, S. Bone mineral density and biochemical markers of bone metabolism in predialysis patients with chronic kidney disease. J. Investig. Med. 2016, 64, 861–866. [Google Scholar] [CrossRef]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.C. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef]
- Ito, K.; Ookawara, S.; Hibino, Y.; Imai, S.; Fueki, M.; Bandai, Y.; Yasuda, M.; Kamimura, T.; Kakuda, H.; Kiryu, S.; et al. Skeletal muscle mass index is positively associated with bone mineral density in hemodialysis patients. Front. Med. 2020, 7, 187. [Google Scholar] [CrossRef]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef]
- Min, H.; Morony, S.; Sarosi, I.; Dunstan, C.R.; Capparelli, C.; Scully, S.; Van, G.; Kaufman, S.; Kostenuik, P.J.; Lacey, D.L.; et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.X.; Li, J.B.; Huang, N.; Huang, X.W.; Li, Y.L.; Huang, F.X. Elevated osteoprotegerin concentration predicts increased risk of cardiovascular mortality in patients with chronic kidney disease: A systematic review and meta-analysis. Kidney Blood Press. Res. 2020, 45, 565–575. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Lok, C.E.; Jamal, S.A. Osteoprotegerin and fractures in men and women with chronic kidney disease. J. Bone Miner. Metab. 2014, 32, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Indridason, O.S.; Franzson, L.; Sigurdsson, G. Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporos. Int. 2005, 16, 417–423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).