Diagnosis of Cardiac Abnormalities in Muscular Dystrophies
Abstract
1. Introduction
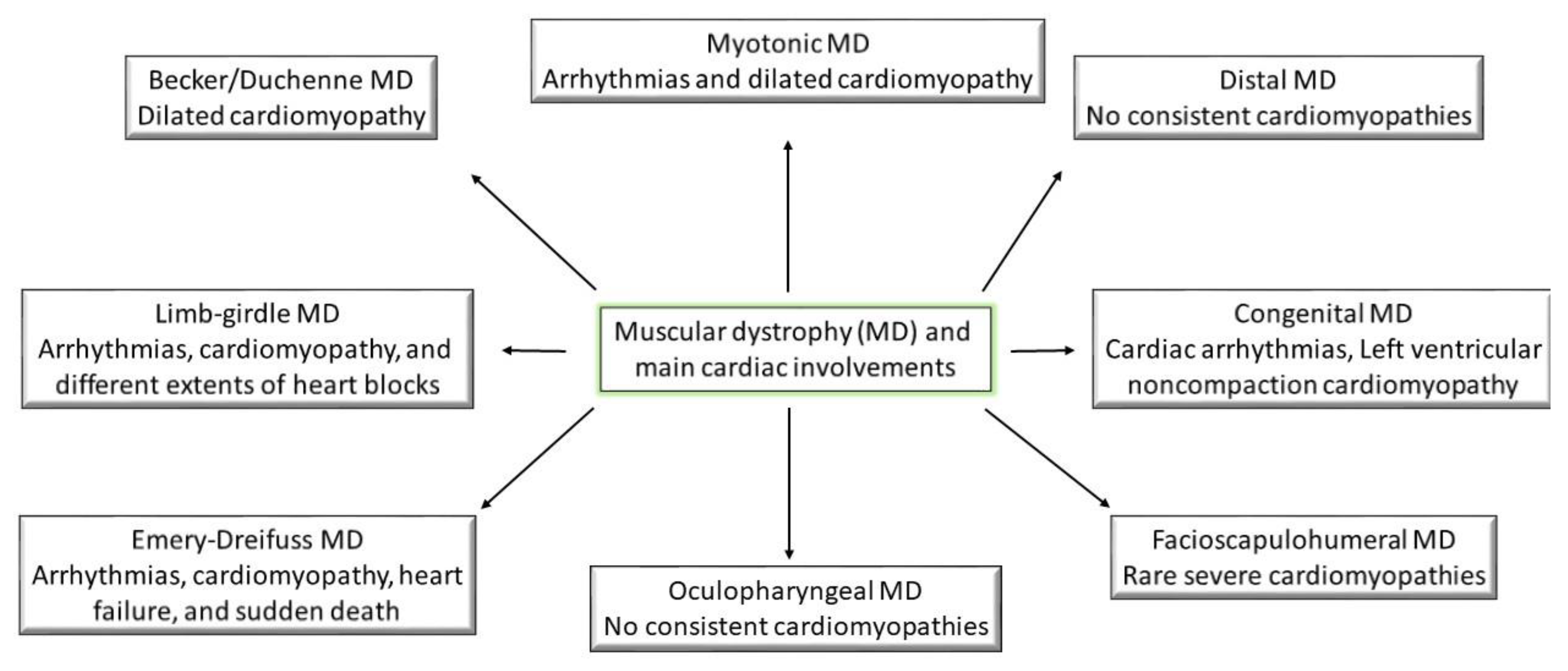
2. Cardiac Abnormalities
2.1. Cardiomyopathies
2.1.1. Dilated Cardiomyopathy (DCM)
2.1.2. Hypertrophic Cardiomyopathy (HCM)
2.1.3. Restrictive Cardiomyopathy (RCM)
2.1.4. Left Ventricular Non-Compaction Cardiomyopathy (LVNC)
2.2. Takotsubo Syndrome (TTS)
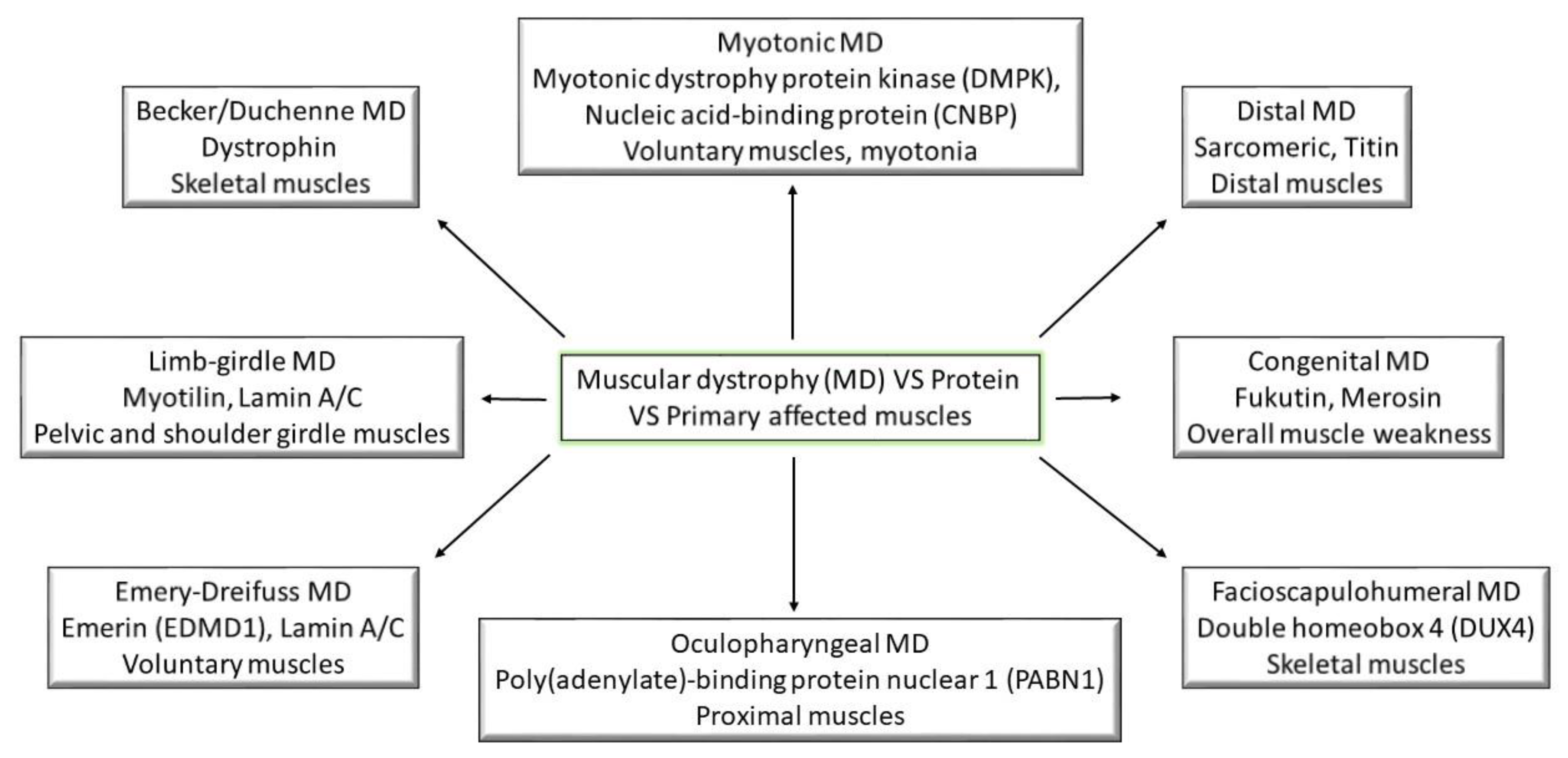
3. Classification and Pathogenesis of Muscular Dystrophies
3.1. Becker and Duchenne Muscular Dystrophy
3.2. Congenital Muscular Dystrophy
3.3. Distal Muscle Dystrophy
3.4. Emery–Dreifuss Muscular Dystrophy
3.5. Facioscapulohumeral Muscular Dystrophy
3.6. Limb–Girdle Muscular Dystrophy
3.7. Myotonic Muscular Dystrophy
3.8. Oculopharyngeal Muscular Dystrophy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozsarlak, O.; Schepens, E.; Parizel, P.M.; Van Goethem, J.W.; Vanhoenacker, F.; De Schepper, A.M.; Martin, J.J. Hereditary Neuromuscular Diseases. Eur. J. Radiol. 2001, 40, 184–197. [Google Scholar] [CrossRef]
- Hermans, M.C.; Pinto, Y.M.; Merkies, I.S.; de Die-Smulders, C.E.; Crijns, H.J.; Faber, C.G. Hereditary Muscular Dystrophies and the Heart. Neuromuscul. Disord. NMD 2010, 20, 479–492. [Google Scholar] [CrossRef]
- Golmohammadi, M.G.; Shahbazi, A.; Asl, M.M.C.; Banaei, S. Calcitriol and Erythropoietin Protect Against Cardiac Injury Induced by Renal Ischemia-Reperfusion. Biointerface Res. Appl. Chem. 2020, 10, 6718–6727. [Google Scholar] [CrossRef]
- Allen, H.D.; Thrush, P.T.; Hoffman, T.M.; Flanigan, K.M.; Mendell, J.R. Cardiac Management in Neuromuscular Diseases. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.; Mahle, W.T.; Auerbach, S.; Clemens, P.; Domenighetti, A.A.; Jefferies, J.L.; Judge, D.P.; Lal, A.K.; Markham, L.W.; Parks, W.J.; et al. Management of Cardiac Involvement Associated with Neuromuscular Diseases: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e200–e231. [Google Scholar] [CrossRef]
- McNally Elizabeth, M.; Mestroni, L. Dilated Cardiomyopathy. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- Bhandari, B.; Quintanilla Rodriguez, B.S.; Masood, W. Ischemic Cardiomyopathy. In StatPearls; Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated Cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef]
- Marian Ali, J.; Braunwald, E. Hypertrophic Cardiomyopathy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Blauwet Lori, A.; Gertz Morie, A. Restrictive Cardiomyopathy. Circ. Res. 2017, 121, 819–837. [Google Scholar] [CrossRef]
- Towbin, J.A.; Ballweg, J.; Johnson, J. Chapter 20–Left Ventricular Noncompaction Cardiomyopathy. In Heart Failure in the Child and Young Adult; Jefferies, J.L., Chang, A.C., Rossano, J.W., Shaddy, R.E., Towbin, J.A., Eds.; Academic Press: Boston, MA, USA, 2018. [Google Scholar]
- Pelliccia, F.; Kaski Juan, C.; Crea, F.; Camici Paolo, G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. Happy Heart Syndrome: Role of Positive Emotional Stress in Takotsubo Syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, K.M. Duchenne and Becker Muscular Dystrophies. Neurol. Clin. 2014, 32, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Coote, D.; Davis, M.R.; Cabrera, M.; Needham, M.; Laing, N.G.; Nowak, K.J. Clinical Utility Gene Card for: Becker Muscular Dystrophy. Eur. J. Hum. Genet. 2018, 26, 1065–1071. [Google Scholar] [CrossRef]
- Das, U.; Joardar, A.; Roy, S.; Guin, D.; Biswas, A.; Gangopadhya, G. A Correlation of Genetic and Clinical Perspective of Becker’s Muscular Dystrophy. Res. Rev. J. Neurosci. 2019, 9, 33–37. [Google Scholar]
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; de Kock, S.; Butt, T.; Jain, M.; Kleijnen, J. The Burden, Epidemiology, Costs and Treatment for Duchenne Muscular Dystrophy: An Evidence Review. Orphanet J. Rare Dis. 2017, 12, 79. [Google Scholar] [CrossRef]
- McCaffrey, T.; Guglieri, M.; Murphy, A.P.; Bushby, K.; Johnson, A.; Bourke, J.P. Cardiac Involvement in Female Carriers of Duchenne or Becker Muscular Dystrophy. Muscle Nerve 2017, 55, 810–818. [Google Scholar] [CrossRef]
- Mori, K.; Hayabuchi, Y.; Inoue, M.; Suzuki, M.; Sakata, M.; Nakagawa, R.; Kagami, S.; Tatara, K.; Hirayama, Y.; Abe, Y. Myocardial Strain Imaging for Early Detection of Cardiac Involvement in Patients with Duchenne’s Progressive Muscular Dystrophy. Echocardiography 2007, 24, 598–608. [Google Scholar] [CrossRef]
- Brunklaus, A.; Parish, E.; Muntoni, F.; Scuplak, S.; Tucker, S.K.; Fenton, M.; Hughes, M.L.; Manzur, A.Y. The Value of Cardiac MRI vs. Echocardiography in the Pre-operative Assessment of Patients with Duchenne Muscular Dystrophy. Eur. J. Paediatr. Neurol. 2015, 19, 395–401. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Markousis-Mavrogenis, G.; Papavasiliou, A.; Papadopoulos, G.; Kolovou, G. Cardiac Involvement in Duchenne Muscular Dystrophy and Related Dystrophinopathies. In Duchenne Muscular Dystrophy: Methods and Protocols; Bernardini, C., Ed.; Springer: New York, NY, USA, 2018; pp. 31–42. [Google Scholar]
- Silvestri, N.J.; Ismail, H.; Zimetbaum, P.; Raynor, E.M. Cardiac Involvement in the Muscular Dystrophies. Muscle Nerve 2018, 57, 707–715. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Respiratory, Cardiac, Bone Health, and Orthopaedic Management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Yamamoto, T.; Awano, H.; Zhang, Z.; Sakuma, M.; Kitaaki, S.; Matsumoto, M.; Nagai, M.; Sato, I.; Imanishi, T.; Hayashi, N.; et al. Cardiac Dysfunction in Duchenne Muscular Dystrophy Is Less Frequent in Patients with Mutations in the Dystrophin Dp116 Coding Region Than in Other Regions. Circ. Genom. Precis. Med. 2018, 11, e001782. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stöllberger, C. Cardiac Involvement in Becker Muscular Dystrophy. Can. J. Cardiol. 2008, 24, 786–792. [Google Scholar] [CrossRef]
- Bertini, E.; D’Amico, A.; Gualandi, F.; Petrini, S. Congenital Muscular Dystrophies: A Brief Review. Semin. Pediatric Neurol. 2011, 18, 277–288. [Google Scholar] [CrossRef]
- Butterfield, R.J. Congenital Muscular Dystrophy and Congenital Myopathy. Continuum 2019, 25, 1640–1661. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Ramaciotti, C.; Wang, C.H.; Wahbi, K.; Rosenthal, D.; Duboc, D.; Melacini, P. Cardiac Findings in Congenital Muscular Dystrophies. Pediatrics 2010, 126, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Taniguchi-Ikeda, M.; Awano, H.; Matsumoto, M.; Lee, T.; Harada, R.; Imanishi, T.; Hayashi, N.; Sakai, Y.; Morioka, I.; et al. Cardiac Involvement in Fukuyama Muscular Dystrophy is Less Severe Than in Duchenne Muscular Dystrophy. Brain Dev. 2017, 39, 861–868. [Google Scholar] [CrossRef]
- Pane, M.; Messina, S.; Vasco, G.; Foley, A.R.; Morandi, L.; Pegoraro, E.; Mongini, T.; D’Amico, A.; Bianco, F.; Lombardo, M.E.; et al. Respiratory and Cardiac Function in Congenital Muscular Dystrophies with Alpha Dystroglycan Deficiency. Neuromuscul. Disord. 2012, 22, 685–689. [Google Scholar] [CrossRef]
- Pasqualin, L.M.; Reed, U.C.; Costa, T.V.; Quedas, E.; Albuquerque, M.A.; Resende, M.B.; Rutkowski, A.; Chadi, G.; Zanoteli, E. Congenital Muscular Dystrophy with Dropped Head Linked to the LMNA Gene in a Brazilian Cohort. Pediatric Neurol. 2014, 50, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Dabaj, I.; Mah, J.K.; Bergounioux, J.; Essid, A.; Bönnemann, C.G.; Rutkowski, A.; Bonne, G.; Quijano-Roy, S.; Wahbi, K. Cardiac Manifestations of Congenital LMNA-related Muscular Dystrophy in Children: Three Case Reports and Recommendations for Care. Cardiol. Young 2016, 27, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Hawkins, C.; Wilson, G.; Yoon, G.; Mertens, L.; Carter, M.T.; Guerin, A. Noncompaction Cardiomyopathy in an Infant with Walker–Warburg Syndrome. Am. J. Med Genet. Part A 2017, 173, 3082–3086. [Google Scholar] [CrossRef]
- Udd, B. Distal Muscular Dystrophies. Handb. Clin. Neurol. 2011, 101, 239–262. [Google Scholar] [CrossRef]
- Dimachkie, M.M.; Barohn, R.J. Distal Myopathies. Neurol. Clin. 2014, 32. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.R.; Bagshaw, J.; Aksmanovic, V.M.; Coomber, E.; McMahon, R.; Whittaker, J.L.; Morrison, P.J.; Kendrick-Jones, J.; Ellis, J.A. Genotype-phenotype analysis in X-linked Emery-Dreifuss muscular dystrophy and identification of a missense mutation associated with a milder phenotype. Neuromuscul. Disord. NMD 1999, 9, 159–165. [Google Scholar] [CrossRef]
- Brisset, M.; Ben Yaou, R.; Carlier, R.-Y.; Chanut, A.; Nicolas, G.; Romero, N.B.; Wahbi, K.; Decrocq, C.; Leturcq, F.; Laforêt, P.; et al. X-linked Emery–Dreifuss Muscular Dystrophy Manifesting with Adult Onset Axial weakness, Camptocormia, and Minimal Joint Contractures. Neuromuscul. Disord. 2019, 29, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, D. Cardiac Involvement in Emery-Dreifuss Muscular Dystrophy and Related Management Strategies. Int. Heart J. 2019, 60, 12–18. [Google Scholar] [CrossRef]
- Boriani, G.; Gallina, M.; Merlini, L.; Bonne, G.; Toniolo, D.; Amati, S.; Biffi, M.; Martignani, C.; Frabetti, L.; Bonvicini, M.; et al. Clinical Relevance of Atrial Fibrillation/Flutter, Stroke, Pacemaker Implant, and Heart Failure in Emery-Dreifuss Muscular Dystrophy: A Long-term Longitudinal Study. Stroke 2003, 34, 901–908. [Google Scholar] [CrossRef]
- Marchel, M.; Madej-Pilarczyk, A.; Tymińska, A.; Steckiewicz, R.; Ostrowska, E.; Wysińska, J.; Russo, V.; Grabowski, M.; Opolski, G. Cardiac Arrhythmias in Muscular Dystrophies Associated with Emerinopathy and Laminopathy: A Cohort Study. J. Clin. Med. 2021, 10, 732. [Google Scholar] [CrossRef]
- Vignier, N.; Mougenot, N.; Bonne, G.; Muchir, A. Effect of Genetic Background on the Cardiac Phenotype in a Mouse Model of Emery-Dreifuss Muscular Dystrophy. Biochem. Biophys. Rep. 2019, 19, 100664. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.A.; Shih, R.; Kalra, R.; Kang, P.B. Emery-Dreifuss Muscular Dystrophy. Muscle Nerve 2020, 61, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.H.; Andersen, C.B.; Tybjaerg-Hansen, A.; Haunso, S.; Svendsen, J.H. Mutation Analysis and Evaluation of the Cardiac Localization of TMEM43 in Arrhythmogenic Right Ventricular Cardiomyopathy. Clin. Genet. 2011, 80, 256–264. [Google Scholar] [CrossRef]
- Tawil, R. Chapter 35–Facioscapulohumeral Muscular Dystrophy. In Handbook of Clinical Neurology; Geschwind, D.H., Paulson, H.L., Klein, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 148, pp. 541–548. [Google Scholar]
- Guien, C.; Blandin, G.; Lahaut, P.; Sanson, B.; Nehal, K.; Rabarimeriarijaona, S.; Bernard, R.; Lévy, N.; Sacconi, S.; Béroud, C. The French National Registry of Patients with Facioscapulohumeral Muscular Dystrophy. Orphanet J. Rare Dis. 2018, 13, 218. [Google Scholar] [CrossRef]
- Labombarda, F.; Maurice, M.; Simon, J.P.; Legallois, D.; Guyant-Marechal, L.; Bedat-Millet, A.L.; Merle, P.; Saloux, E.; Chapon, F.; Milliez, P. Cardiac Abnormalities in Type 1 Facioscapulohumeral Muscular Dystrophy. J. Clin. Neuromuscul. Dis. 2017, 18, 199–206. [Google Scholar] [CrossRef]
- Domingos, J.; Sarkozy, A.; Scoto, M.; Muntoni, F. Dystrophinopathies and Limb-Girdle Muscular Dystrophies. Neuropediatrics 2017, 48, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Groh, W.J. 100–Arrhythmias in Patients with Neurologic Disorders. In Cardiac Electrophysiology: From Cell to Bedside, 6th ed.; Zipes, D.P., Jalife, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 993–999. [Google Scholar]
- Sveen, M.-L.; Thune, J.J.; Køber, L.; Vissing, J. Cardiac Involvement in Patients with Limb-Girdle Muscular Dystrophy Type 2 and Becker Muscular Dystrophy. Arch. Neurol. 2008, 65, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Ki, C.S.; Kim, J.W.; Suh, Y.L.; Kim, J.S.; Baek, K.K.; Kim, B.J.; Ahn, K.J.; Kim, D.K. Cardiac Dysrhythmias, Cardiomyopathy and Muscular Dystrophy in Patients with Emery-Dreifuss Muscular Dystrophy and Limb-girdle Muscular Dystrophy Type 1B. J. Korean Med Sci. 2005, 20, 283–290. [Google Scholar] [CrossRef][Green Version]
- Rosales, X.Q.; Moser, S.J.; Tran, T.; McCarthy, B.; Dunn, N.; Habib, P.; Simonetti, O.P.; Mendell, J.R.; Raman, S.V. Cardiovascular Magnetic Resonance of Cardiomyopathy in Limb Girdle Muscular Dystrophy 2B and 2I. J. Cardiovasc. Magn. Reson. 2011, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pérez, J.; González-Quereda, L.; Bello, L.; Guglieri, M.; Straub, V.; Gallano, P.; Semplicini, C.; Pegoraro, E.; Zangaro, V.; Nascimento, A.; et al. New Genotype-phenotype Correlations in a Large European Cohort of Patients with Sarcoglycanopathy. Brain J. Neurol. 2020, 143, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Pratte, A.; Prévost, C.; Puymirat, J.; Mathieu, J. Anticipation in Myotonic Dystrophy Type 1 Parents with Small CTG Expansions. Am. J. Med Genet. Part A 2015, 167, 708–714. [Google Scholar] [CrossRef]
- Meola, G.; Cardani, R. Myotonic Dystrophy Type 2 and Modifier Genes: An Update on Clinical and Pathomolecular Aspects. Neurol. Sci. 2017, 38, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A.; Wang, E.; Carrell, E.M. Myotonic Dystrophy: Approach to Therapy. Curr. Opin. Genet. Dev. 2017, 44, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wahbi, K.; Furling, D. Cardiovascular Manifestations of Myotonic Dystrophy. Trends Cardiovasc. Med. 2019. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Gialafos, E.; Gatzonis, S.; Papadopoulos, G.; Kolovou, G. Muscular Dystrophies and the Heart: The Emerging Role of Cardiovascular Magnetic Resonance Imaging. Curr. Res. Cardiol. 2015, 2, 53–62. [Google Scholar] [CrossRef]
- Cardona, A.; Arnold, W.D.; Kissel, J.T.; Raman, S.V.; Zareba, K.M. Myocardial Fibrosis by Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Myotonic Muscular Dystrophy Type 1: Highly Prevalent but not Associated with Surface Conduction Abnormality. J. Cardiovasc. Magn. Reson. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Abdul Razak, K.; Hawkins, A.; Khoo, T.; Burrage, M.; Challa, V.; Atherton, J.; Jackson, R. Early Detection of Cardiac Structural and Functional Abnormalities in Adult Myotonic Dystrophy Type 1 Patients Using Advanced Cardiac Magnetic Resonance Imaging. Heart Lung Circ. 2018, 27, S236. [Google Scholar] [CrossRef]
- Khadilkar, S.V.; Yadav, R.S.; Patel, B.A. Oculopharyngeal Muscular Dystrophy. In Neuromuscular Disorders: A Comprehensive Review with Illustrative Cases; Khadilkar, S.V., Yadav, R.S., Patel, B.A., Eds.; Springer: Singapore, 2018; pp. 189–194. [Google Scholar]
- Goyal, N.A.; Mozaffar, T.; Chui, L.A. Oculopharyngeal Muscular Dystrophy, an Often Misdiagnosed Neuromuscular Disorder: A Southern California Experience. J. Clin. Neuromuscul. Dis. 2019, 21, 61–68. [Google Scholar] [CrossRef]
- Silva, M.C.; Magalhães, T.A.; Meira, Z.M.A.; Rassi, C.H.R.E.; Andrade, A.C.d.S.; Gutierrez, P.S.; Azevedo, C.F.; Gurgel-Giannetti, J.; Vainzof, M.; Zatz, M.; et al. Myocardial Fibrosis Progression in Duchenne and Becker Muscular Dystrophy: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 190–199. [Google Scholar] [CrossRef]
- Johnston, P.; Cripe, L.; Hor, K. Blinded Late Gadolinium Enhancement Quantification of Age Matched Adolescent and Young Adult Becker and Duchenne Muscular Dystrophy Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, A896. [Google Scholar] [CrossRef]
- Aikawa, T.; Takeda, A.; Oyama-Manabe, N.; Naya, M.; Yamazawa, H.; Koyanagawa, K.; Ito, Y.M.; Anzai, T. Progressive Left Ventricular Dysfunction and Myocardial Fibrosis in Duchenne and Becker Muscular Dystrophy: A Longitudinal Cardiovascular Magnetic Resonance Study. Pediatric Cardiol. 2019, 40, 384–392. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Giannakopoulou, A.; Papavasiliou, A.; Markousis-Mavrogenis, G.; Pons, R.; Karanasios, E.; Noutsias, M.; Kolovou, G.; Papadopoulos, G. Cardiac Profile of Asymptomatic Children with Becker and Duchenne Muscular Dystrophy under Treatment with Steroids and with/without Perindopril. BMC Cardiovasc. Disord. 2017, 17, 197. [Google Scholar] [CrossRef]
- Keller, H.; Neuhold, U.; Weidinger, F.; Gatterer, E.; Stöllberger, C.; Huber, K.; Finsterer, J. Takotsubo as Initial Manifestation of Non-Myopathic Cardiomyopathy Due to the Titin Variant c.1489G> T. Medicines 2018, 5, 80. [Google Scholar] [CrossRef]
- Savarese, M.; Palmio, J.; Poza, J.J.; Weinberg, J.; Olive, M.; Cobo, A.M.; Vihola, A.; Jonson, P.H.; Sarparanta, J.; García-Bragado, F.; et al. Actininopathy: A New Muscular Dystrophy Caused by ACTN2 Dominant Mutations. Ann. Neurol. 2019, 85, 899–906. [Google Scholar] [CrossRef]
- Ten Dam, L.; Frankhuizen, W.S.; Linssen, W.H.J.P.; Straathof, C.S.; Niks, E.H.; Faber, K.; Fock, A.; Kuks, J.B.; Brusse, E.; de Coo, R.; et al. Autosomal Recessive Limb-girdle and Miyoshi Muscular Dystrophies in the Netherlands: The Clinical and Molecular Spectrum of 244 Patients. Clin. Genet. 2019, 96, 126–133. [Google Scholar] [CrossRef]
- Roy, B.; Raynor, E. Cardioembolic Stroke in a 23-year-old Man with Elbow Contracture, an Unusual Presentation of Emery Dreifuss Muscular Dystrophy (EDMD) (P5.451). Neurology 2018, 90, P5.451. [Google Scholar] [CrossRef] [PubMed]
- Sandra, M.; Maria Pia, L.; Stefano, C.; Pietro, P.; Crociani, P.; Aldo, R.; Giuseppe, D.S.; Massimo, C. Emery-Dreifuss Muscular Dystrophy Type 4: A New SYNE1 Mutation Associated with Hypertrophic Cardiomyopathy Masked by a Perinatal Distress-related Spastic Diplegia. Clin. Case Rep. 2019, 7, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, D. Heart Failure, Atrial Standstill, and Non-sustained Ventricular Tachycardia Due to Emery-Dreiffus Muscular Dystrophy. J. Am. Coll. Cardiol. 2016, 67, 1247. [Google Scholar] [CrossRef]
- Sato, M.; Shirasawa, H.; Makino, K.; Miura, H.; Sato, W.; Shimizu, D.; Sato, N.; Kumagai, J.; Sato, A.; Terada, Y. Perinatal Management of Pregnancy Complicated by Autosomal Dominant Emery-Dreifuss Muscular Dystrophy. AJP Rep. 2016, 6, e145–e147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blaszczyk, E.; Grieben, U.; von Knobelsdorff-Brenkenhoff, F.; Kellman, P.; Schmacht, L.; Funk, S.; Spuler, S.; Schulz-Menger, J. Subclinical Myocardial Injury in Patients with Facioscapulohumeral Muscular Dystrophy 1 and Preserved Ejection Fraction–Assessment by Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Svahn, J.; Laforêt, P.; Vial, C.; Streichenberger, N.; Romero, N.; Bouchet-Séraphin, C.; Bruneel, A.; Dupré, T.; Seta, N.; Menassa, R.; et al. Dilated Cardiomyopathy and Limb-girdle Muscular Dystrophy-dystroglycanopathy Due to Novel Pathogenic Variants in the DPM3 Gene. Neuromuscul. Disord. 2019, 29, 497–502. [Google Scholar] [CrossRef]
- Miskew Nichols, B.; Nikhanj, A.; Wang, F.; Freed Darren, H.; Oudit Gavin, Y. Advanced Dilated Cardiomyopathy in a Patient with Hutterite Limb-Girdle Muscular Dystrophy. Circ. Heart Fail. 2018, 11, e004960. [Google Scholar] [CrossRef] [PubMed]
- Im, S.I.; Kim, E.J.; Kim, S.W. Abdominal Paradox Encountered in Neuromuscular Disease: A Possible Clue for Cor Pulmonale. J. Cardiol. Cases 2013, 7, e71–e73. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Type of MD | Cardiac Involvement | Imagistic Technique | No. Patients (Age of Patients) | Reference |
|---|---|---|---|---|
| Becker and Duchenne MD |
| Echocardiography | 130 (39 ± 15.7 years) | [19] |
| Cardiac magnetic resonance imaging | 76 (12.1 ± 2.7 years) | [63] | |
| Late-gadolinium enhanced cardiac magnetic imaging | 35 DMD/33 BMD(17.4 ± 6/18.3 ± 3.9 years) | [64] | |
| Cardiac magnetic resonance imaging | 34 (IQR, 6.4–15.9 years) | [65] | |
| Electrocardiography Echocardiography Cardiac magnetic resonance imaging | 34 (10.5 ± 1.5 years) | [66] | |
| Myocardial strain imaging | 25 (14.8 ± 3.1 years) | [20] | |
| Holter monitoring | N/A | [24] | |
| Congenital MD |
| Electrocardiography Holter monitor Cardiac magnetic resonance imaging | 3 case reports | [33] |
| Fetal echocardiography Electrocardiography Echocardiography | 1 (4 months) | [34] | |
| Distal MD |
| Electrocardiography Echocardiography Cardiac ventriculography Cardiac magnetic resonance imaging | 1 case report | [67] |
| Echocardiography Holter monitor Magnetic resonance imaging | 18 | [68] | |
| Cardiac ultrasound Electrocardiography | 244 (5–74 years) | [69] | |
| Emery–Dreifuss MD |
| Electrocardiography Echocardiography Cardiac magnetic resonance imaging | 1 (23 years) | [70] |
| Electrocardiography Echocardiography | 1 (18 years) | [71] | |
| Electrocardiography Echocardiography | 1 case report | [72] | |
| Electrocardiography Echocardiography Chest X-ray | 1 (36 years) | [73] | |
| Facioscapulohumeral MD |
| Electrocardiography Holter monitor Echocardiography | 56 (21–86 years) | [47] |
| Electrocardiography Holter monitor Cardiac magnetic resonance imaging | 52 (48 ± 15 years) | [74] | |
| Limb–Girdle MD |
| Electrocardiography Echocardiography Holter monitor Cardiac magnetic resonance imaging | 2 | [75] |
| Electrocardiography Transthoracic echocardiography Cardiac magnetic resonance imaging | 1 (39 years) | [76] | |
| Computer tomography | 181 (10.1 ± 4.6 years) | [25] | |
| Transthoracic echocardiography | N/A | [26] | |
| Myotonic MD |
| Cardiac magnetic resonance imaging Electrocardiography Holter monitor | N/A | [29] |
| Cardiac magnetic resonance imaging Transthoracic echocardiography | 115(1–68 years) | [31] | |
| Cardiac magnetic resonance imaging Electrocardiography | N/A | [42] | |
| Late-gadolinium enhanced cardiac magnetic imaging Electrocardiography | 52 (41 ± 14 years) | [59] | |
| Oculopharyngeal MD |
| Transthoracic echocardiography Electrocardiography | 1 (35 years) | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bădilă, E.; Lungu, I.I.; Grumezescu, A.M.; Scafa Udriște, A. Diagnosis of Cardiac Abnormalities in Muscular Dystrophies. Medicina 2021, 57, 488. https://doi.org/10.3390/medicina57050488
Bădilă E, Lungu II, Grumezescu AM, Scafa Udriște A. Diagnosis of Cardiac Abnormalities in Muscular Dystrophies. Medicina. 2021; 57(5):488. https://doi.org/10.3390/medicina57050488
Chicago/Turabian StyleBădilă, Elisabeta, Iulia Ioana Lungu, Alexandru Mihai Grumezescu, and Alexandru Scafa Udriște. 2021. "Diagnosis of Cardiac Abnormalities in Muscular Dystrophies" Medicina 57, no. 5: 488. https://doi.org/10.3390/medicina57050488
APA StyleBădilă, E., Lungu, I. I., Grumezescu, A. M., & Scafa Udriște, A. (2021). Diagnosis of Cardiac Abnormalities in Muscular Dystrophies. Medicina, 57(5), 488. https://doi.org/10.3390/medicina57050488







