Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with rTMS on Upper Limb Motor Recovery after Stroke. A Study Protocol for a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
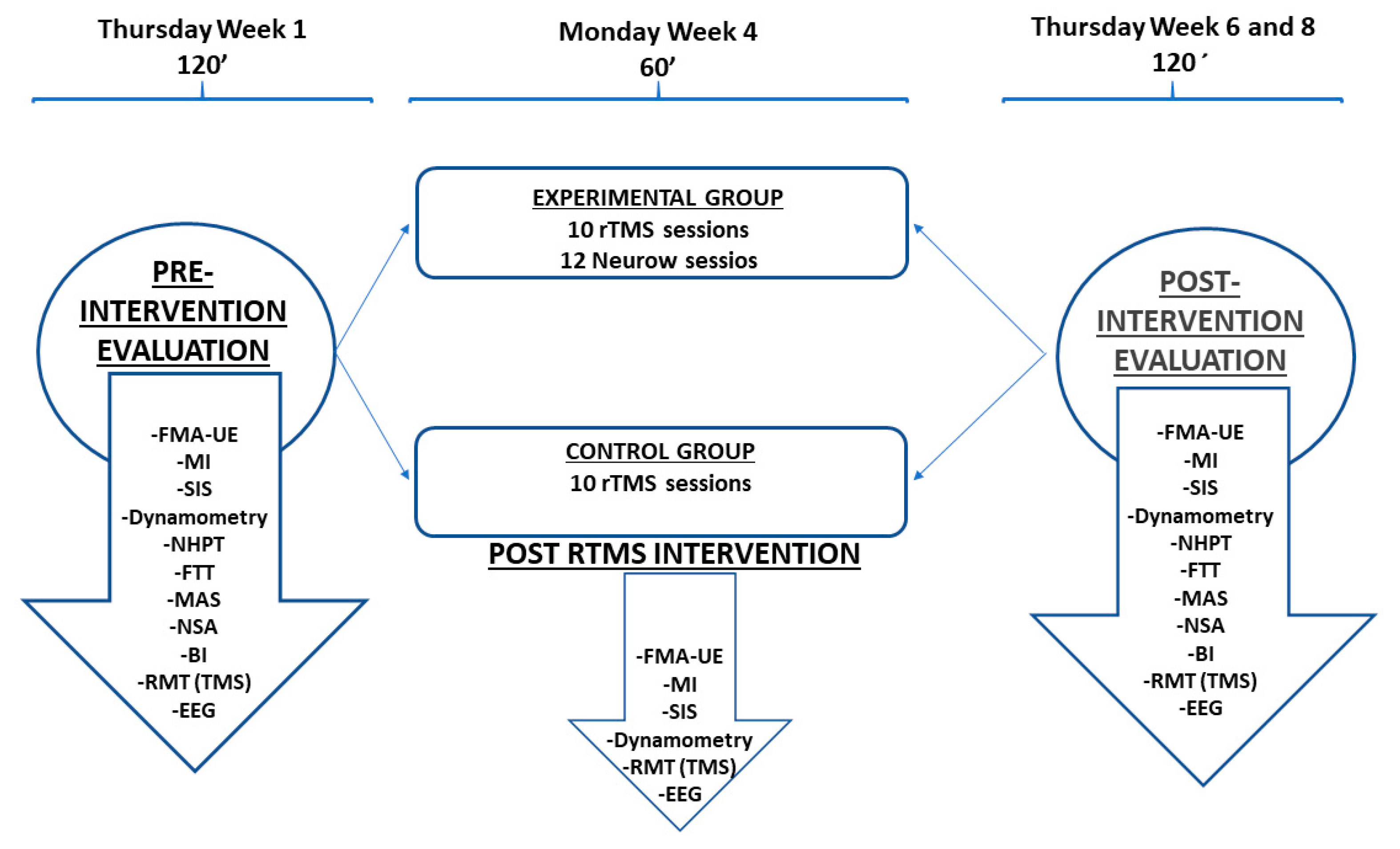
2.1. Study Design and Participants
2.2. Intervention Protocol
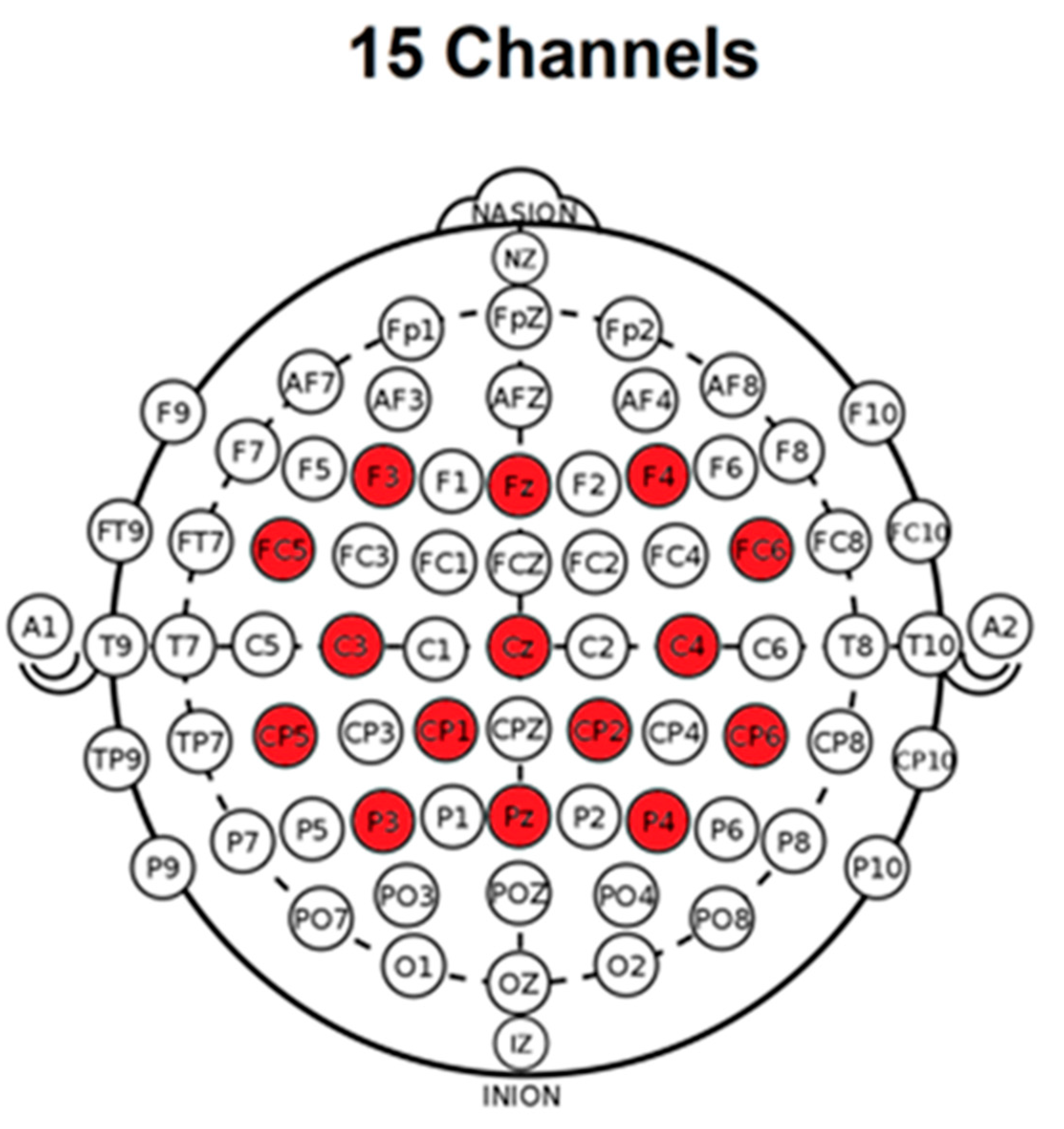
2.2.1. rTMS Stimulation
2.2.2. Immersive Multimodal BCI-VR Training
- Ask the patient to perform the rowing movement with both upper limbs with external facilitation of the paretic side.
- Ask the patient to imagine the movement with eyes closed, focusing on his internal perspective and on the sensation of rotation. Imagine the hand closed in a fist and feel the arm weight and contraction of arm muscles.
- Imagine the movement slowly and increase their speed.
- The best strategies will be identified for each participant. The patient reports in detail what he felt/tried to visualize; the researcher will give feedback and will also give a description of the sensation of the movement to the participant during the motor imagination, describing the sequence of movements required for rowing (elbow stretched, closed hand grasping the paddle, etc.).
- The patient will be asked if he succeeds in imagining the tasks.
2.3. Outcomes Measurement
2.3.1. Main Outcomes
2.3.2. Secondary Outcomes
2.4. Data Analysis
2.5. Dissemination Plans
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Panel, O. Ottawa Panel Evidence-Based Clinical Practice Guidelines for Post-Stroke Rehabilitation. Top. Stroke Rehabil. 2006, 13, 1–269. [Google Scholar] [CrossRef]
- Alia, C.; Spalletti, C.; Lai, S.; Panarese, A.; Lamola, G.; Bertolucci, F.; Vallone, F.; Di Garbo, A.; Chisari, C.; Micera, S.; et al. Neuroplastic Changes Following Brain Ischemia and Their Contribution to Stroke Recovery: Novel Approaches in Neurorehabilitation. Front. Cell Neurosci. 2017, 11, 76. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5352696/ (accessed on 26 December 2020). [CrossRef]
- Dobkin, B.H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef]
- Gorelick, P.B. The global burden of stroke: Persistent and disabling. Lancet Neurol. 2019, 18, 417–418. [Google Scholar] [CrossRef]
- Duarte, E.; Alonso, B.; Fernández, M.J. Rehabilitación del ictus: Modelo asistencial. Recomendaciones de la Sociedad Española de Rehabilitación y Medicina Física, 2009. Rev. Soc. Española Rehabil. Med. Física 2010, 44, 60–68. [Google Scholar] [CrossRef]
- Dionísio, A.; Duarte, I.C.; Patrício, M.; Castelo-Branco, M. The Use of Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation: A Systematic Review. J. Stroke Cereb. Dis. 2018, 27, 1–31. [Google Scholar] [CrossRef]
- Takeuchi, N.; Tada, T.; Toshima, M.; Matsuo, Y.; Ikoma, K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J. Rehabil. Med. 2009, 41, 1049–1054. [Google Scholar] [CrossRef]
- Klein, M.M.; Treister, R.; Raij, T.; Pascual-Leone, A.; Park, L.; Nurmikko, T.; Lenz, F.; Lefaucheur, J.P.; Lang, M.; Hallett, M.; et al. Transcranial magnetic stimulation of the brain: Guidelines for pain treatment research. Pain 2015, 156, 1601–1614. [Google Scholar] [CrossRef]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 35–48. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5846193/ (accessed on 11 June 2021). [CrossRef]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive Transcranial Magnetic Stimulation in Stroke Rehabilitation: Review of the Current Evidence and Pitfalls. Ther. Adv. Neurol. Disord. 2019, 12. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6763938/ (accessed on 11 June 2021). [CrossRef]
- Stockley, R.C.; O’Connor, D.A.; Smith, P.; Moss, S.; Allsop, L.; Edge, W. A Mixed Methods Small Pilot Study to Describe the Effects of Upper Limb Training Using a Virtual Reality Gaming System in People with Chronic Stroke. Rehabil. Res. Pract. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Kodama, T.; Murata, S.; Nakamoto, T.; Fujihara, T.; Ito, Y. Effect of Auditory Neurofeedback Training on Upper Extremity Function and Motor Imagery Ability in a Stroke Patient: A Single Case Study. Int. J. Clin. Res. Trials 2018, 3. Available online: https://www.graphyonline.com/archives/IJCRT/2018/IJCRT-126/index.php?page=abstract (accessed on 8 August 2018). [CrossRef][Green Version]
- Polli, A.; Moseley, G.L.; Gioia, E.; Beames, T.; Baba, A.; Agostini, M.; Tonin, P.; Turolla, A. Graded motor imagery for patients with stroke: A non-randomized controlled trial of a new approach. Eur. J. Phys. Rehabil. Med. 2017, 53, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, T.; Li, B.-J.; Zhang, W.; Zhao, J.; Song, L.-P. Motor imagery training induces changes in brain neural networks in stroke patients. Neural Regen. Res. 2018, 13, 1771–1781. [Google Scholar] [PubMed]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Paolucci, S.; Inghilleri, M.; Astolfi, L.; Cincotti, F.; et al. Brain-computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, W.; Abo, M.; Sasanuma, J.; Shimizu, M.; Okamoto, T.; Kimura, C.; Kakita, K.; Hara, H. Combination Protocol of Low-Frequency rTMS and Intensive Occupational Therapy for Post-stroke Upper Limb Hemiparesis: A 6-year Experience of More Than 1700 Japanese Patients. Transl. Stroke Res. 2016, 7, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation in neurorehabilitation. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780444534972000401 (accessed on 26 March 2020).
- Sato, S.; Bergmann, T.O.; Borich, M.R. Opportunities for concurrent transcranial magnetic stimulation and electroencephalography to characterize cortical activity in stroke. Front. Hum. Neurosci. 2015, 9, 250. [Google Scholar] [CrossRef][Green Version]
- Liew, S.L.; Rana, M.; Cornelsen, S.; Fortunato de Barros Filho, M.; Birbaumer, N.; Sitaram, R.; Cohen, L.G.; Soekadar, S.R. Improving motor cortico-thalamic communication after stroke using real-time fMRI connectivity-based neurofeedback. Neurorehabil. Neural Repair 2016, 30, 671–675. [Google Scholar] [CrossRef]
- Linden, D.E.J.; Turner, D.L. Real-time functional magnetic resonance imaging neurofeedback in motor neurorehabilitation. Curr. Opin. Neurol. 2016, 29, 412–418. [Google Scholar] [CrossRef]
- Jang, S.H.; Kwon, Y.H.; Lee, M.Y.; Lee, D.Y.; Hong, J.H. Difference of neural connectivity for motor function in chronic hemiparetic stroke patients with intracerebral hemorrhage. Neurosci. Lett. 2012, 531, 80–85. [Google Scholar] [CrossRef]
- Yin, D.; Song, F.; Xu, D.; Peterson, B.S.; Sun, L.; Men, W.; Yan, X.; Fan, M. Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS ONE. 2012, 7, e52727. [Google Scholar] [CrossRef]
- Abo, M.; Kakuda, W. Rehabilitation with rTMS; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Available online: https://www.springer.com/gp/book/9783319209814 (accessed on 11 June 2021).
- Hirakawa, Y.; Takeda, K.; Tanabe, S.; Koyama, S.; Motoya, I.; Sakurai, H.; Kanada, Y.; Kawamura, N.; Kawamura, M.; Nagata, J.; et al. Effect of intensive motor training with repetitive transcranial magnetic stimulation on upper limb motor function in chronic post-stroke patients with severe upper limb motor impairment. Top. Stroke Rehabil. 2018, 25, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Vongvaivanichakul, P.; Tretriluxana, J.; Bovonsunthonchai, S.; Pakaprot, N.; Laksanakorn, W. Reach-to-grasp training in individuals with chronic stroke augmented by low-frequency repetitive transcranial magnetic stimulation. J. Med. Assoc. Thai. 2014, 97 (Suppl. 7), S45–S49. [Google Scholar] [PubMed]
- Etoh, S.; Noma, T.; Ikeda, K.; Jonoshita, Y.; Ogata, A.; Matsumoto, S.; Shimodozono, M.; Kawahira, K. Effects of repetitive trascranial magnetic stimulation on repetitive facilitation exercises of the hemiplegic hand in chronic stroke patients. J. Rehabil. Med. 2013, 45, 843–847. [Google Scholar] [CrossRef]
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain-computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Ferreira, A.; Bermúdez i Badia, S. NeuRow: An Immersive VR Environment for Motor-Imagery Training with the Use of Brain-Computer Interfaces and Vibrotactile Feedback. In Proceedings of the 3rd International Conference on Physiological Computing Systems, Lisbon, Portugal, 27–28 July 2016. [Google Scholar]
- Vourvopoulos, A.; Jorge, C.; Abreu, R.; Figueiredo, P.; Fernandes, J.-C.; Bermúdez IBadia, S. Efficacy and Brain Imaging Correlates of an Immersive Motor Imagery BCI-Driven VR System for Upper Limb Motor Rehabilitation: A Clinical Case Report. Front. Hum. Neurosci. 2019, 13, 244. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, Ö.; Msc, F.L.B.; Liberati, G.; Curado, M.R.; Garcia-Cossio, E.; Vyziotis, A.; et al. Brain-Machine Interface in Chronic Stroke Rehabilitation: A Controlled Study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Bermúdez I Badia, S. Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. J. Neuroeng. Rehabil. 2016, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Bermúdez i Badia, S. Usability and Cost-effectiveness in Brain-Computer Interaction: Is it User Throughput or Technology Related? In Proceedings of the 7th Augmented Human International Conference 2016, Geneva, Switzerland, 25–27 February 2016. [Google Scholar]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hrobjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. Declaración SPIRIT 2013, definición de los elementos estándares del protocolo de un ensayo clínico. Rev. Panam. Salud Publ. 2015, 38, 506–514. [Google Scholar]
- Spontaneous and Therapeutic-Induced Mechanisms of Functional Recovery After Stroke|SpringerLink [Internet]. Available online: https://link.springer.com/article/10.1007%2Fs12975-016-0467-5 (accessed on 30 May 2021).
- Sivan, M.; O’Connor, R.J.; Makower, S.; Levesley, M.; Bhakta, B. Systematic review of outcome measures used in the evaluation of robot-assisted upper limb exercise in stroke. J. Rehabil. Med. 2011, 43, 181–189. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Valiengo, L.; Baccaro, A.; Zanao, T.A.; de Oliveira, J.F.; Vieira, G.P.; Bueno, V.F.; Goulart, A.C.; Boggio, P.; Lotufo, P.; et al. Sertraline vs. ELectrical Current Therapy for Treating Depression Clinical Trial--SELECT TDCS: Design, rationale and objectives. Contemp. Clin. Trials 2011, 32, 90–98. [Google Scholar] [CrossRef]
- Hiragami, S.; Inoue, Y.; Harada, K. Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J. Phys. Ther. Sci. 2019, 31, 917–921. [Google Scholar] [CrossRef]
- Aleatorizador de Investigación [Internet]. Available online: https://www.randomizer.org/ (accessed on 29 March 2020).
- Wassermann, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 1–16. [Google Scholar] [CrossRef]
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Lécuyer, A. OpenViBE: An Open-Source Software Platform to Design, Test and Use Brain-Computer Interfaces in Real and Virtual Environments. Presence Teleoperators Virtual Environ. Presence Teleoperators Virtual Environ. 2010, 19, 35–53. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C.; Muller, G.; Obermaier, B.; Krausz, G.; Schlogl, A.; Scherer, R.; Graimann, B.; Keinrath, C.; Skliris, D.; et al. Graz-BCI: State of the art and clinical applications. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 177–180. [Google Scholar] [CrossRef]
- Bohannon, R. Motricity Index Scores are Valid Indicators of Paretic Upper Extremity Strength Following Stroke. J. Phys. Ther. Sci. 1999, 11, 59–61. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Taveggia, G.; Galeri, S.; Bissolotti, L.; Mullè, C.; Imperio, G.; Valdes, K.; Borboni, A.; Negrini, S. Efficacy of Short-Term Robot-Assisted Rehabilitation in Patients With Hand Paralysis After Stroke: A Randomized Clinical Trial. HAND 2018, 13, 95–102. [Google Scholar] [CrossRef]
- Hsieh, Y.; Wu, C.; Lin, K.; Chang, Y.; Chen, C.; Liu, J. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke 2009, 40, 1386–1391. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]
- Oxford Grice, K.; Vogel, K.A.; Le, V.; Mitchell, A.; Muniz, S.; Vollmer, M.A. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am. J. Occup. Ther. 2003, 57, 570–573. [Google Scholar] [CrossRef]
- Johansson, G.M.; Häger, C.K. A modified standardized nine hole peg test for valid and reliable kinematic assessment of dexterity post-stroke. J. Neuroeng. Rehabil. 2019, 16, 8. [Google Scholar] [CrossRef]
- Chen, H.-M.; Chen, C.C.; Hsueh, I.-P.; Huang, S.-L.; Hsieh, C.-L. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil. Neural Repair 2009, 23, 435–440. [Google Scholar] [CrossRef]
- Lin, K.; Chuang, L.; Wu, C.; Hsieh, Y.; Chang, W. Responsiveness and validity of three dexterous function measures in stroke rehabilitation. J. Rehabil. Res. Dev. 2010, 47, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Van Wijck, F.M.; Pandyan, A.D.; Johnson, G.R.; Barnes, M.P. Assessing motor deficits in neurological rehabilitation: Patterns of instrument usage. Neurorehabil. Neural Repair 2001, 15, 23–30. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Li, X. Test-retest reliability and inter-rater reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in hemiplegic patients with stroke. Eur. J. Phys. Rehabil. Med. 2014, 50, 9–15. [Google Scholar]
- Chen, C.-L.; Chen, C.-Y.; Chen, H.-C.; Wu, C.-Y.; Lin, K.-C.; Hsieh, Y.-W.; Shen, I.-H. Responsiveness and minimal clinically important difference of Modified Ashworth Scale in patients with stroke. Eur. J. Phys. Rehabil. Med. 2019, 55, 754–760. [Google Scholar] [CrossRef]
- Villepinte, C.; Catella, E.; Martin, M.; Hidalgo, S.; Téchené, S.; Lebely, C.; Castel-Lacanal, E.; de Boissezon, X.; Chih, H.; Gasq, D. Validation of French upper limb Erasmus modified Nottingham Sensory Assessment in stroke. Ann. Phys. Rehabil. Med. 2019, 62, 35–42. [Google Scholar] [CrossRef]
- Rayegani, S.M.; Raeissadat, S.A.; Sedighipour, L.; Rezazadeh, I.M.; Bahrami, M.H.; Eliaspour, D.; Khosrawi, S. Effect of neurofeedback and electromyographic-biofeedback therapy on improving hand function in stroke patients. Top. Stroke Rehabil. 2014, 21, 137–151. [Google Scholar] [CrossRef]
- Liepert, J.; Greiner, J.; Nedelko, V.; Dettmers, C. Reduced upper limb sensation impairs mental chronometry for motor imagery after stroke: Clinical and electrophysiological findings. Neurorehabil. Neural Repair 2012, 26, 470–478. [Google Scholar] [CrossRef]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Monge-Pereira, E.; Molina-Rueda, F.; Rivas-Montero, F.; Ibáñez, J.; Serrano, J.; Alguacil-Diego, I.; Miangolarra-Page, J. Electroencephalography as a post-stroke assessment method: An updated review. Neurología 2017, 32, 40–49. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Cheng, C.-H.; Liao, K.-K.; Lee, I.-H.; Lin, Y.-Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: A meta-analysis. Stroke 2012, 43, 1849–1857. [Google Scholar] [CrossRef]
- Wang, C.; Tsai, P.; Yang, T.F.; Yang, K.; Wang, C. Differential Effect of Conditioning Sequences in Coupling Inhibitory/Facilitatory Repetitive Transcranial Magnetic Stimulation for PostStroke Motor Recovery. CNS Neurosci. Ther. 2014, 20, 355–363. [Google Scholar] [CrossRef]
- Fuentes, M.A.; Borrego, A.; Latorre, J.; Colomer, C.; Alcañiz, M.; Sánchez-Ledesma, M.J.; Noé, E.; Llorens, R. Combined Transcranial Direct Current Stimulation and Virtual Reality-Based Paradigm for Upper Limb Rehabilitation in Individuals with Restricted Movements. A Feasibility Study with a Chronic Stroke Survivor with Severe Hemiparesis. J. Med. Syst. 2018, 42, 87. [Google Scholar] [CrossRef]
- Graef, P.; Dadalt, M.L.R.; Rodrigués, D.A.M.D.S.; Stein, C.; Pagnussat, A.S. Transcranial magnetic stimulation combined with upper-limb training for improving function after stroke: A systematic review and meta-analysis. J. Neurol. Sci. 2016, 369, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yim, J. Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation Combined with Task-Oriented Mirror Therapy Training on Hand Rehabilitation of Acute Stroke Patients. Med. Sci. Monit. 2018, 24, 743–750. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| Older than 18 years old. | History of seizure or brain aneurysm |
| Ischemic or hemorrhagic cerebrovascular injury diagnosed by a neurologist and who have at least one brain-imaging test | Pacemakers, medication pumps, metal implants in the head (except dental implants) |
| Onset of hemispheric ischemic or hemorrhagic stroke >3 months | Clinical instability |
| Kinesthetic and Visual Imagery Questionnaire (KVIQ) >55. | Muscle tone in the wrist and hand with a modified Ashworth scale (MAS) score equal to or higher than 3 in the wrist |
| Stability in antispastic medication for more than 5 days | Other pre-existing neurological diseases or previous cerebrovascular accidents with sequelae |
| Able to read and write | Aphasia |
| Sufficient cognitive ability to understand and perform tasks: Token Test >11 | Previous TMS after stroke Hemispatial neglect (Bells Test >6 omissions on one side) Visual problems |
| Flaccid paralysis Brunnstrom’s stage = 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cuesta, F.J.; Arroyo-Ferrer, A.; González-Zamorano, Y.; Vourvopoulos, A.; Badia, S.B.i.; Figuereido, P.; Serrano, J.I.; Romero, J.P. Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with rTMS on Upper Limb Motor Recovery after Stroke. A Study Protocol for a Randomized Controlled Trial. Medicina 2021, 57, 736. https://doi.org/10.3390/medicina57080736
Sánchez-Cuesta FJ, Arroyo-Ferrer A, González-Zamorano Y, Vourvopoulos A, Badia SBi, Figuereido P, Serrano JI, Romero JP. Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with rTMS on Upper Limb Motor Recovery after Stroke. A Study Protocol for a Randomized Controlled Trial. Medicina. 2021; 57(8):736. https://doi.org/10.3390/medicina57080736
Chicago/Turabian StyleSánchez-Cuesta, Francisco José, Aida Arroyo-Ferrer, Yeray González-Zamorano, Athanasios Vourvopoulos, Sergi Bermúdez i Badia, Patricia Figuereido, José Ignacio Serrano, and Juan Pablo Romero. 2021. "Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with rTMS on Upper Limb Motor Recovery after Stroke. A Study Protocol for a Randomized Controlled Trial" Medicina 57, no. 8: 736. https://doi.org/10.3390/medicina57080736
APA StyleSánchez-Cuesta, F. J., Arroyo-Ferrer, A., González-Zamorano, Y., Vourvopoulos, A., Badia, S. B. i., Figuereido, P., Serrano, J. I., & Romero, J. P. (2021). Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with rTMS on Upper Limb Motor Recovery after Stroke. A Study Protocol for a Randomized Controlled Trial. Medicina, 57(8), 736. https://doi.org/10.3390/medicina57080736











