Follow-Up of Patients Who Achieved Sustained Virologic Response after Interferon-Free Treatment against Hepatitis C Virus: Focus on Older Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Drug Regimens Used for SVR24 in the Present Study
2.3. Laboratory Tests
2.4. Diagnosis of Cirrhosis and HCC
2.5. Statistical Analysis
3. Results
3.1. Comparison of Pretreatment Factors for Patients Aged 75 Years or Older and Younger Than 75 Years
3.2. Comparison of Post-Treatment Factors for Patients Aged 75 Years or Older and Younger Than 75 Years
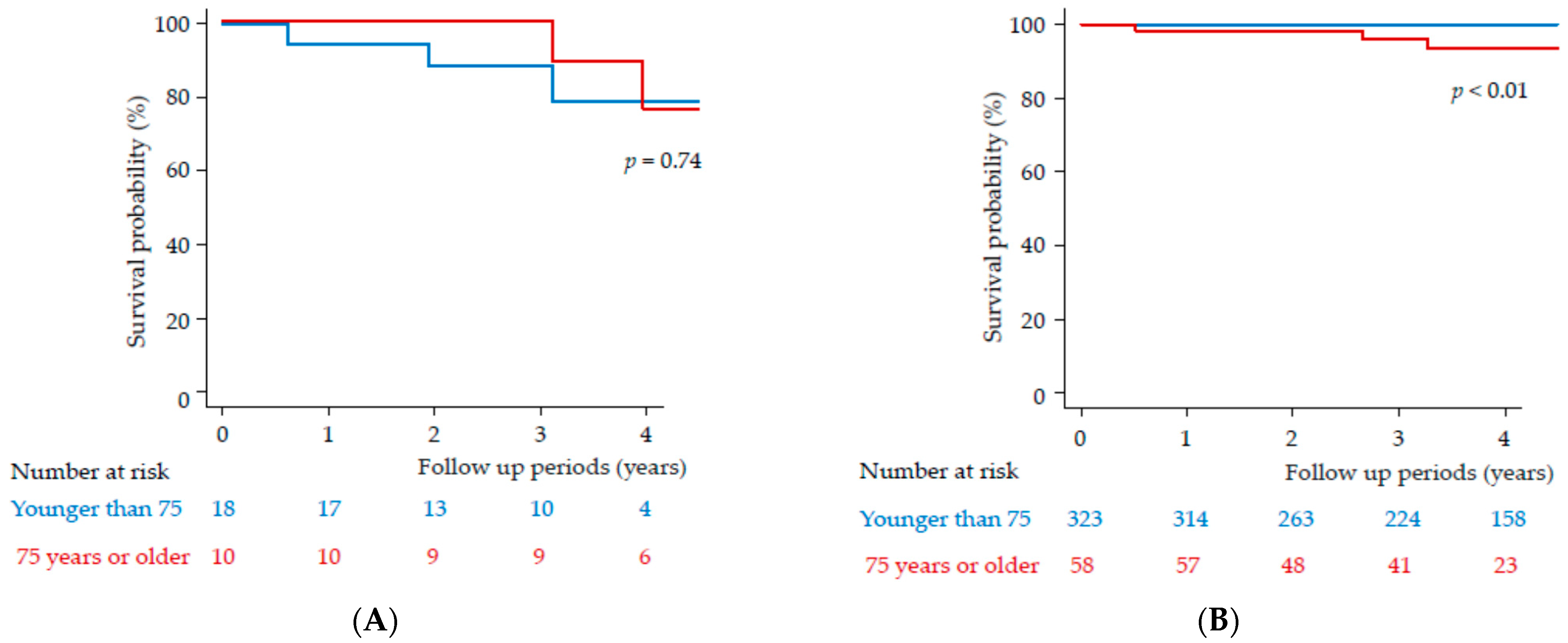
3.3. Occurrence of HCC in Patients with or without a History of HCC
3.4. Clinical Outcomes of Patients with or without a History of HCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afdhal, N.; Reddy, R.; Nelson, D.R.; Lawitz, E.; Gordon, S.C.; Schiff, E.; Nahass, R.; Ghalib, R.; Gitlin, N.; Herring, R.; et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1483–1493. [Google Scholar] [CrossRef] [Green Version]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, K.; Kurosaki, M.; Itakura, J.; Mori, N.; Takaki, S.; Hasebe, C.; Akahane, T.; Joko, K.; Yagisawa, H.; Takezawa, J.; et al. Real-world efficacy and safety of ledipasvir and sofosbuvir in patients with hepatitis C virus genotype 1 infection: A nationwide multicenter study by the Japanese Red Cross Liver Study Group. J. Gastroenterol. 2018, 53, 1142–1150. [Google Scholar] [CrossRef]
- Kobayashi, M.; Suzuki, F.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J. Med. Virol. 2017, 89, 476–483. [Google Scholar] [CrossRef]
- Tamai, H.; Shingaki, N.; Ida, Y.; Shimizu, R.; Maeshima, S.; Okamura, J.; Kawashima, A.; Nakao, T.; Hara, T.; Matsutani, H.; et al. Real-world safety and efficacy of sofosbuvir and ledipasvir for elderly patients. JGH Open 2018, 2, 300–306. [Google Scholar] [CrossRef]
- Mizokami, M.; Yokosuka, O.; Takehara, T.; Sakamoto, N.; Korenaga, M.; Mochizuki, H.; Nakane, K.; Enomoto, H.; Ikeda, F.; Yanase, M.; et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: An open-label, randomised, phase 3 trial. Lancet Infect. Dis. 2015, 15, 645–653. [Google Scholar] [CrossRef]
- Mizokami, M.; Dvory-Sobol, H.; Izumi, N.; Nishiguchi, S.; Doehle, B.; Svarovskaia, E.S.; De-Oertel, S.; Knox, S.; Brainard, D.M.; Miller, M.D.; et al. Resistance Analyses of Japanese Hepatitis C-Infected Patients Receiving Sofosbuvir or Ledipasvir/Sofosbuvir Containing Regimens in Phase 3 Studies. J. Viral Hepat. 2016, 23, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Nishiguchi, S.; Ueno, Y.; Mochizuki, H.; Izumi, N.; Ikeda, F.; Toyoda, H.; Yokosuka, O.; Nirei, K.; Genda, T.; et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: An open-label, phase 3 trial. J. Viral Hepat. 2014, 21, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Haga, Y.; Sasaki, R.; Wu, S.; Nakamoto, S.; Imazeki, F.; et al. Daclatasvir plus Asunaprevir Treatment for Real-World HCV Genotype 1-Infected Patients in Japan. Int. J. Med. Sci. 2016, 13, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chayama, K.; Notsumata, K.; Kurosaki, M.; Sato, K.; Rodrigues, L.; Setze, C.; Badri, P.; Pilot-Matias, T.; Vilchez, R.A.; Kumada, H. Randomized trial of interferon- and ribavirin-free ombitasvir/paritaprevir/ritonavir in treatment-experienced hepatitis C virus-infected patients. Hepatology 2015, 61, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL clinical practice recommendation: How to treat HCV-infected patients with renal impairment? Hepatol. Int. 2019, 13, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, T.; Kanda, T.; Nirei, K.; Matsumoto, N.; Yamazaki, M.; Shibata, T.; Tamura, A.; Ogawa, M.; Nakajima, N.; Matsuoka, S.; et al. Follow-up Results of HCV GT2 Patients after Sofosbuvir/Ribavirin Therapy: Careful Attention to Occurrence of HCC. Anticancer Res. 2019, 39, 3855–3862. [Google Scholar] [CrossRef] [Green Version]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Yoshimasu, Y.; Furuichi, Y.; Kasai, Y.; Takeuchi, H.; Sugimoto, K.; Nakamura, I.; Itoi, T. Predictive factors for hepatocellular carcinoma occurrence or recurrence after direct-acting antiviral agents in patients with chronic hepatitis C. J. Gastrointest. Liver Dis. JGLD 2019, 28, 63–71. [Google Scholar] [CrossRef]
- Ouchi, Y.; Rakugi, H.; Arai, H.; Akishita, M.; Ito, H.; Toba, K.; Kai, I. Joint Committee of Japan Gerontological Society (JGLS); Japan Geriatrics Society (JGS) on the Definition and Classification of the Elderly. Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr. Gerontol. Int. 2017, 17, 1045–1047. [Google Scholar]
- Ohno, O.; Mizokami, M.; Wu, R.R.; Saleh, M.G.; Ohba, K.; Orito, E.; Mukaide, M.; Williams, R.; Lau, J.Y. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J. Clin. Microbiol. 1997, 35, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirei, K.; Nakamura, H.; Matsuoka, S.; Yamana, Y.; Yoda, S.; Hirayama, A.; Moriyama, M. Ventricular Tachycardia as a Complication of Ledipasvir and Sofosbuvir Treatment for HCV Infection. Intern. Med. 2017, 56, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Li, D.K.; Ren, Y.; Fierer, D.S.; Rutledge, S.; Shaikh, O.S.; Lo Re, V., 3rd; Simon, T.; Abou-Samra, A.B.; Chung, R.T.; Butt, A.A. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 2018, 67, 2244–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Asahina, Y.; Tsuchiya, K.; Tamaki, N.; Hirayama, I.; Tanaka, T.; Sato, M.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Kuzuya, T.; et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010, 52, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reig, M.; Marino, Z.; Perello, C.; Inarrairaegui, M.; Ribeiro, A.; Lens, S.; Diaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Cheung, M.C.M.; Walker, A.J.; Hudson, B.E.; Verma, S.; McLauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.H.; MacDonald, D.C.; Agarwal, K.; et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 65, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Virlogeux, V.; Pradat, P.; Hartig-Lavie, K.; Bailly, F.; Maynard, M.; Ouziel, G.; Poinsot, D.; Lebosse, F.; Ecochard, M.; Radenne, S.; et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int. 2017, 37, 1122–1127. [Google Scholar] [CrossRef]
- Ikeda, K.; Kawamura, Y.; Kobayashi, M.; Kominami, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Akuta, N.; Saitoh, S.; Suzuki, F.; et al. Direct-Acting Antivirals Decreased Tumor Recurrence After Initial Treatment of Hepatitis C Virus-Related Hepatocellular Carcinoma. Dig. Dis. Sci. 2017, 62, 2932–2942. [Google Scholar] [CrossRef]
- Lleo, A.; Aglitti, A.; Aghemo, A.; Maisonneuve, P.; Bruno, S.; Persico, M. Predictors of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Dig. Liver Dis. 2019, 51, 310–317. [Google Scholar] [CrossRef]
- Nagata, H.; Nakagawa, M.; Asahina, Y.; Sato, A.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Otani, S.; Kawai-Kitahata, F.; et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017, 67, 933–939. [Google Scholar] [CrossRef]
- Backus, L.I.; Belperio, P.S.; Shahoumian, T.A.; Mole, L.A. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology 2019, 69, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Shiratori, Y.; Shiina, S.; Teratani, T.; Imamura, M.; Obi, S.; Sato, S.; Koike, Y.; Yoshida, H.; Omata, M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann. Intern. Med. 2003, 138, 299–306. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Center Japan. Available online: https://ganjoho.jp/reg_stat/statistics/stat/cancer/8_liver.html#anchor4 (accessed on 24 July 2021).
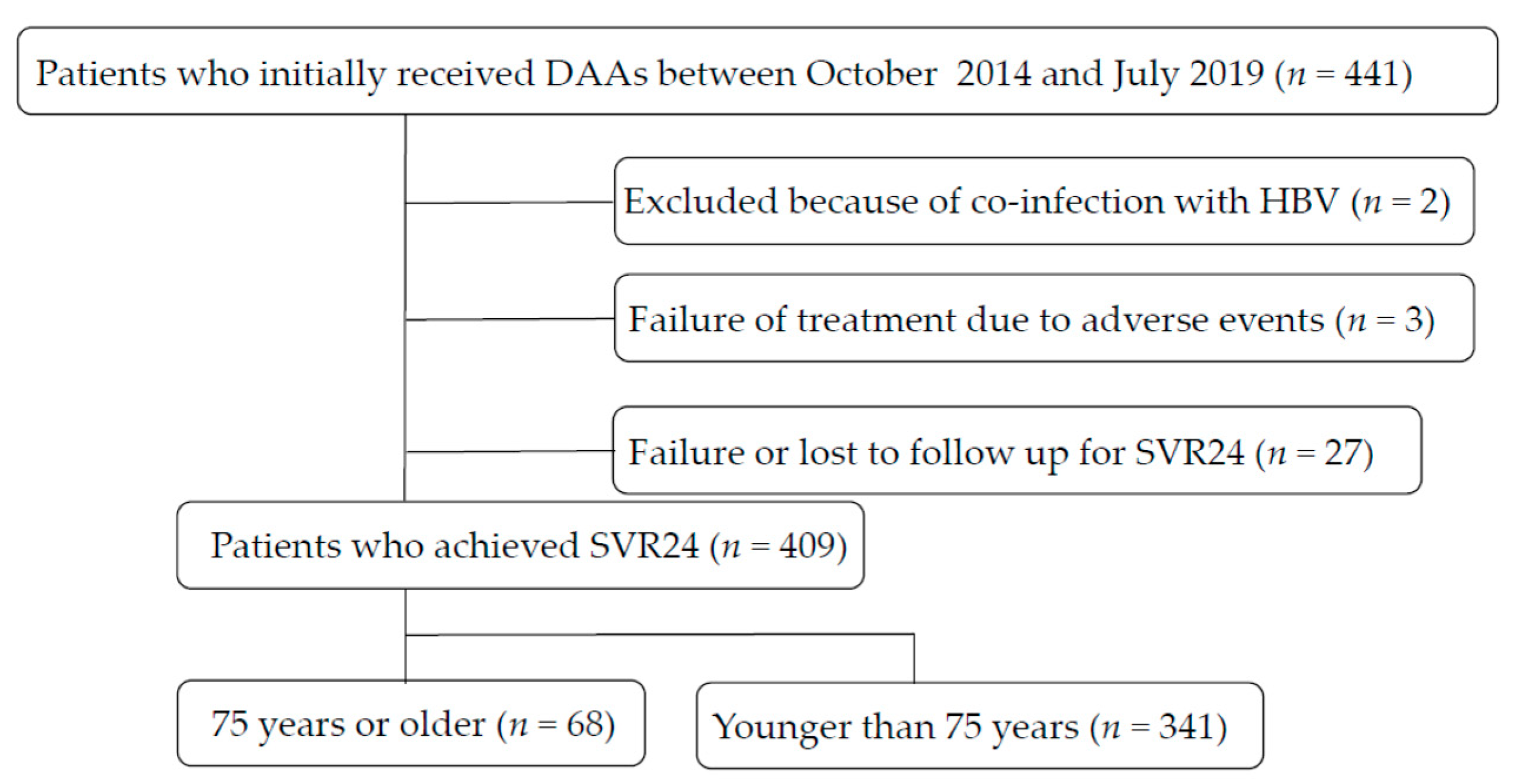

| Combination Regimens | Total Patients (n) | 75 Years or Older (n) | Younger Than 75 Years (n) |
|---|---|---|---|
| Asunaprevir plus daclatasvir | 62 | 15 | 47 |
| Sofosbuvir plus ribavirin | 95 | 8 | 87 |
| Sofosbuvir plus ledipasvir | 146 | 22 | 124 |
| Ombitasvir plus paritaprevir/ritonavir | 19 | 5 | 14 |
| Glecaprevir plus pibrentasvir | 82 | 16 | 66 |
| Elbasvir plus grazoprevir | 5 | 2 | 3 |
| Total SVR patients | 409 | 68 | 341 |
| Items | Total | 75 Years or Older | Younger Than 75 Years | p-Value |
|---|---|---|---|---|
| Number | 409 | 68 | 341 | |
| Age (years) | 62.53 ± 11.56 | 78.69 ± 2.94 | 59.31 ± 9.80 | <0.01 |
| Male/Female | 185/224 | 19/49 | 166/175 | <0.01 |
| AST (IU/L) | 51.95 ± 38.60 | 49.02 ± 28.09 | 52.20 ± 40.37 | 0.34 |
| ALT (IU/L) | 53.02 ± 47.11 | 39.00 ± 21.70 | 54.82 ± 50.32 | 0.06 |
| Hemoglobin (g/dL) | 13.88 ± 6.74 | 12.63 ± 1.19 | 14.14 ± 7.04 | <0.01 |
| Platelet count (×10⁴/mm3) | 17.75 ± 7.15 | 16.02 ± 4.69 | 18.09 ± 8.05 | 0.02 |
| eGFR (mL/min/1.73 m2) | 74.79 ± 18.97 | 63.94 ± 15.47 | 77.31 ± 18.45 | <0.01 |
| Prothrombin time (%) | 93.81 ± 13.87 | 95.51 ± 6.85 | 93.60 ± 14.38 | 0.09 |
| HCV RNA (log IU/mL) | 5.92 ± 0.91 | 5.94 ± 0.81 | 5.92 ± 0.93 | 0.80 |
| HCV genotypes (1/2/others) | 276/130/3 | 58/10/0 | 218/120/3 | <0.01 |
| Diabetes mellitus (%) | 66 | 10 (13.69%) | 56 (13.74%) | 0.85 |
| Liver cirrhosis (%) | 138 (33.74%) | 40 (58.82%) | 98 (28.73%) | <0.01 |
| History of interferon (%) | 80 (19.56%) | 16 (23.52%) | 64 (19.06%) | 0.62 |
| History of HCC (%) | 28 (0.01%) | 10 (18.8%) | 18 (5.5%) | 0.01 |
| 75 Years or Older (n = 68) | Pre-Treatment | Post-SVR24 | p-Value |
|---|---|---|---|
| ALT (IU/L) | 39.00 ± 21.70 | 16.07 ± 6.87 | <0.01 |
| AST (IU/L) | 49.02 ± 28.09 | 26.02 ± 7.38 | <0.01 |
| Platelet count (×104/mm3) | 16.02 ± 4.69 | 16.76 ± 5.30 | 0.06 |
| Total bilirubin (mg/dL) | 0.59 ± 0.22 | 0.60 ± 0.24 | 0.69 |
| Younger Than 75 Years (n = 341) | Pre-Treatment | Post-SVR24 | p-Value |
| ALT (IU/L) | 54.82 ± 50.32 | 28.38 ± 170.95 | <0.01 |
| AST (IU/L) | 52.20 ± 40.37 | 25.54 ± 17.76 | <0.01 |
| Platelet count (×104/mm3) | 18.09 ± 8.05 | 18.96 ± 7.43 | <0.01 |
| Total bilirubin (mg/dL) | 0.69 ± 0.51 | 0.84 ± 2.19 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirei, K.; Kanda, T.; Masuzaki, R.; Mizutani, T.; Moriyama, M. Follow-Up of Patients Who Achieved Sustained Virologic Response after Interferon-Free Treatment against Hepatitis C Virus: Focus on Older Patients. Medicina 2021, 57, 761. https://doi.org/10.3390/medicina57080761
Nirei K, Kanda T, Masuzaki R, Mizutani T, Moriyama M. Follow-Up of Patients Who Achieved Sustained Virologic Response after Interferon-Free Treatment against Hepatitis C Virus: Focus on Older Patients. Medicina. 2021; 57(8):761. https://doi.org/10.3390/medicina57080761
Chicago/Turabian StyleNirei, Kazushige, Tatsuo Kanda, Ryota Masuzaki, Taku Mizutani, and Mitsuhiko Moriyama. 2021. "Follow-Up of Patients Who Achieved Sustained Virologic Response after Interferon-Free Treatment against Hepatitis C Virus: Focus on Older Patients" Medicina 57, no. 8: 761. https://doi.org/10.3390/medicina57080761
APA StyleNirei, K., Kanda, T., Masuzaki, R., Mizutani, T., & Moriyama, M. (2021). Follow-Up of Patients Who Achieved Sustained Virologic Response after Interferon-Free Treatment against Hepatitis C Virus: Focus on Older Patients. Medicina, 57(8), 761. https://doi.org/10.3390/medicina57080761








