Leriche Syndrome Misdiagnosed as Complex Regional Pain Syndrome in a Patient with Neuropathic Pain Caused by a Chip Fracture: A Case Report
Abstract
1. Introduction
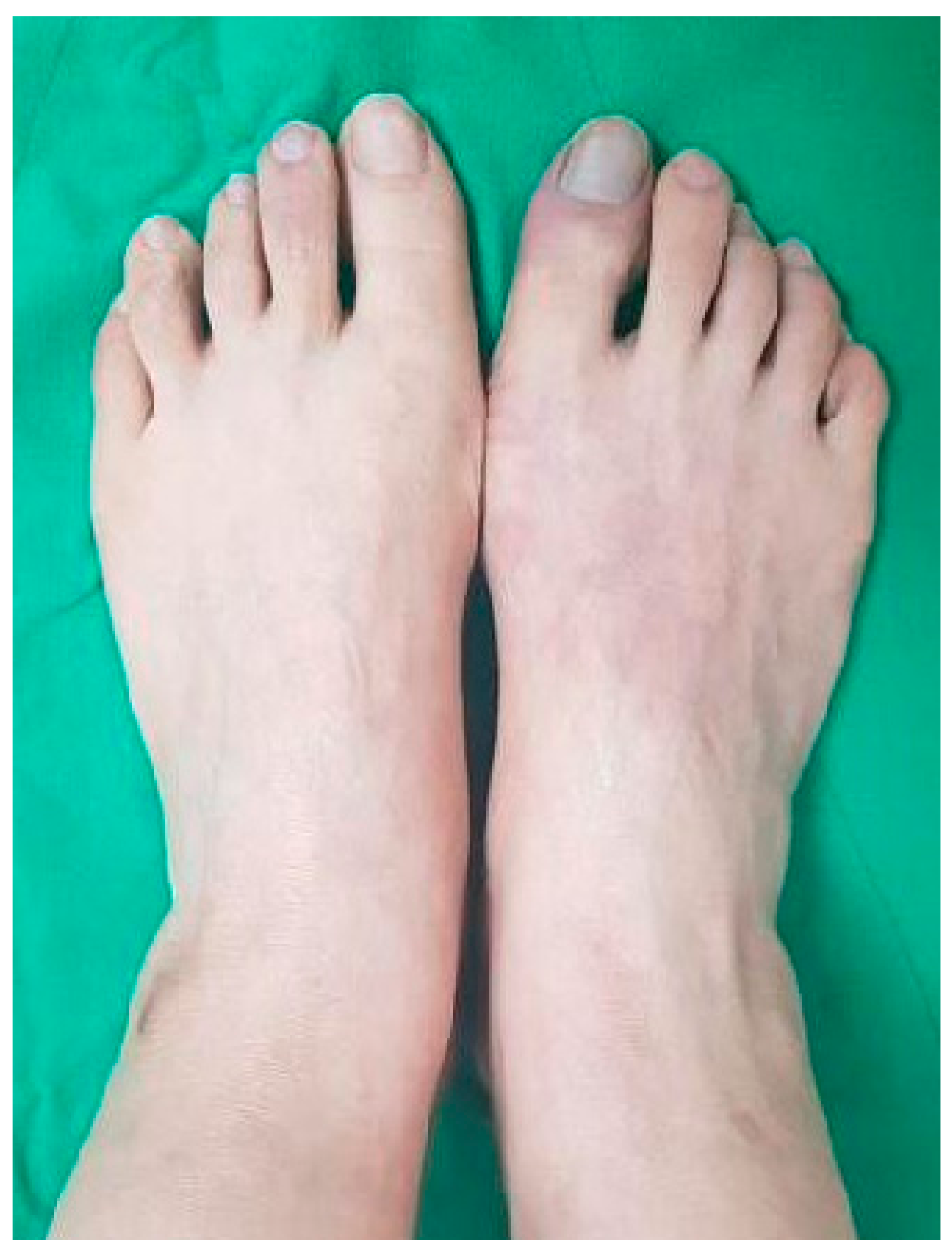
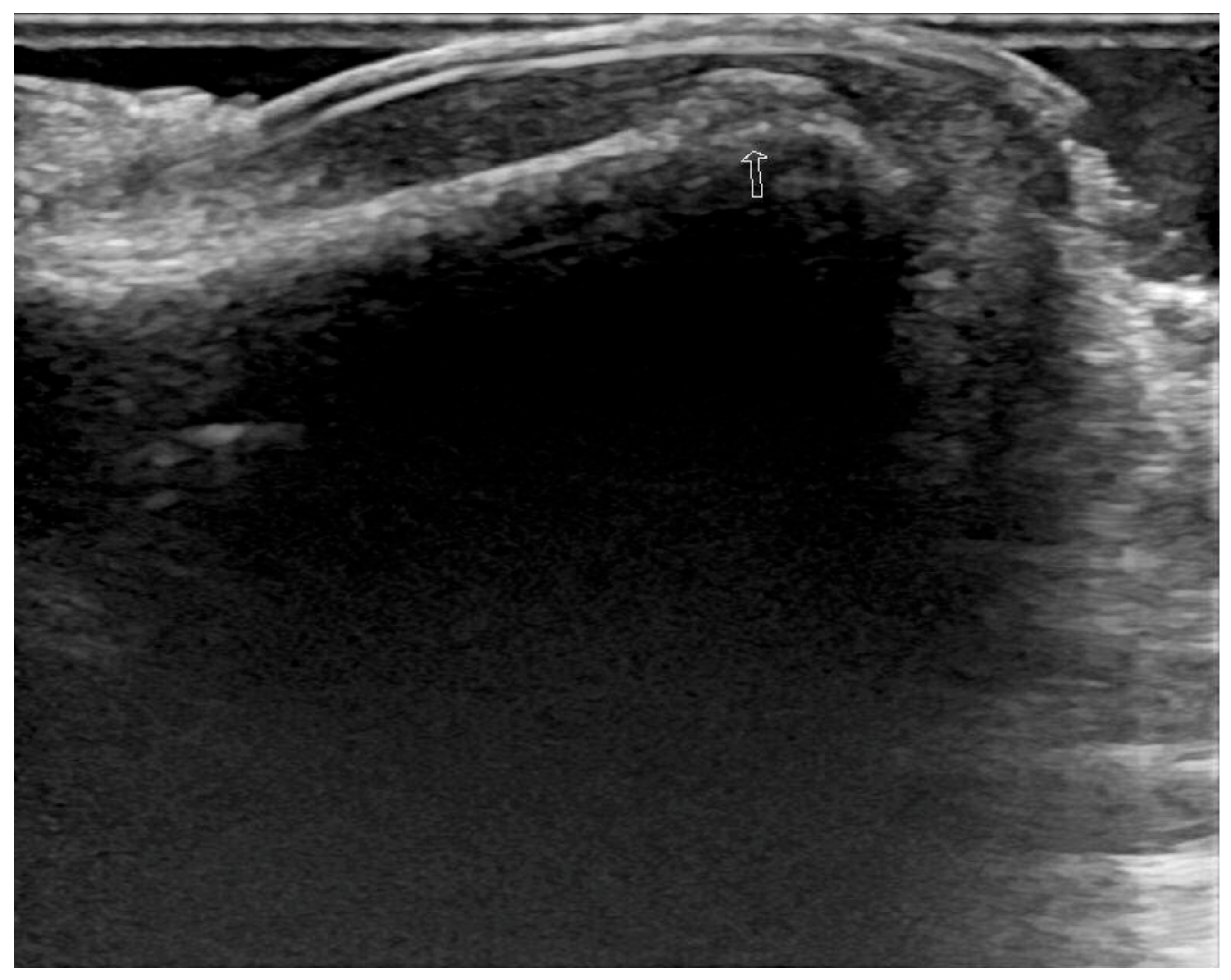
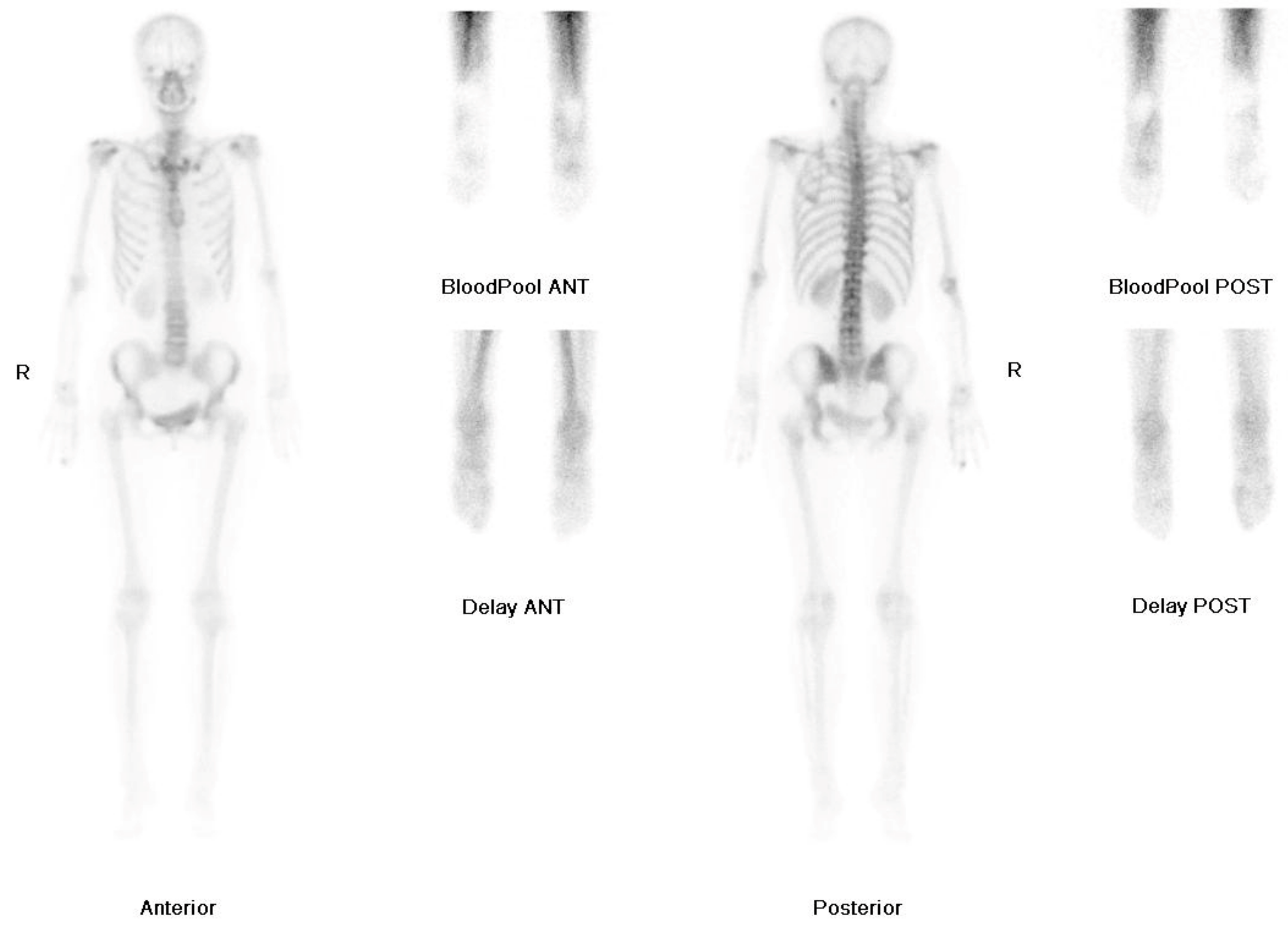
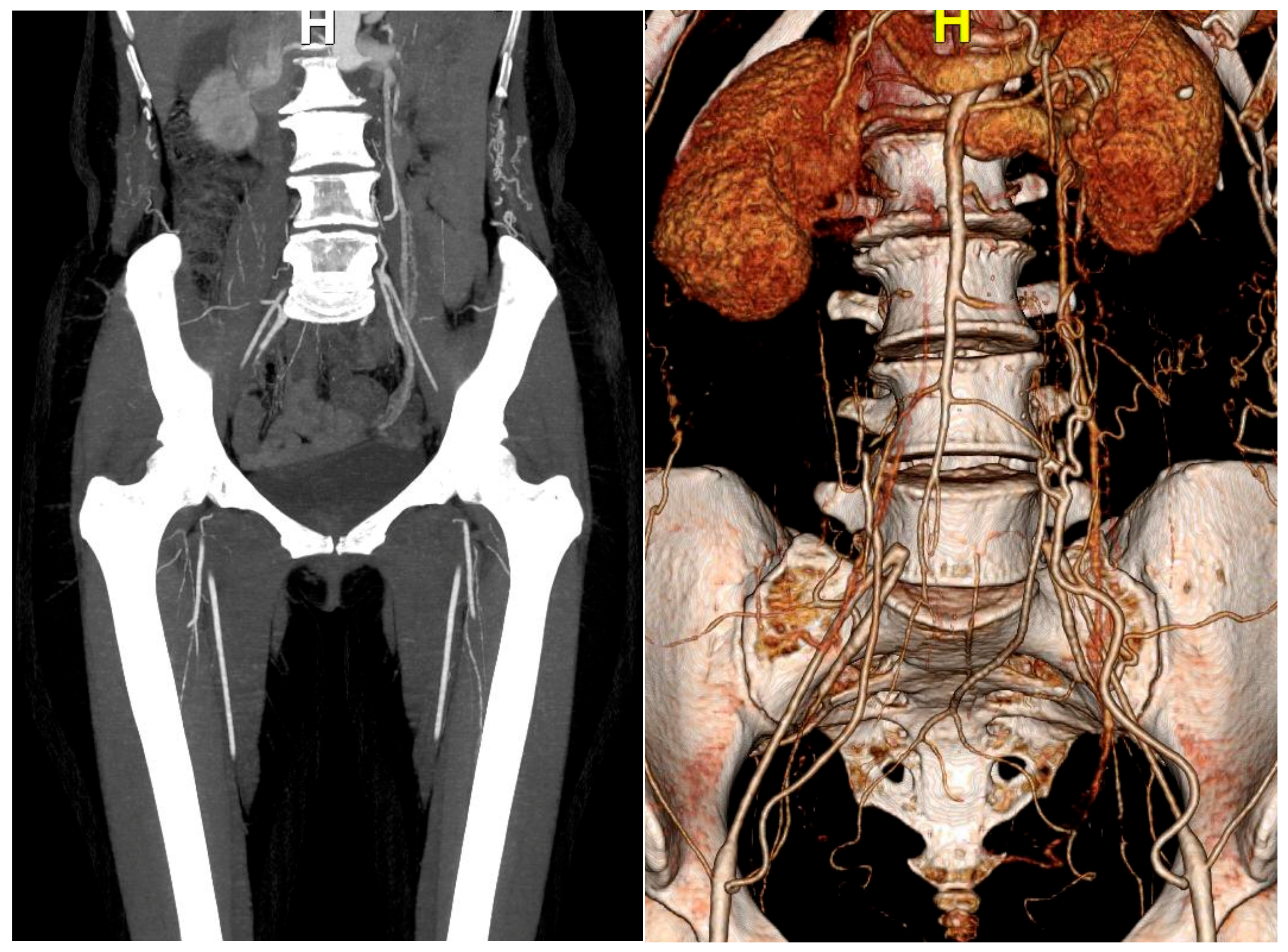
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.-J.; Cheng, Y.-Z.; Lin, H.-J. Leriche syndrome. Int. J. Emerg. Med. 2008, 1, 223. [Google Scholar] [CrossRef] [PubMed]
- Frederick, M.; Newman, J.; Kohlwes, J. Leriche syndrome. J. Gen. Intern. Med. 2010, 25, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Leriche, R.; Morel, A. The syndrome of thrombotic obliteration of the aortic bifurcation. Ann. Surg. 1948, 127, 193. [Google Scholar] [CrossRef] [PubMed]
- Kullo, I.J.; Rooke, T.W. Peripheral Artery Disease. N. Engl. J. Med. 2016, 374, 861–871. [Google Scholar] [CrossRef]
- Schorr, E.N.; Treat-Jacobson, D. Methods of symptom evaluation and their impact on peripheral artery disease (PAD) symptom prevalence: A review. Vasc. Med. 2013, 18, 95–111. [Google Scholar] [CrossRef]
- Weinberg, D.H.; Simovic, D.; Isner, J.; Ropper, A.H. Chronic ischemic monomelic neuropathy from critical limb ischemia. Neurology 2001, 57, 1008. [Google Scholar] [CrossRef]
- Weber, F.; Ziegler, A. Axonal neuropathy in chronic peripheral arterial occlusive disease. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2002, 26, 471–476. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, E.S.; Hwang, H.K.; Lee, K.B.; Lee, S.; Jung, J.W.; Kwon, Y.J.; Cho, D.H.; Park, S.S.; Yoon, J.; et al. Prevalence and risk factors for the peripheral neuropathy in patients with peripheral arterial occlusive disease. Vasc. Spec. Int. 2014, 30, 125. [Google Scholar] [CrossRef]
- Coraci, D.; Giovannini, S.; Loreti, C.; Fusco, A.; Padua, L. Management of neuropathic pain: A graph theory-based presentation of literature review. Breast J. 2020, 26, 581–5822. [Google Scholar] [CrossRef]
- Giovannini, S.; Coraci, D.; Brau, F.; Galluzzo, V.; Loreti, C.; Caliandro, P.; Padua, L.; Maccauro, G.; Biscotti, L.; Bernabei, R. Neuropathic Pain in the Elderly. Diagnostics 2021, 11, 613. [Google Scholar] [CrossRef]
- Dillon, R.S. Patient Assessment and Examples Patient Assessment and Examples of a Method of Treatment. Angiology 1997, 48, S35–S58. [Google Scholar] [CrossRef]
- Groth, M.; Much, C.; Fiehler, J. Manifestation of Acute Leriche Syndrome as Cauda Equina Syndrome. Rofo 2013, 185, 375–376. [Google Scholar] [CrossRef]
- Do Hyun Yoon, H.C.; Seol, S.J.; Kim, T. Right calf claudication revealing leriche syndrome presenting as right sciatic neuropathy. Ann. Rehabil. Med. 2014, 38, 132. [Google Scholar] [CrossRef][Green Version]
- Carfì, A.; Liperoti, R.; Fusco, D.; Giovannini, S.; Brandi, V.; Vetrano, D.L.; Meloni, E.; Mascia, D.; Villani, E.R.; Manes Gravina, E.; et al. Bone mineral density in adults with Down syndrome. Osteoporos. Int. 2017, 28, 2929–2934. [Google Scholar] [CrossRef]
- England, J.D.; Ferguson, M.A.; Hiatt, W.R.; Regensteiner, J.G. Progression of neuropathy in peripheral arterial disease. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1995, 18, 380–387. [Google Scholar] [CrossRef]
- Rüger, L.J.; Irnich, D.; Abahji, T.N.; Crispin, A.; Hoffmann, U.; Lang, P.M. Characteristics of chronic ischemic pain in patients with peripheral arterial disease. Pain 2008, 139, 201–208. [Google Scholar] [CrossRef]
- Carter, S.A.; Tate, R.B. The value of toe pulse waves in determination of risks for limb amputation and death in patients with peripheral arterial disease and skin ulcers or gangrene. J. Vasc. Surg. 2001, 33, 708–714. [Google Scholar] [CrossRef]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Silva, M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Harden, R.N.; Bruehl, S.; Stanton-Hicks, M.; Wilson, P.R. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007, 8, 326–331. [Google Scholar] [CrossRef]
- Maihöfner, C.; Seifert, F.; Markovic, K. Complex regional pain syndromes: New pathophysiological concepts and therapies. Eur. J. Neurol. 2010, 17, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, S.J. Bone scintigraphy in patients with pain. Korean J. Pain 2017, 30, 165. [Google Scholar] [CrossRef]
- Lai, C.-H.; Wang, C.-H.; Wu, S.-Y.; Shih, H.-M. Aortoiliac occlusive disease presenting as sudden onset paraplegia. Ann. Vasc. Surg. 2014, 28, 1321.e5–1321.e7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-C.; Oh, D.-S.; Lee, H.-S.; Kim, S.-H.; Park, J.-H.; Lee, K.-H.; Kim, H.-J.; Yang, J.-H.; Lee, S.-E. Leriche Syndrome Misdiagnosed as Complex Regional Pain Syndrome in a Patient with Neuropathic Pain Caused by a Chip Fracture: A Case Report. Medicina 2021, 57, 486. https://doi.org/10.3390/medicina57050486
Lee B-C, Oh D-S, Lee H-S, Kim S-H, Park J-H, Lee K-H, Kim H-J, Yang J-H, Lee S-E. Leriche Syndrome Misdiagnosed as Complex Regional Pain Syndrome in a Patient with Neuropathic Pain Caused by a Chip Fracture: A Case Report. Medicina. 2021; 57(5):486. https://doi.org/10.3390/medicina57050486
Chicago/Turabian StyleLee, Byeong-Cheol, Dae-Seok Oh, Hyun-Seong Lee, Se-Hun Kim, Jae-Hong Park, Ki-Hwa Lee, Hyo-Joong Kim, Ji-Hyun Yang, and Sang-Eun Lee. 2021. "Leriche Syndrome Misdiagnosed as Complex Regional Pain Syndrome in a Patient with Neuropathic Pain Caused by a Chip Fracture: A Case Report" Medicina 57, no. 5: 486. https://doi.org/10.3390/medicina57050486
APA StyleLee, B.-C., Oh, D.-S., Lee, H.-S., Kim, S.-H., Park, J.-H., Lee, K.-H., Kim, H.-J., Yang, J.-H., & Lee, S.-E. (2021). Leriche Syndrome Misdiagnosed as Complex Regional Pain Syndrome in a Patient with Neuropathic Pain Caused by a Chip Fracture: A Case Report. Medicina, 57(5), 486. https://doi.org/10.3390/medicina57050486





