To Enhance or Not to Enhance? The Role of Contrast Medium 18F-FDG PET/CT in Recurrent Ovarian Carcinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Image Acquisition
2.3. Image Analysis
2.4. Standard of Reference
2.5. Statistical Analysis
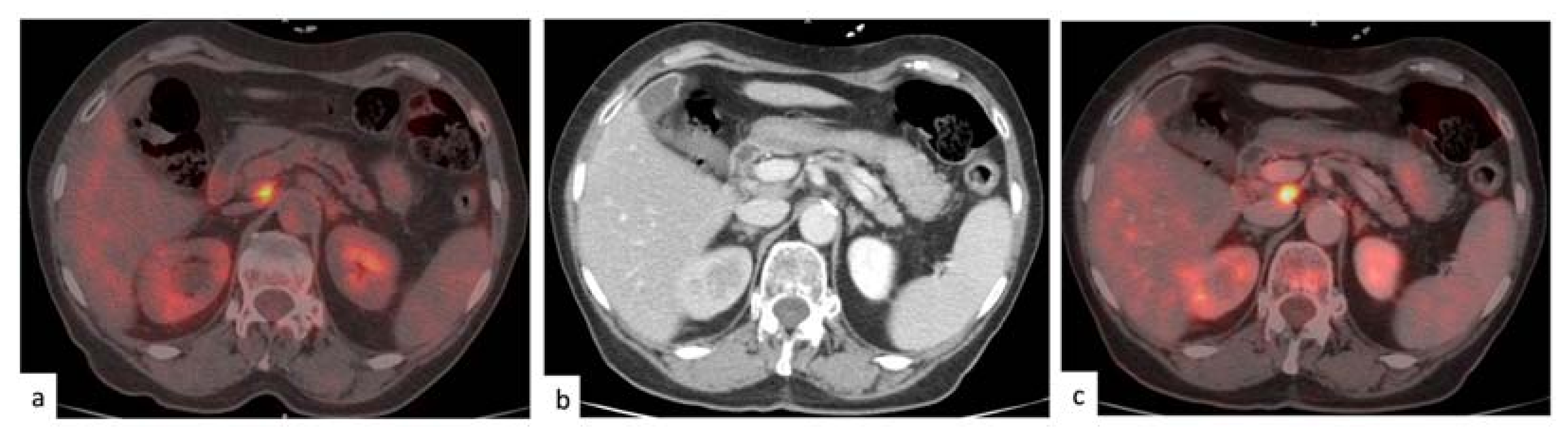
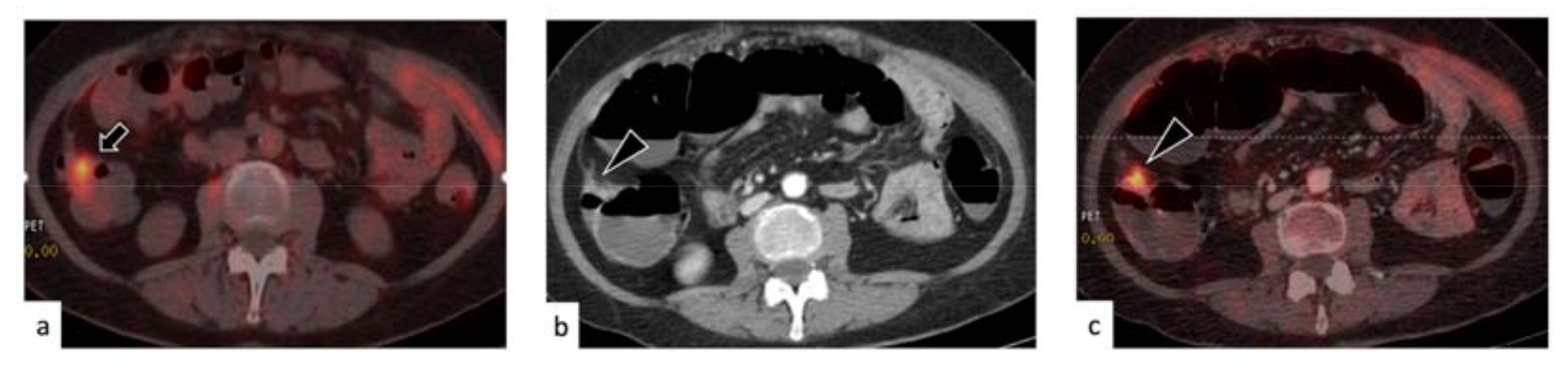
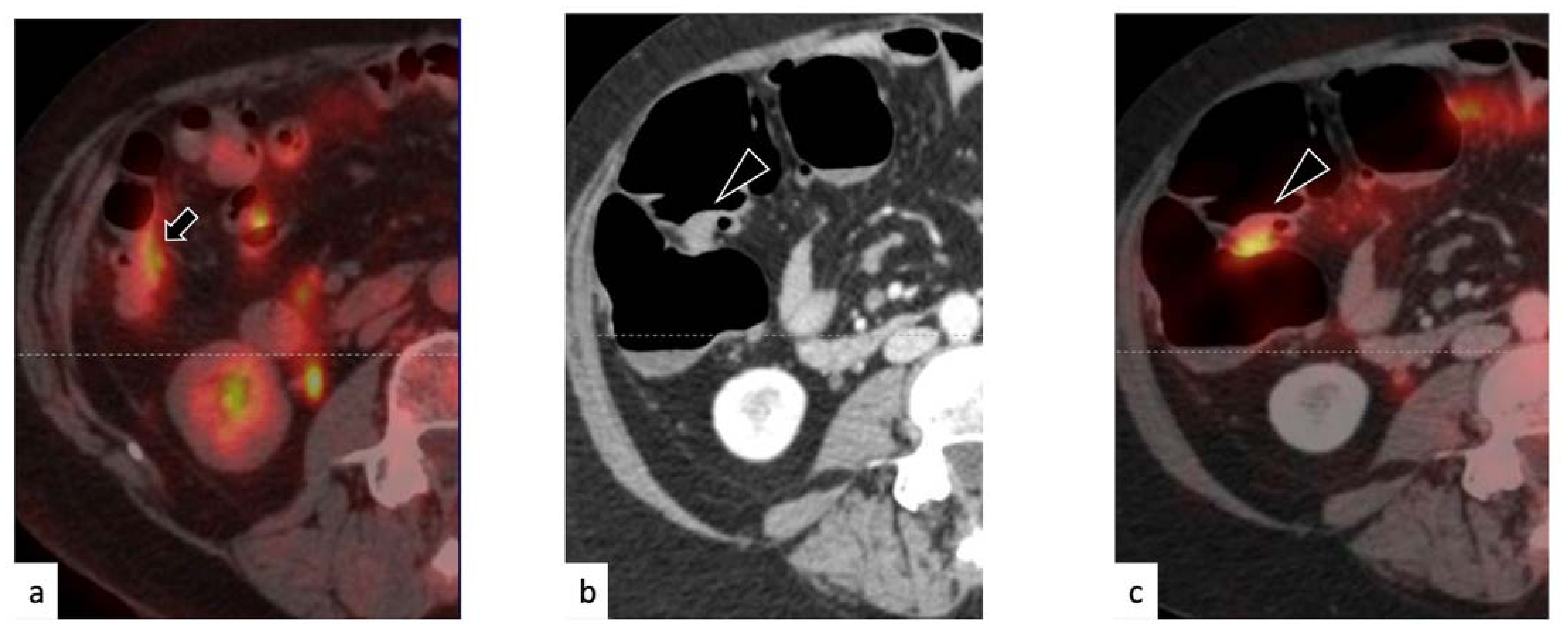
3. Results
3.1. Characteristics of the Selected Population
3.2. Rate of Disease Identification
3.3. Patient-Based Analysis
3.4. Lesion-Based Analysis
3.5. Change of Management Due to PET/ceCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Grunewald, T.; Ledermann, J.A. Targeted Therapies for Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 139–152. [Google Scholar] [CrossRef]
- Greenlee, R.T.; Hill-Harmon, M.B.; Murray, T.; Thun, M. Cancer Statistics. CA Cancer J. Clin. 2001, 51, 15–36. [Google Scholar] [CrossRef]
- Moufarrij, S.; Dandapani, M.; Arthofer, E.; Gomez, S.; Srivastava, A.; Lopez-Acevedo, M.; Villagra, A.; Chiappinelli, K.B. Epigenetic therapy for ovarian cancer: Promise and progress. Clin. Epigenet. 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A. Distribution of intra-abdominal malignant seeding: Dependency on dynamics of flow of ascitic fluid. Am. J. Roentgenol. 1973, 119, 198–206. [Google Scholar] [CrossRef]
- Berek, J.S.; Kehoe, S.T.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2018, 143, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.; Kim, S.W.; Wu, S.Y.; Nishimura, M.; Chaluvally-Raghavan, P.; Miyake, T.; Pecot, C.V.; Kim, S.-J.; Choi, H.J.; Bischoff, F.Z.; et al. Hematogenous Metastasis of Ovarian Cancer: Rethinking Mode of Spread. Cancer Cell 2014, 26, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F. Treatment goals in ovarian cancer. Int. J. Gynecol. Cancer 2005, 15, 3–11. [Google Scholar] [CrossRef]
- Turlakow, A.; Yeung, H.W.; Salmon, A.S.; Macapinlac, H.A.; Larson, S.M. Peritoneal carcinomatosis: Role of (18)F-FDG PET. J. Nucl. Med. 2003, 44, 1407–1412. [Google Scholar]
- Scholler, N.; Urban, N. CA125 in ovarian cancer. Biomark. Med. 2007, 1, 513–523. [Google Scholar] [CrossRef]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.M.; Calcagni, M.L.; Rufini, V.; Valenza, V.; Giordano, A.; Bonomo, L. Imaging of peritoneal carcinomatosis with FDG PET-CT: Diagnostic patterns, case examples and pitfalls. Abdom. Imaging 2009, 34, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.M.; Willmann, J.K.; Drescher, C.W.; Ray, P.; Cochran, F.V.; Urban, N.; Gambhir, S.S. Early Diagnosis of Ovarian Carcinoma: Is a Solution in Sight? Radiology 2011, 259, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Ludovisi, M.; Mascilini, F.; Di Legge, A.; Malaggese, M.; Fagotti, A.; Fanfani, F.; Salerno, M.G.; Ercoli, A.; Scambia, G.; et al. Ultrasound evaluation of intra-abdominal sites of disease to predict likelihood of suboptimal cytoreduction in advanced ovarian cancer: A prospective study. Ultrasound Obstet. Gynecol. 2011, 39, 99–105. [Google Scholar] [CrossRef]
- Forstner, R. Radiological staging of ovarian cancer: Imaging findings and contribution of CT and MRI. Eur. Radiol. 2007, 17, 3223–3235. [Google Scholar] [CrossRef] [PubMed]
- Funicelli, L.; Travaini, L.L.; Landoni, F.; Trifirò, G.; Bonello, L.; Bellomi, M. Peritoneal carcinomatosis from ovarian cancer: The role of CT and [18F]FDG-PET/CT. Abdom. Imaging 2009, 35, 701–707. [Google Scholar] [CrossRef]
- Low, R.N.; Barone, R.M.; Lacey, C.; Sigeti, J.S.; Alzate, G.D.; Sebrechts, C.P. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology 1997, 204, 513–520. [Google Scholar] [CrossRef]
- Klumpp, B.D.; Aschoff, P.; Schwenzer, N.; Fenchel, M.; Koenigsrainer, I.; Falch, C.; Bruecher, B.; Claussen, C.D.; Koenigsrainer, A.; Pfannenberg, C.; et al. Peritoneal carcinomatosis: Comparison of dynamic contrast-enhanced magnetic resonance imaging with surgical and histopathologic findings. Abdom. Radiol. 2011, 37, 834–842. [Google Scholar] [CrossRef]
- Klumpp, B.D.; Schwenzer, N.; Aschoff, P.; Miller, S.; Kramer, U.; Claussen, C.D.; Bruecher, B.; Koenigsrainer, A.; Pfannenberg, C. Preoperative assessment of peritoneal carcinomatosis: Intraindividual comparison of 18F-FDG PET/CT and MRI. Abdom. Imaging 2012, 38, 64–71. [Google Scholar] [CrossRef]
- Pfannenberg, C.; Königsrainer, I.; Aschoff, P.; Öksüz, M.Ö.; Zieker, D.; Beckert, S.; Symons, S.; Nieselt, K.; Glatzle, J.; Weyhern, C.V.; et al. 18F-FDG-PET/CT to Select Patients with Peritoneal Carcinomatosis for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2009, 16, 1295–1303. [Google Scholar] [CrossRef]
- Sebastian, S.; Lee, S.I.; Horowitz, N.S.; Scott, J.A.; Fischman, A.J.; Simeone, J.F.; Fuller, A.F.; Hahn, P.F. PET–CT vs. CT alone in ovarian cancer recurrence. Abdom. Imaging 2007, 33, 112–118. [Google Scholar] [CrossRef]
- Risum, S.; Høgdall, C.; Markova, E.; Berthelsen, A.K.; Loft, A.; Jensen, F.; Høgdall, E.; Roed, H.; Engelholm, S.A. Influence of 2-(18F) Fluoro-2-Deoxy-d-Glucose Positron Emission Tomography/Computed Tomography on Recurrent Ovarian Cancer Diagnosis and on Selection of Patients for Secondary Cytoreductive Surgery. Int. J. Gynecol. Cancer 2009, 19, 600–604. [Google Scholar] [CrossRef]
- Bhosale, P.; Peungjesada, S.; Wei, W.; Levenback, C.F.; Schmeler, K.; Rohren, E.; Macapinlac, H.A.; Iyer, R.B. Clinical Utility of Positron Emission Tomography/Computed Tomography in the Evaluation of Suspected Recurrent Ovarian Cancer in the Setting of Normal CA-125 Levels. Int. J. Gynecol. Cancer 2010, 20, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Addley, H.C.; Colombo, P.E.; Fujii, S.; Al Sharif, S.S.; Tirumani, S.H.; Jardon, K.; Sala, E.; Reinhold, C. Ovarian Carcinomatosis: How the Radiologist Can Help Plan the Surgical Approach. Radiographics 2012, 32, 1775–1800. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Dirisamer, A.; Schima, W.; Heinisch, M.; Weber, M.; Lehner, H.P.; Haller, J.; Langsteger, W. Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur. J. Radiol. 2009, 69, 536–541. [Google Scholar] [CrossRef]
- Kitajima, K.; Ueno, Y.; Suzuki, K.; Kita, M.; Ebina, Y.; Yamada, H.; Senda, M.; Maeda, T.; Sugimura, K. Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT scans for diagnosing ovarian cancer recurrence. Eur. J. Radiol. 2012, 81, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Domeki, Y.; Kaji, Y.; Fukasawa, I.; Inaba, N.; Suganuma, N.; Sugimura, K. Performance of integrated FDG–PET/contrast-enhanced CT in the diagnosis of recurrent ovarian cancer: Comparison with integrated FDG–PET/non-contrast-enhanced CT and enhanced CT. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1439–1448. [Google Scholar] [CrossRef]
- Gadducci, A.; Simonetti, E.; Manca, G.; Guidoccio, F.; Fanucchi, A.; Cosio, S.; Volterrani, D. Positron Emission Tomography/Computed Tomography in Platinum-sensitive Recurrent Ovarian Cancer: A Single-center Italian Study. Anticancer Res. 2020, 40, 2191–2197. [Google Scholar] [CrossRef]
- Antoch, G.; Freudenberg, L.S.; Beyer, T.; Bockisch, A.; Debatin, J.F. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual-modality 18F-FDG PET/CT. J. Nucl. Med. 2004, 45, 56S–65S. [Google Scholar]
- Morbelli, S.; Conzi, R.; Campus, C.; Cittadini, G.; Bossert, I.; Massollo, M.; Fornarini, G.; Calamia, I.; Marini, C.; Fiz, F.; et al. Contrast-enhanced [18 F] fluorodeoxyglucose-positron emission tomography/computed tomography in clinical oncology: Tumor-, site-, and question-based comparison with standard positron emission tomography/computed tomography. Cancer Imaging 2014, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Boellaard, R.; O’Doherty, M.J.; Weber, W.A.; Mottaghy, F.M.; Lonsdale, M.N.; Stroobants, S.G.; Oyen, W.J.; Kotzerke, J.; Hoekstra, O.S.; Pruim, J.; et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2009, 37, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Willowson, K.P.; Bailey, E.A.; Bailey, D.L. A retrospective evaluation of radiation dose associated with low dose FDG protocols in whole-body PET/CT. Australas. Phys. Eng. Sci. Med. 2011, 35, 49–53. [Google Scholar] [CrossRef]
- Reske, S.U.; Braunschweig, R.; Reske, A.W.; Loose, R.; Wucherer, M. Whole-Body CT in Multiple Trauma Patients: Clinically Adapted Usage of Differently Weighted CT Protocols. RöFo Fortschr. Gebiet Röntgenstrahlen Bildgeb. Verfahr. 2018, 190, 1141–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.; Baumgartner, R.; Shavit, L.; Barash, I.; Michael, J.; Puzanov, I.; Kopolovic, J.; Rosengarten, O.; Blank, S.; Curtin, J.P.; et al. Is Renal Thrombotic Angiopathy an Emerging Problem in the Treatment of Ovarian Cancer Recurrences? Oncologist 2012, 17, 1534–1540. [Google Scholar] [CrossRef]
- Massollo, M.; Treglia, G.; Trimboli, P.; Fiz, F.; Ugolini, M.; Piccardo, A. Head-to-head comparison between 18F-FDG PET/low-dose CT and 18F-FDG PET/contrast-enhanced CT in relapsing ovarian carcinoma: A systematic review and meta-analysis. Clin. Transl. Imaging 2021, 9, 73–81. [Google Scholar] [CrossRef]
- Tawakol, A.; Abdelhafez, Y.; Osama, A.; Hamada, E.; El Refaei, S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl. Med. Commun. 2016, 37, 453–460. [Google Scholar] [CrossRef]
- Sala, E.; Kataoka, M.; Pandit-Taskar, N.; Ishill, N.; Mironov, S.; Moskowitz, C.S.; Mironov, O.; Collins, M.A.; Chi, D.S.; Larson, S.; et al. Recurrent Ovarian Cancer: Use of Contrast-enhanced CT and PET/CT to Accurately Localize Tumor Recurrence and to Predict Patients’ Survival. Radiology 2010, 257, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-M.; Zhang, J.; Niu, S.-H.; Li, K.-X.; Song, C.-Z. Secondary cytoreductive surgery in recurrent epithelial ovarian cancer: A prognostic analysis with 103 cases. Int. J. Surg. 2017, 38, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tovey, H.; Bowen, R.; Folkerd, E.; Kilburn, L.; McLachlan, J.; Hall, M.; Tunariu, N.; Attygalle, A.; Lima, J.P.D.S.N.; et al. Abiraterone in patients with recurrent epithelial ovarian cancer: Principal results of the phase II Cancer of the Ovary Abiraterone (CORAL) trial (CRUK–A16037). Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Definition According to Follow Up |
|---|---|
| True positive (TP) | Disappearance after treatment; Morphological increase without treatment. Progress (immediate or delayed) under therapy |
| True negative (TN) | No appearance of abnormal fluorodeoxyglucose (FDG) uptakes or computed tomography (CT)-evident lesions |
| False positive (FP) | Disappearance without treatment |
| False negative (FN) | Morphological increase of the FDG-negative lesion |
| Age | |
| Median | 57 |
| Range | 27–85 |
| The Stage at Diagnosis (FIGO Staging System) | |
| I | 19 (16%) |
| II | 11 (9%) |
| III | 69 (56%) |
| IV | 23 (19%) |
| Tumor Grade | |
| 1 | 7 (6%) |
| 2 | 19 (15%) |
| 3 | 97 (79%) |
| Tumor Histology | |
| Serous | 79 (65%) |
| Endometroid | 23 (19%) |
| Other/nonspecified | 20 (16%) |
| The Main Indication for positron emission tomography/X-ray computed tomography (PET/CT) Scan | |
| Rising tumor markers levels | 65 (53%) |
| Suspect CT and/or magnetic resonance (MR) findings | 19 (16%) |
| Rising tumor marker levels, suspicious CT and/or MR imaging findings | 23(19%) |
| Suspicious findings at physical examination | 15 (12%) |
| Follow up (Months) | |
| Median | 36 |
| Range | 18–108 |
| Peritoneal Lesions | |||||
| PET/ldCT | PET/ceCT | PET/ldCT | PET/ceCT | ||
| TP | 37 | 41 | Sensitivity | 88.10% | 95.35% |
| TN | 76 | 77 | Specificity | 95.00% | 97.47% |
| FP | 4 | 2 | PPV | 90.24% | 95.35% |
| FN | 5 | 2 | NPV | 93.83% | 97.47% |
| Total | 122 | 122 | Accuracy | 92.62% | 96.72% |
| Nodal Lesions | |||||
| PET/ldCT | PET/ceCT | PET/ldCT | PET/ceCT | ||
| TP | 26 | 27 | Sensitivity | 92.86% | 96.43% |
| TN | 94 | 94 | Specificity | 98.95% | 98.95% |
| FP | 1 | 1 | PPV | 96.30% | 96.43% |
| FN | 2 | 1 | NPV | 97.92% | 98.95% |
| Total | 122 | 122 | Accuracy | 98.36% | 99.18% |
| Peritoneal Lesions | |||||
| PET/ldCT | PET/ceCT | PET/ldCT | PET/ceCT | ||
| TP | 293 | 310 | Sensitivity | 97.99% | 99.04% |
| TN | 75 | 77 | Specificity | 91.46% | 96.25% |
| FP | 7 | 3 | PPV | 97.67% | 99.04% |
| FN | 6 | 3 | NPV | 92.59% | 96.25% |
| Total | 380 | 393 | Accuracy | 96.84% | 98.47% |
| Nodal Lesions | |||||
| PET/ldCT | PET/ceCT | PET/ldCT | PET/ceCT | ||
| TP | 162 | 164 | Sensitivity | 98.18% | 98.80% |
| TN | 92 | 92 | Specificity | 94.85% | 94.85% |
| FP | 5 | 5 | PPV | 97.01% | 97.04% |
| FN | 3 | 2 | NPV | 96.84% | 97.87% |
| Total | 262 | 263 | Accuracy | 96.95% | 97.34% |
| All Lesions | |||||
| PET/ldCT | PET/ceCT | PET/ldCT | PET/ceCT | ||
| TP | 455 | 474 | Sensitivity | 98.06% | 98.96% |
| TN | 167 | 169 | Specificity | 93.30% | 95.48% |
| FP | 12 | 8 | PPV | 97.43% | 98.34% |
| FN | 9 | 5 | NPV | 94.89% | 97.13% |
| Total | 642 | 656 | Accuracy | 96.88% | 98.02% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massollo, M.; Fiz, F.; Bottoni, G.; Ugolini, M.; Paparo, F.; Puppo, C.; Provinciali, N.; Iacozzi, M.; Altrinetti, V.; Cistaro, A.; et al. To Enhance or Not to Enhance? The Role of Contrast Medium 18F-FDG PET/CT in Recurrent Ovarian Carcinomas. Medicina 2021, 57, 561. https://doi.org/10.3390/medicina57060561
Massollo M, Fiz F, Bottoni G, Ugolini M, Paparo F, Puppo C, Provinciali N, Iacozzi M, Altrinetti V, Cistaro A, et al. To Enhance or Not to Enhance? The Role of Contrast Medium 18F-FDG PET/CT in Recurrent Ovarian Carcinomas. Medicina. 2021; 57(6):561. https://doi.org/10.3390/medicina57060561
Chicago/Turabian StyleMassollo, Michela, Francesco Fiz, Gianluca Bottoni, Martina Ugolini, Francesco Paparo, Cristina Puppo, Nicoletta Provinciali, Massimiliano Iacozzi, Vania Altrinetti, Angelina Cistaro, and et al. 2021. "To Enhance or Not to Enhance? The Role of Contrast Medium 18F-FDG PET/CT in Recurrent Ovarian Carcinomas" Medicina 57, no. 6: 561. https://doi.org/10.3390/medicina57060561
APA StyleMassollo, M., Fiz, F., Bottoni, G., Ugolini, M., Paparo, F., Puppo, C., Provinciali, N., Iacozzi, M., Altrinetti, V., Cistaro, A., Cabria, M., DeCensi, A., Treglia, G., & Piccardo, A. (2021). To Enhance or Not to Enhance? The Role of Contrast Medium 18F-FDG PET/CT in Recurrent Ovarian Carcinomas. Medicina, 57(6), 561. https://doi.org/10.3390/medicina57060561








