Evaluation of the Effectiveness of Helicobacter pylori Eradication Regimens in Lithuania during the Years 2013–2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg)
Abstract
:1. Introduction
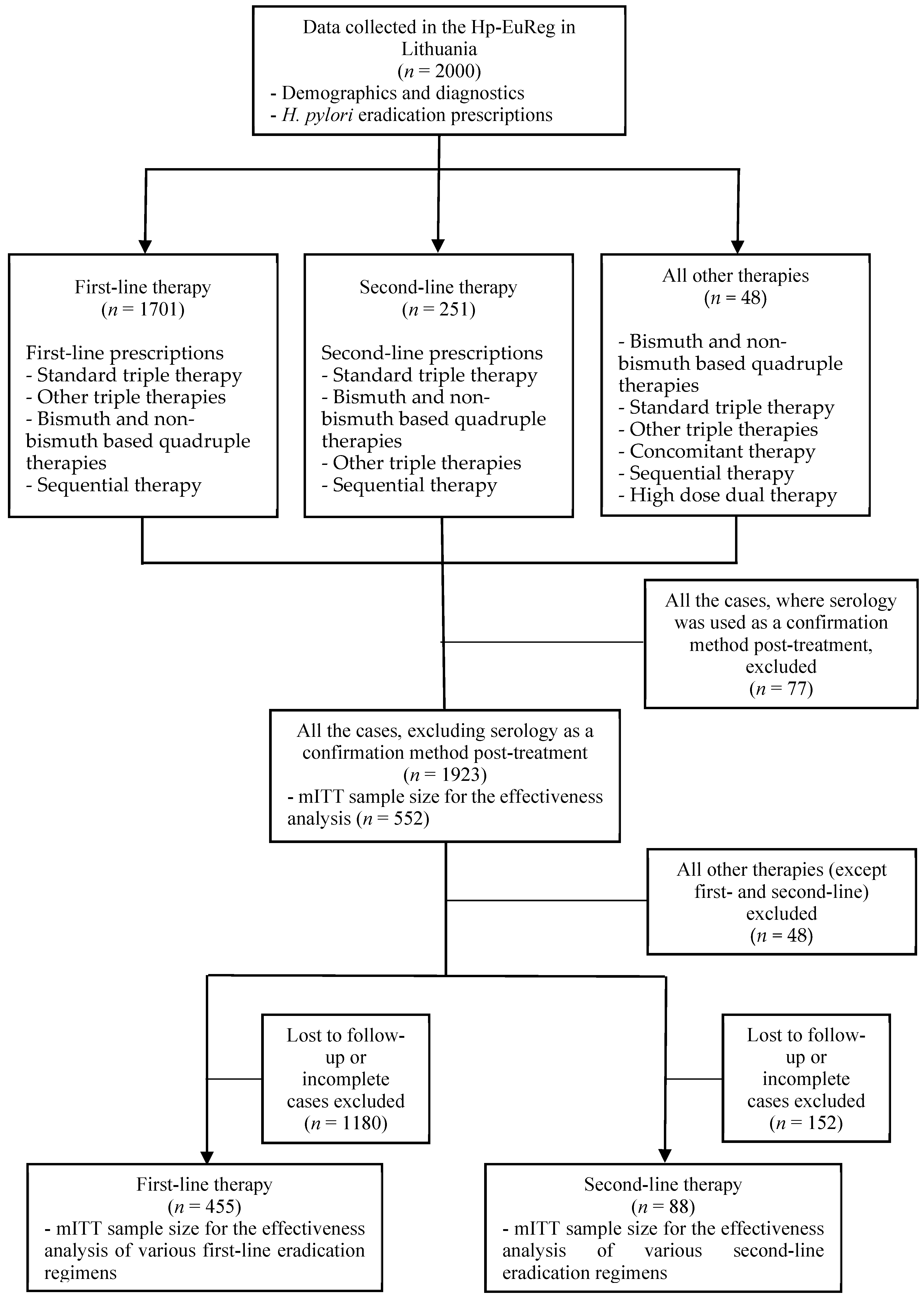
2. Materials and Methods
2.1. European Registry on H. pylori Management (Hp-EuReg) Setting and Ethics
2.2. Participants
2.3. Data Extraction and Analysis
2.4. Effectiveness Analysis
2.5. Statistical Analysis
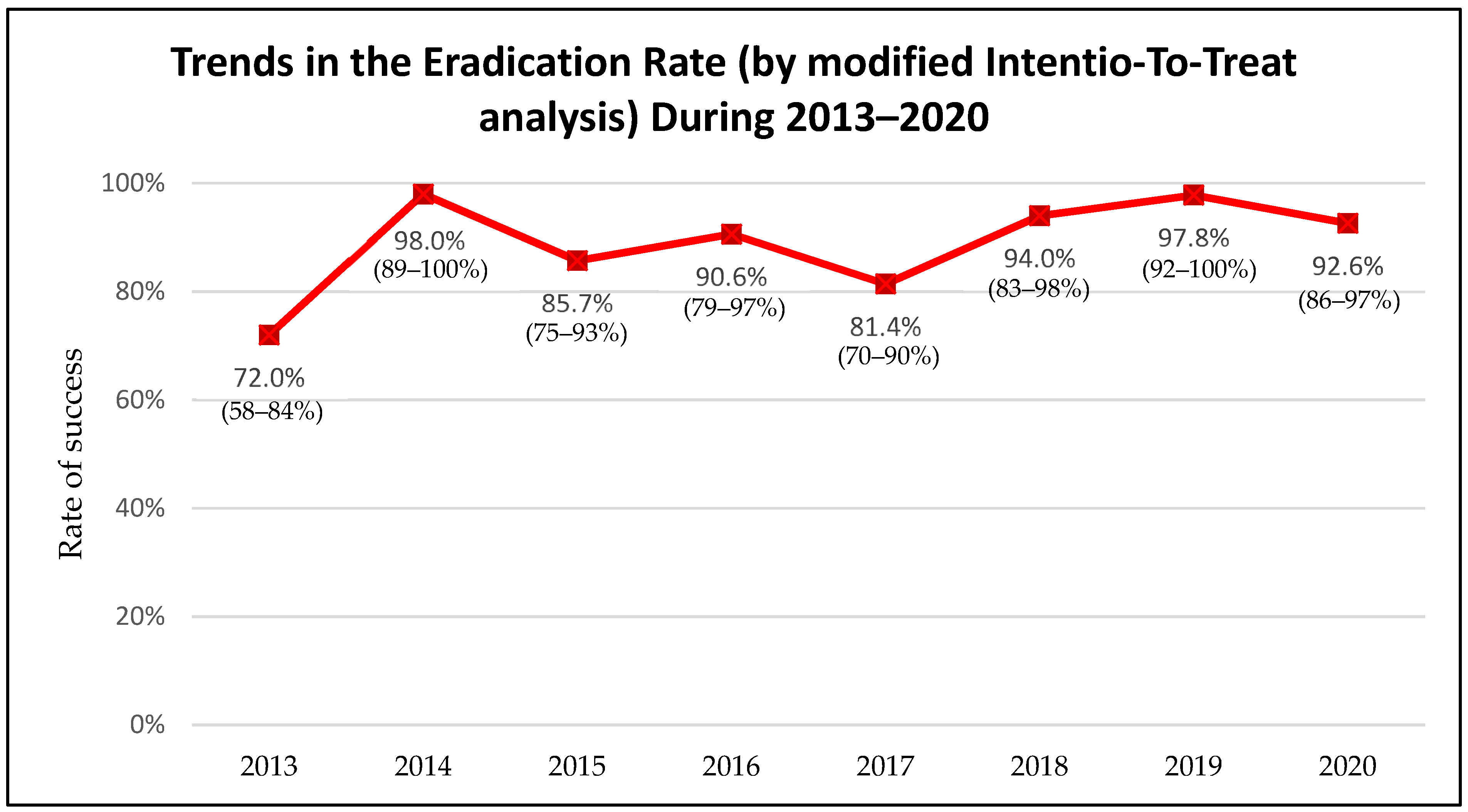
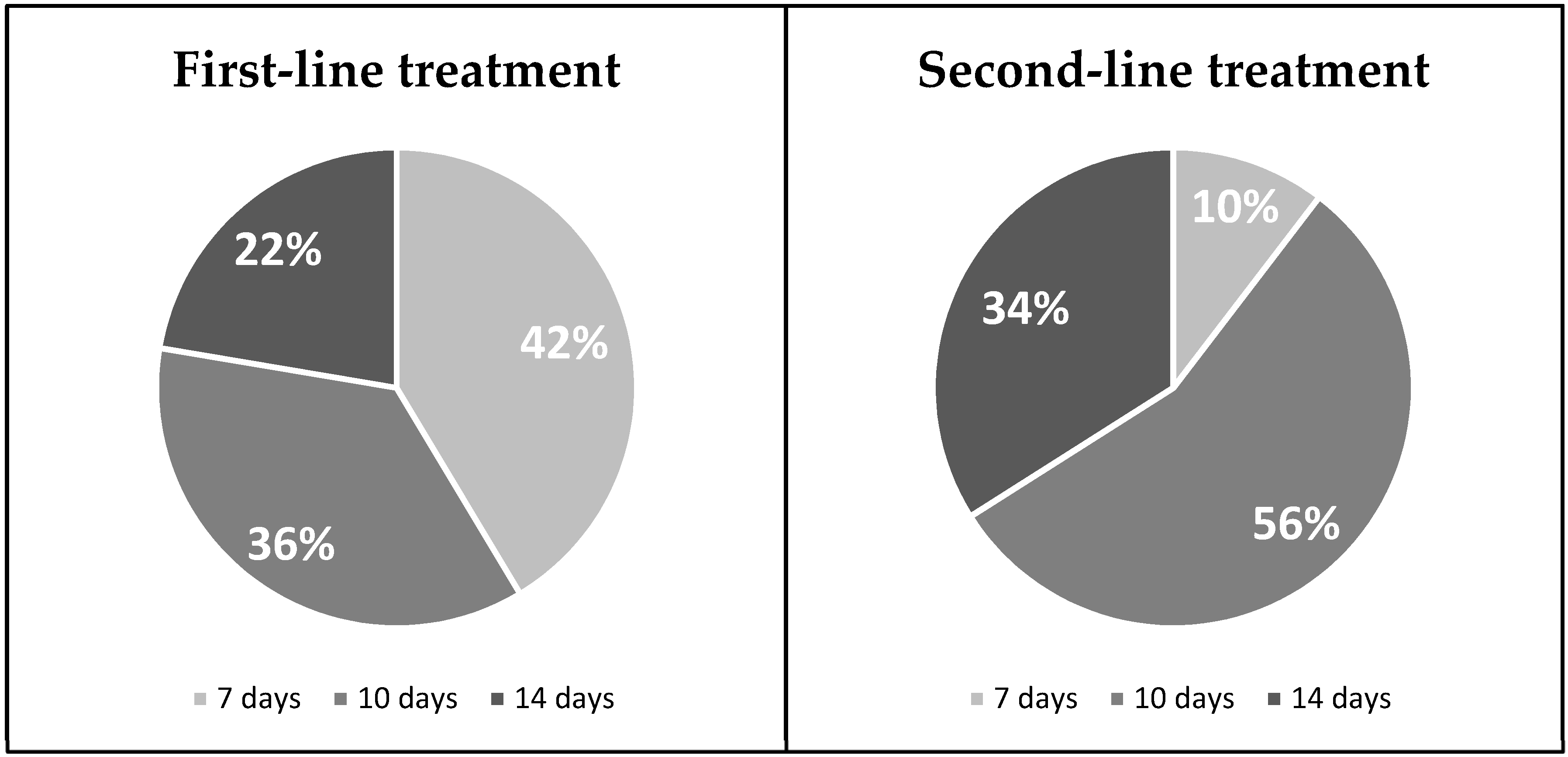
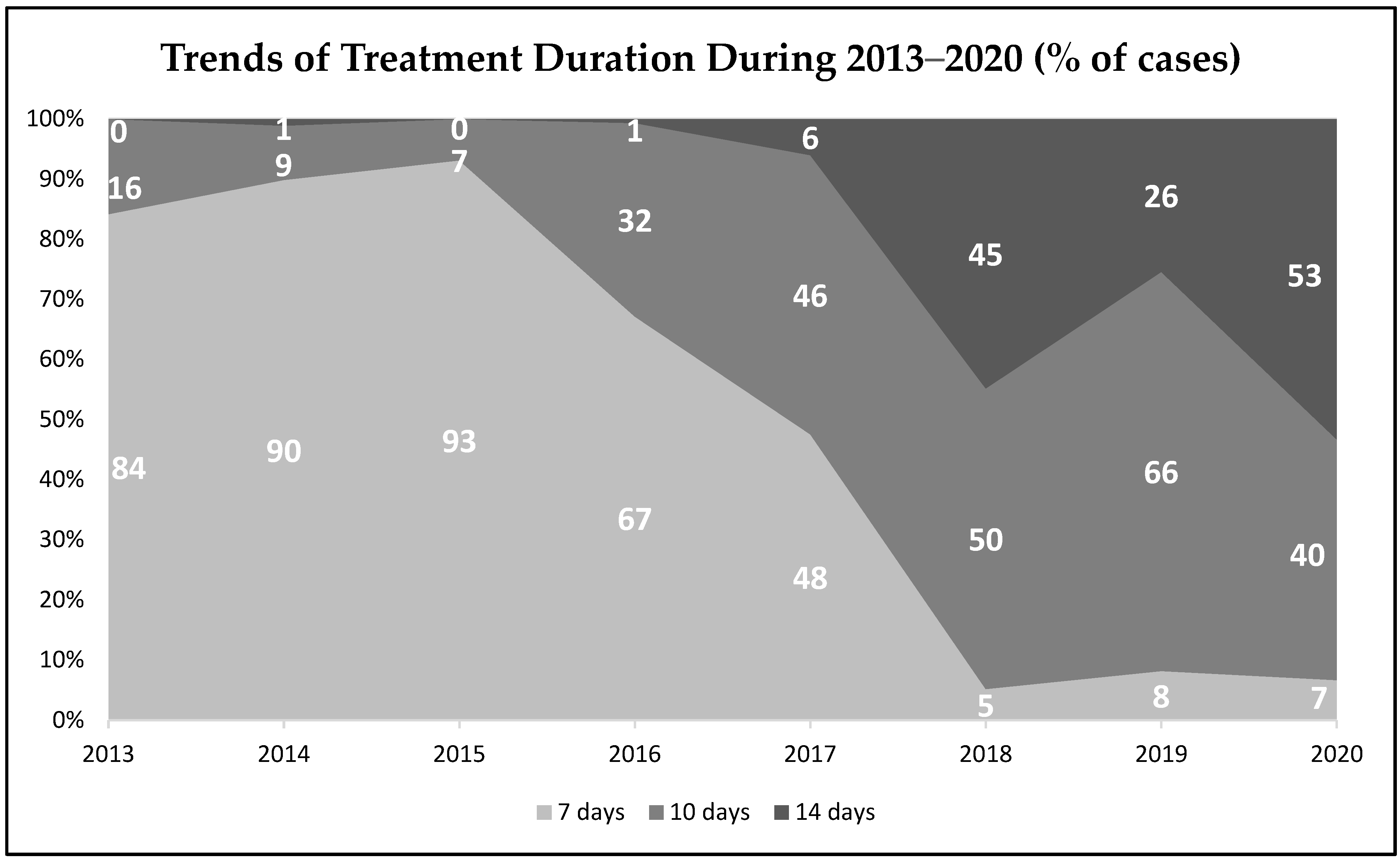
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Malfertheiner, P.; Megraud, F.; O’morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.-L.; Chan, W.-K.; Shiota, S.; Yamaoka, Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 2011, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23 (Suppl. S1), e12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonaitis, L.; Pellicano, R.; Kupcinskas, L. Helicobacter pylori and nonmalignant upper gastrointestinal diseases. Helicobacter 2018, 23 (Suppl. S1), e12522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.F. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Data on CYP2C19 Alleles [Internet]. Available online: https://www.pharmvar.org/gene/CYP2C19 (accessed on 2 January 2021).
- Šterbenc, A.; Jarc, E.; Poljak, M.; Homan, M. Helicobacter pylori virulence genes. World J. Gastroenterol. 2019, 25, 4870–4884. [Google Scholar] [CrossRef]
- Hooi, J.K.; Lai, W.Y.; Ng, W.K.; Suen, M.M.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.; Wu, J.C.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22 (Suppl. S1). [Google Scholar] [CrossRef]
- Mezmale, L.; Coelho, L.G.; Bordin, D.; Leja, M. Review: Epidemiology of Helicobacter pylori. Helicobacter 2020, 25 (Suppl. S1), e12734. [Google Scholar] [CrossRef]
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. S1), e12635. [Google Scholar] [CrossRef] [Green Version]
- Leja, M.; Axon, A.; Brenner, H. Epidemiology of Helicobacter pylori infection. Helicobacter 2016, 21 (Suppl. S1), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ruibys, G.; Denapiene, G.; Wright, R.A.; Irnius, A. Prevalence of Helicobacter Pylori Infection in Lithuanian Children. Off. J. Am. Coll. Gastroenterol. 2004, 99. Available online: https://journals.lww.com/ajg/Fulltext/2004/10001/PREVALENCE_OF_HELICOBACTER_PYLORI_INFECTION_IN.95.aspx (accessed on 2 January 2021).
- Jonaitis, L.; Balzekaite, V.; Bruzaite, S.; Gipas, J. Prevalence of H.pylori Among 40-60 Years Old Patients, Referring to Family Medicine Doctors. In Conference of Young Scientists and Investigators, Abstract Book; Student Scientific Society: Kaunas, Lithuania, 2013; pp. 274–275. [Google Scholar]
- Jonaitis, L.; Ivanauskas, A.; Jančiauskas, D.; Funka, K.; Sudraba, A.; Tolmanis, I.; Krams, A.; Vanags, A.; Kupčinskas, L.; Leja, M.; et al. Precancerous gastric conditions in high Helicobacter pylori prevalence areas: Comparison between Eastern European (Lithuanian, Latvian) and Asian (Taiwanese) patients. Medicina 2007, 43, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonaityte, I.R.; Ciupkeviciene, E.; Jonaitis, P.; Kupcinskas, J.; Petkeviciene, J.; Jonaitis, L. Changes in the Seroprevalence of Helicobacter pylori among the Lithuanian Medical Students over the Last 25 Years and Its Relation to Dyspeptic Symptoms. Medicina 2021, 57, 254. [Google Scholar] [CrossRef]
- Jonaitis, L.; Kiudelis, G.; Slepavicius, P.; Kupcinskas, L. High rate of Helicobacter pylori reinfection in Lithuanian peptic ulcer patients. World J. Gastrointest. Pathophysiol. 2016, 7, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.S.; Lee, H.J.; Jung, E.J.; Kim, M.A.; Nam, K.T.; Goldenring, J.R.; Yang, H.K.; Kim, W.H. The combined expression of metaplasia biomarkers predicts the prognosis of gastric cancer. Ann. Surg. Oncol. 2012, 19, 1240–1249. [Google Scholar] [CrossRef] [Green Version]
- Leja, M.; Kupcinskas, L.; Funka, K.; Sudraba, A.; Jonaitis, L.; Ivanauskas, A.; Janciauskas, D.; Kiudelis, G.; Chiu, H.M.; Lin, J.T. The validity of a biomarker method for indirect detection of gastric mucosal atrophy versus standard histopathology. Dig. Dis. Sci. 2009, 54, 2377–2384. [Google Scholar] [CrossRef]
- Pichon, M.; Pichard, B.; Barrioz, T.; Plouzeau, C.; Croquet, V.; Fotsing, G.; Chéron, A.; Vuillemin, É.; Wangermez, M.; Haineaux, P.A.; et al. Diagnostic Accuracy of a Noninvasive Test for Detection of Helicobacter pylori and Resistance to Clarithromycin in Stool by the Amplidiag H. pylori+ClariR Real-Time PCR Assay. J. Clin. Microbiol. 2020, 58, e01787-19. [Google Scholar] [CrossRef] [PubMed]
- Attumi, T.A.; Graham, D.Y. Follow-up testing after treatment of Helicobacter pylori infections: Cautions, caveats, and recommendations. Clin. Gastroenterol. Hepatol. 2011, 9, 373–375. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. Correction: ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2018, 113, 1102. [Google Scholar] [CrossRef]
- Kato, M.; Ota, H.; Okuda, M.; Kikuchi, S.; Satoh, K.; Shimoyama, T.; Suzuki, H.; Handa, O.; Furuta, T.; Mabe, K.; et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter 2019, 24, e12597. [Google Scholar] [CrossRef]
- O’Morain, N.R.; Dore, M.P.; O’Connor, A.J.P.; Gisbert, J.P.; O’Morain, C.A. Treatment of Helicobacter pylori infection in 2018. Helicobacter 2018, 23 (Suppl. S1), e12519. [Google Scholar]
- Kamboj, A.K.; Cotter, T.G.; Oxentenko, A.S. Helicobacter pylori: The Past, Present, and Future in Management. Mayo Clin. Proc. 2017, 92, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megraud, F. European Survey of Helicobacter Pylori Primary Resistance to Antibiotics-Evolution Over the Last 20 Years; UEG Week Barcelona: Catalonia, Spain, 2019. [Google Scholar]
- Dargiene, G.; Kupcinskas, J.; Jonaitis, L.; Vezbavicius, M.; Kadusevicius, E.; Kupcinskiene, E.; Frandsen, T.H.; Kucinskiene, R.; Kupcinskas, L.; Andersen, L.P. Primary antibiotic resistance of Helicobacter pylori strains among adults and children in a tertiary referral centre in Lithuania. APMIS 2018, 126, 21–28. [Google Scholar] [CrossRef]
- Nijevitch, A.A.; Idrisov, B.; Akhmadeeva, E.N.; Graham, D.Y. Choosing optimal first-line Helicobacter pylori therapy: A view from a region with high rates of antibiotic resistance. Curr. Pharm. Des. 2014, 20, 4510–4516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Zhang, W.; Graham, D.Y. Bismuth-containing quadruple therapy for Helicobacter pylori: Lessons from China. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1134–1140. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lee, Y.-C.; Wu, M.-S. Rational Helicobacter pylori therapy: Evidence-based medicine rather than medicine-based evidence. Clin. Gastroenterol. Hepatol. 2014, 12, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori Management (Hp-EuReg): Patterns and Trends in First-line Empirical Eradication Prescription and Outcomes of 5 years and 21 533 patients. Gut 2021, 70, 40LP–54LP. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, J.M.; Cheung, D.Y.; Oh, J.H. Comparison of 7- and 14-Day Eradication Therapy for Helicobacter pylori with First- and Second-Line Regimen: Randomized Clinical Trial. J. Korean Med. Sci. 2020, 35, e33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Park, M.I.; Park, S.J.; Moon, W.; Choi, Y.J.; Cheon, J.H.; Kwon, H.J.; Ku, K.H.; Yoo, C.H.; Kim, J.H.; et al. Trends in Helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J. Intern. Med. 2015, 30, 801–807. [Google Scholar] [CrossRef]
- Fallone, C.A.; Barkun, A.N.; Szilagyi, A.; Herba, K.M.; Sewitch, M.; Martel, M.; Fallone, S.S. Prolonged treatment duration is required for successful Helicobacter pylori eradication with proton pump inhibitor triple therapy in Canada. Can. J. Gastroenterol. 2013, 27, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Ford, A.C.; Khan, K.J.; Gisbert, J.P.; Forman, D.; Leontiadis, G.I.; Tse, F.; Calvet, X.; Fallone, C.; Fischbach, L.; et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2013, CD008337. [Google Scholar] [CrossRef]
- Arama, S.S.; Tiliscan, C.; Negoita, C.; Croitoru, A.; Arama, V.; Mihai, C.M.; Pop, F.; Garg, A. Efficacy of 7-Day and 14-Day Triple Therapy Regimens for the Eradication of Helicobacter pylori: A Comparative Study in a Cohort of Romanian Patients. Gastroenterol. Res. Pract. 2016, 2016, 5061640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholl, A.G.; O’Morain, C.A.; Megraud, F.; Gisbert, J.P. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter 2019, 24, e12630. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ierardi, E.; Losurdo, G.; La Fortezza, R.F.; Principi, M.; Barone, M.; Di Leo, A. Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness. World J. Gastroenterol. 2019, 25, 5097–5104. [Google Scholar] [CrossRef]
- Vallve, M.; Vergara, M.; Gisbert, J.P.; Calvet, X. Single vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: A meta-analysis. Aliment. Pharmacol. Ther. 2002, 16, 1149–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulkhakov, S.R.; Bordin, D.S.; Abdulkhakov, R.A.; Safina, D.D.; Gizdatullina, A.R.; Gimadieva, L.Z.; Safina, G.M.; Ziyatdinov, A.I.; Maturina, A.M.; Nyssen, O.P.; et al. European Registry on the management of Helicobacter pylori infection: Features of diagnosis and treatment in Kazan. Ter. Arkh. 2020, 92, 52–59. [Google Scholar]
- Bordin, D.S.; Embutnieks, Y.V.; Vologzhanina, L.G.; Ilchishina, T.A.; Voynovan, I.N.; Sarsenbaeva, A.S.; Zaitsev, O.V.; Alekseenko, S.A.; Abdulkhakov, R.A.; Dehnich, N.N.; et al. European registry Helicobacter pylori (Hp-EuReg): How has clinical practice changed in Russia from 2013 to 2018 years. Ter. Arkh. 2019, 91, 16–24. [Google Scholar] [CrossRef]
- Buzás, G.M.; Nyssen, O.P.; Mégraud, F.; O’Morain, C.; Gisbert, J.P. Pan-European Registry on Helicobacter pylori management. Results from Ferencváros, Budapest, 2013–2019. Orv. Hetil. 2019, 160, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Tepes, B.; Kastelic, M.; Vujasinovic, M.; Lampic, P.; Seruga, M.; Jurecic, N.B.; Nyssen, O.P.; Donday, M.G.; O’Morain, C.; Megraud, F.; et al. Helicobacter Pylori Treatment Results in Slovenia in the Period 2013–2015 as a Part of European Registry on Helicobacter Pylori Management. Radiol. Oncol. 2018, 52, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepes, B.; Jurecic, N.B.; Tepes, K.; Sanchez, M.E.; Nyssen, O.P.; O’Morain, C.; Mégraud, F.; Gisbert, J. Helicobacter pylori eradication rates in Slovenia in the period from 2017 to 2019-data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Dig. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
| First-Line Treatment | No. of Patients (% of All Cases) | No. of Patients Tested Post-Treatment | mITT (95% CI) |
| PPI+C+A | 1550 (91%) | 416 | 90% (86–92%) |
| PPI+C+A+B | 66 (3.9%) | 9 | 89% (52–100%) |
| PPI+C+M | 34 (2%) | 12 | 100% (74–100%) |
| PPI+A+L | 16 (0.9%) | 6 | 100% (54–100%) |
| PPI+A+M+B | 10 (0.6%) | 3 | 100% (29–100%) |
| PPI+A+L+B | 8 (0.5%) | 1 | 100% (3–100%) |
| PPI+A+M | 8 (0.5%) | 6 | 100% (54–100%) |
| PPI+C+L | 2 (0.1%) | - | - |
| PPI+A+C+T (Sequential) | 1 (0.1%) | 1 | 100% (3–100%) |
| PPI+C+M+B | 1 (0.1%) | - | - |
| PPI+C+A+M | 1 (0.1%) | 1 | 100% (3–100%) |
| Second-Line Treatment |
No. of Patients (% of All Cases) |
No. of Patients Tested Post-Treatment | mITT (95% CI) |
| PPI+A+L | 118 (47.0%) | 49 | 92% (80–98%) |
| PPI+A+L+B | 58 (23.1%) | 8 | 100% (63–100%) |
| PPI+C+A | 25 (10%) | 14 | 71% (42–92%) |
| PPI+A+M+B | 14 (5.6%) | 1 | 100% (3–100%) |
| PPI+A+M | 13 (5.2%) | 7 | 71% (29–96%) |
| PPI+C+A+B | 11 (4.4%) | 3 | 100% (29–100%) |
| PPI+C+M | 5 (2%) | 3 | 100% (29–100%) |
| PPI+A+C+T (Sequential) | 3 (1.2%) | 2 | 100% (16–100%) |
| PPI+C+M+B | 2 (0.8%) | 1 | 100% (3–100%) |
| PPI+C+L+B | 1 (0.4%) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonaitis, P.; Kupcinskas, J.; Nyssen, O.P.; Puig, I.; Gisbert, J.P.; Jonaitis, L. Evaluation of the Effectiveness of Helicobacter pylori Eradication Regimens in Lithuania during the Years 2013–2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Medicina 2021, 57, 642. https://doi.org/10.3390/medicina57070642
Jonaitis P, Kupcinskas J, Nyssen OP, Puig I, Gisbert JP, Jonaitis L. Evaluation of the Effectiveness of Helicobacter pylori Eradication Regimens in Lithuania during the Years 2013–2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Medicina. 2021; 57(7):642. https://doi.org/10.3390/medicina57070642
Chicago/Turabian StyleJonaitis, Paulius, Juozas Kupcinskas, Olga P. Nyssen, Ignasi Puig, Javier P. Gisbert, and Laimas Jonaitis. 2021. "Evaluation of the Effectiveness of Helicobacter pylori Eradication Regimens in Lithuania during the Years 2013–2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg)" Medicina 57, no. 7: 642. https://doi.org/10.3390/medicina57070642
APA StyleJonaitis, P., Kupcinskas, J., Nyssen, O. P., Puig, I., Gisbert, J. P., & Jonaitis, L. (2021). Evaluation of the Effectiveness of Helicobacter pylori Eradication Regimens in Lithuania during the Years 2013–2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Medicina, 57(7), 642. https://doi.org/10.3390/medicina57070642






