Trends of Stomach Cancer in Central Serbia
Abstract
:1. Introduction
2. Material and Method
Statistical Analysis
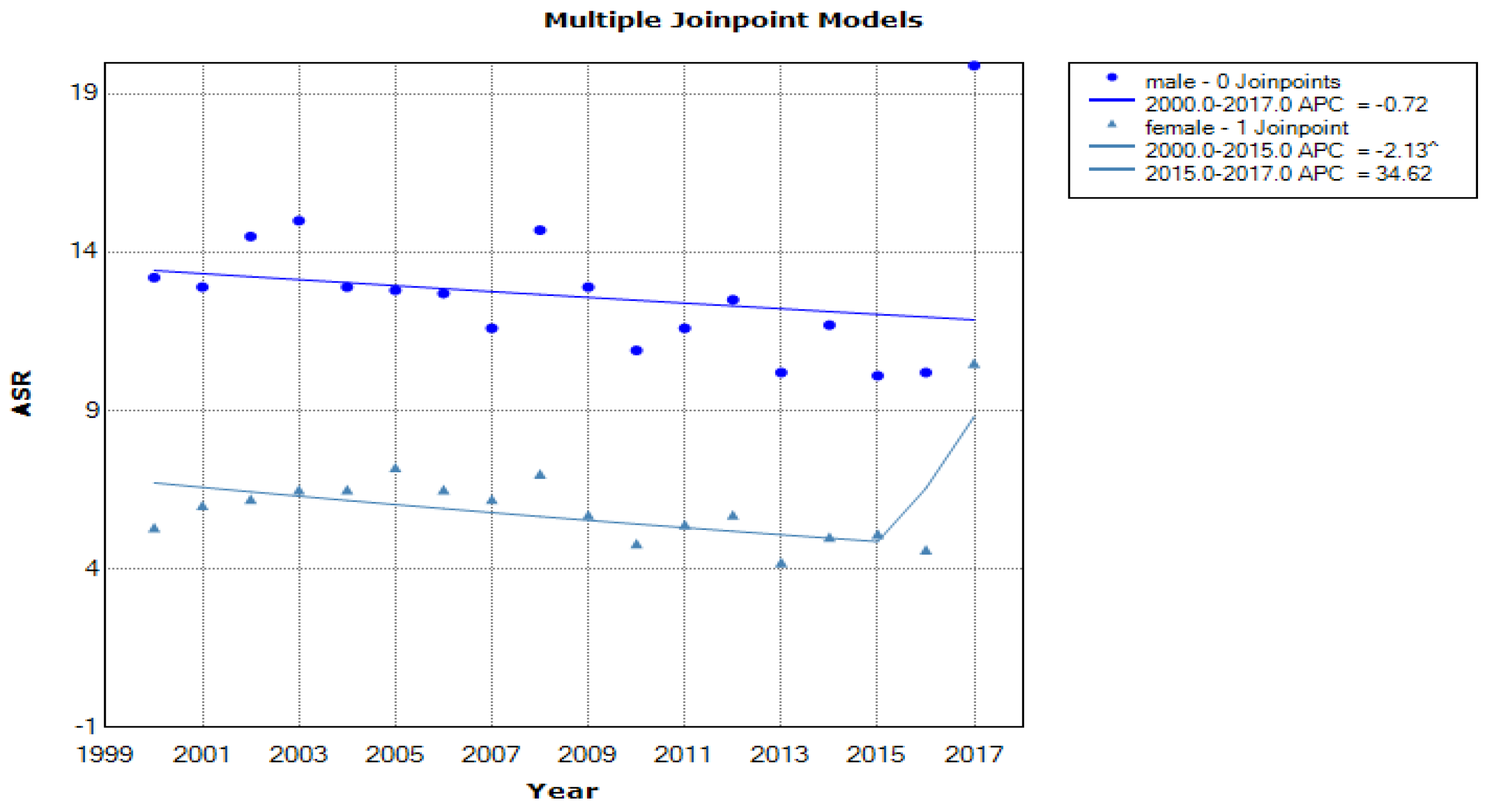
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLOBOCAN | Global Cancer Observatory |
| FDA | Food and Drug Agency |
| CR | crude rate |
| ASR | age-standardized rate |
| APC | annual percentage change |
| CI | confidence interval |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR). Continuous Update Project Report: Diet, Nutrition, Physical Activity and Stomach Cancer 2016. Revised 2018; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France; Available online: https://gco.iarc.fr/today (accessed on 9 October 2018).
- National Comprehensive Cancer Network. Guidelines for Patients: Diffuse Large B-Cell Lymphomas (Version 3.2020). Available online: https://www.nccn.org/patients/guidelines/nhl-diffuse/index.html (accessed on 9 August 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F. (Eds.) SEER Cancer Statistics Review, 1975–2013, Bethesda: National Cancer Institute. 2016. Available online: http://seer.cancer.gov/csr/1975_2013/ (accessed on 9 June 2017).
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing trends in stomach cancer throughout the world. Curr Gastroenterol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Ma, J.; Ward, E.M.; Siegel, R.L.; Jemal, A. Temporal trends in mortality in the United States, 1969–2013. JAMA 2015, 314, 1731–1739. [Google Scholar] [CrossRef] [Green Version]
- Katanoda, K.; Hori, M.; Matsuda, T.; Shibata, A.; Nishino, Y.; Hattori, M.; Soda, M.; Ioka, A.; Sobue, T.; Nishimoto, H. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn. J. Clin. Oncol. 2015, 45, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Coggon, D.A.U.; Barker, D.J.; Cole, R.B.; Nelson, M.S.O.J. Stomach cancer and food storage. Natl. Cancer Inst. 1989, 81, 1178. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M., Jr.; Keith, T.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Jha, H.C. Status of Epstein-Barr virus coinfection with Helicobacter pylori in gastric cancer. J. Oncol. 2017, 2017, 3456264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, L.; Wang, L.; Zhang, Y.; Chen, G.; Lin, L.; Jin, X.; Huang, Y.; Chen, J. Sex difference in incidence of gastric cancer: An international comparative study based on the Global Burden of Disease Study 2017. BMJ Open 2020, 10, e033323. [Google Scholar] [CrossRef] [Green Version]
- Hunt, R.H.; Xiao, S.D.; Megraud, F.; Leon-Barua, R.; Bazzoli, F.; Van der Merwe, S.; Coelho, L.V.; Fock, M.; Fedail, S.; Cohen, H.; et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J. Gastrointestin. Liver Dis. 2011, 20, 299–304. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 96: Alcoholic Beverage Consumption and Ethyl Carbamate (Urethane); International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Perez-Perez, G.I.; Rothenbacher, D.; Brenner, H. Epidemiology of Helicobacter pylori infection. Helicobacter 2004, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Spurnic, A.R.; Bukumiric, Z.; Jevtovic, D.; Brmbolic, B.; Pekmezovic, T.; Salemovic, D.; Pesic Pavlovic, I.; Milosevic, I.; Ranin, J.; Korac, M. Helicobacter pylori infection rates in dyspeptic Serbian HIV-infected patients compared to HIV-negative controls. PLoS ONE 2021. [Google Scholar] [CrossRef]
- Fang, X.; Wei, J.; He, X.; An, P.; Wang, H.; Jiang, L.; Shao, D.; Liang, H.; Li, Y.; Wang, F. Landscape of dietary factors associated with risk of stomach cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Cancer 2015, 51, 2820–2832. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Xiao, F.T.; Gong, B.C.; Liu, F.N. Alcohol drinking and gastric cancer risk: A meta-analysis of observational studies. Oncotarget 2017, 8, 99013–99023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everatt, R.; Tamosiunas, A.; Kuzmickiene, I.; Virviciute, D.; Radisauskas, R.; Reklaitiene, R.; Milinaviciene, E. Alcohol consumption and risk of stomach cancer: A cohort study of men in Kaunas, Lithuania, with up to 30 years follow-up. BMC Cancer 2012, 12, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute for Public Health of Serbia Dr. Milan Jovanović “Batut”. Malignant tumors in Republic of Serbia 2018; Institute for Public Health of Serbia Dr. Milan Jovanović “Batut”: Belgrade, Serbia, 2020. [Google Scholar]
- Segi, M. Cancer Mortality for Selected Sites in 24 Countries (1950–1957); Tohoku University School of Public Health: Sendai, Japan, 1960. [Google Scholar]
- World Health Organization. International Classification of Diseases and Related Health Problems, 10 th Revision. Available online: www.who.int/classifications/icd/en/ (accessed on 25 June 2021).
- Vlajinac, H.; Šipetić-Grujičić, S.; Janković, S.; Marinković, J.; Kocev, N.; Marković-Denić, L.; Bjegović, V. Burden of cancer in Serbia. Croat. Med. J. 2006, 47, 134–141. [Google Scholar]
- Ilić, M.; Ilic, I. Cancer mortality in Serbia, 1991–2015: An age-period-cohort and joinpoint regression analysis. Cancer Commun. 2018, 38, 10. [Google Scholar] [CrossRef]
- Mihajlović, J.; Pechlivanoglou, P.; Miladinov-Mikov, M.; Živković, S.; Postma, M.J. Cancer incidence and mortality in Serbia 1999–2009. BMC Cancer 2013, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Statistical Office of the Republic of Serbia. Mortality Databases 1996–2017; Statistical Office of the Republic of Serbia: Belgrade, Seriba, 2017.
- Official Gazette of Republic Serbia. Law on Protection of the Population from Exposure to Tobacco Smoke. Number: 30/2010. 7 May 2010. [Google Scholar]
- Knežević, T.; Miljuš, D.; Živković, S.; Plavšić, V.; Janković, J.; Savković, S. Attributable Causes of Cancer in Serbia in the Year 2005; Institute of Public Health of Serbia “Dr Milan Jovanović-Batut: Belgrade, Seriba, 2008. [Google Scholar]
- Castillejo, M.M. Commentary: Gastric cancer: Reducing the gap. Atencio Primaria 2001, 28, 634–641. [Google Scholar]
- Dassen, A.E. Stomach Cancer Trends and Treatment Strategies in the Netherlands: Challenges Ahead. Available online: http://hdl.handle.net/1765/50826 (accessed on 25 June 2021).
- Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/stomach-cancer/survival#heading-Two (accessed on 3 October 2018).
- Olar, L.; Mitrut, P.; Florou, C.; Mălăescu, G.D.; Predescu, O.I.; Rogozea, L.M.; Mogoantă, L.; Ionovici, N.; Pirici, I. Evaluation of Helicobacter pylori infection in patients with eso-gastro-duodenal pathology. Rom. J. Morphol. Embryol. 2017, 58, 809–815. [Google Scholar]
- Ciobanu, L.; Taulescu, M.; Dumitraşcu, D.L. Helicobacter pylori in Romania: Epidemiology, Diagnosis and Treatment. In Helicobacter pylori: A Worldwide Perspective; Buzas, G.M., Ed.; Bentham Science: Oak Park, IL, USA; pp. 183–201.
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Stomach cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Country | Incidence | Country | Mortality | ||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| South Korea | 57.8 | 23.8 | Mongolia | 36.7 | 15.6 |
| Mongolia | 47.2 | 23.5 | Kyrgyzstan | 26.7 | 9.1 |
| Japan | 40.7 | 16.0 | China | 25.0 | 10.4 |
| China | 29.5 | 12.3 | Vietnam | 19.7 | 8.7 |
| Chile | 26.9 | 10.3 | Chile | 17.9 | 6.4 |
| The UK | 5.3 | 2.6 | Australia | 3.1 | 1.5 |
| Canada | 2.9 | 5.2 | Canada | 2.8 | 1.7 |
| Philippines | 4.8 | 3.0 | Sweden | 2.7 | 1.6 |
| Sweden | 4.2 | 2.4 | Egypt | 7.1 | 2.5 |
| Egypt | 3.1 | 2.7 | The USA | 2.3 | 1.7 |
| Incidence | Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||
| Year | New cases | CR | ASR | New cases | CR | ASR | Fatal cases | CR | ASR | New cases | CR | ASR |
| 2000 | 599 | 22.3 | 13.2 | 290 | 10.3 | 5.3 | 591 | 22.1 | 12.5 | 313 | 11.1 | 5.3 |
| 2001 | 595 | 22.3 | 12.9 | 328 | 11.7 | 6.0 | 557 | 20.9 | 11.7 | 337 | 10.5 | 5.1 |
| 2002 | 667 | 25 | 14.5 | 344 | 12.2 | 6.2 | 577 | 21.7 | 11.6 | 300 | 10.7 | 4.8 |
| 2003 | 705 | 26.5 | 15.0 | 373 | 13.3 | 6.5 | 556 | 21.0 | 11.2 | 303 | 11.4 | 5.9 |
| 2004 | 617 | 23.3 | 12.9 | 383 | 13.7 | 6.5 | 566 | 21.4 | 11.4 | 353 | 12.6 | 5.8 |
| 2005 | 616 | 23.3 | 12.8 | 415 | 14.9 | 7.2 | 559 | 21.2 | 11.3 | 335 | 12.0 | 5.5 |
| 2006 | 609 | 23.1 | 12.7 | 359 | 12.9 | 6.5 | 487 | 18.5 | 9.9 | 283 | 10.2 | 4.6 |
| 2007 | 542 | 20.5 | 11.6 | 348 | 12.5 | 6.2 | 515 | 19.6 | 10.4 | 300 | 10.8 | 4.8 |
| 2008 | 698 | 26.6 | 14.7 | 399 | 14.4 | 7 | 506 | 19.4 | 9.8 | 283 | 10.3 | 4.4 |
| 2009 | 609 | 23.3 | 12.9 | 329 | 11.9 | 5.7 | 312 | 21.1 | 10.7 | 311 | 11.3 | 4.9 |
| 2010 | 536 | 20.6 | 10.9 | 284 | 10.3 | 4.8 | 517 | 19.9 | 10 | 279 | 10.2 | 4.3 |
| 2011 | 567 | 21.9 | 11.6 | 304 | 11.1 | 5.4 | 533 | 20.6 | 10.4 | 276 | 10.1 | 4.4 |
| 2012 | 615 | 23.8 | 12.5 | 336 | 12.3 | 5.7 | 497 | 19.3 | 9.2 | 311 | 11.5 | 4.4 |
| 2013 | 517 | 20.1 | 10.2 | 255 | 9.4 | 4.2 | 457 | 18.1 | 8.7 | 325 | 12.1 | 5.3 |
| 2014 | 591 | 22.1 | 11.7 | 314 | 11.7 | 5 | 460 | 18.1 | 8.2 | 248 | 9.2 | 3.7 |
| 2015 | 512 | 20.1 | 10.1 | 307 | 11.4 | 5.1 | 435 | 17.2 | 8.0 | 229 | 8.6 | 3.5 |
| 2016 | 538 | 21.2 | 10.2 | 273 | 21.5 | 10.6 | 632 | 18.4 | 16.8 | 320 | 11.0 | 4.6 |
| 2017 | 747 | 21.8 | 10.4 | 467 | 13.0 | 5.7 | 591 | 17.3 | 15.8 | 336 | 9.3 | 8.6 |
| Age-Group | Total | Male | Female | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0–19 | 22 | 0.10 | 12 | 0.11 | 10 | 0.17 | 0.340 |
| 20–29 | 160 | 0.90 | 104 | 0.96 | 56 | 0.93 | 0.847 |
| 30–39 | 810 | 4.80 | 533 | 4.90 | 277 | 4.59 | 0.352 |
| 40–49 | 2422 | 14.30 | 1387 | 12.76 | 1035 | 17.13 | <0.001 |
| 50–59 | 3635 | 21.50 | 2371 | 21.82 | 1264 | 20.92 | 0.171 |
| 60–69 | 5200 | 30.80 | 3447 | 31.72 | 1753 | 29.02 | <0.001 |
| 70–79 | 4660 | 27.60 | 3014 | 27.73 | 1646 | 27.25 | 0.434 |
| ∑ | 16,909 | 10,868 | 6041 | ||||
| Age-Group | Total | Male | Female | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0–19 | 5 | 0.03 | 4 | 0.04 | 1 | 0.02 | 0.436 |
| 20–29 | 42 | 0.28 | 22 | 0.24 | 20 | 0.37 | 0.145 |
| 30–39 | 212 | 1.43 | 120 | 1.28 | 92 | 1.69 | 0.045 |
| 40–49 | 916 | 6.19 | 577 | 6.17 | 339 | 6.23 | 0.889 |
| 50–59 | 1501 | 10.15 | 901 | 9.64 | 600 | 11.03 | 0.007 |
| 60–69 | 4250 | 28.74 | 2758 | 29.50 | 1492 | 27.42 | 0.007 |
| 70–79 | 7864 | 53.17 | 4966 | 53.12 | 2898 | 53.25 | 0.891 |
| ∑ | 14,790 | 100.00% | 9348 | 100.00% | 5442 | 100.00% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, M.M.; Rancic, N.K.; Andjelkovic Apostolovic, M.R.; Ignjatovic, A.M.; Ilic, M.V. Trends of Stomach Cancer in Central Serbia. Medicina 2021, 57, 665. https://doi.org/10.3390/medicina57070665
Stojanovic MM, Rancic NK, Andjelkovic Apostolovic MR, Ignjatovic AM, Ilic MV. Trends of Stomach Cancer in Central Serbia. Medicina. 2021; 57(7):665. https://doi.org/10.3390/medicina57070665
Chicago/Turabian StyleStojanovic, Miodrag M., Natasa K. Rancic, Marija R. Andjelkovic Apostolovic, Aleksandra M. Ignjatovic, and Mirko V. Ilic. 2021. "Trends of Stomach Cancer in Central Serbia" Medicina 57, no. 7: 665. https://doi.org/10.3390/medicina57070665
APA StyleStojanovic, M. M., Rancic, N. K., Andjelkovic Apostolovic, M. R., Ignjatovic, A. M., & Ilic, M. V. (2021). Trends of Stomach Cancer in Central Serbia. Medicina, 57(7), 665. https://doi.org/10.3390/medicina57070665







