Pancreatic Disorders in Children with Inflammatory Bowel Disease
Abstract
1. Introduction
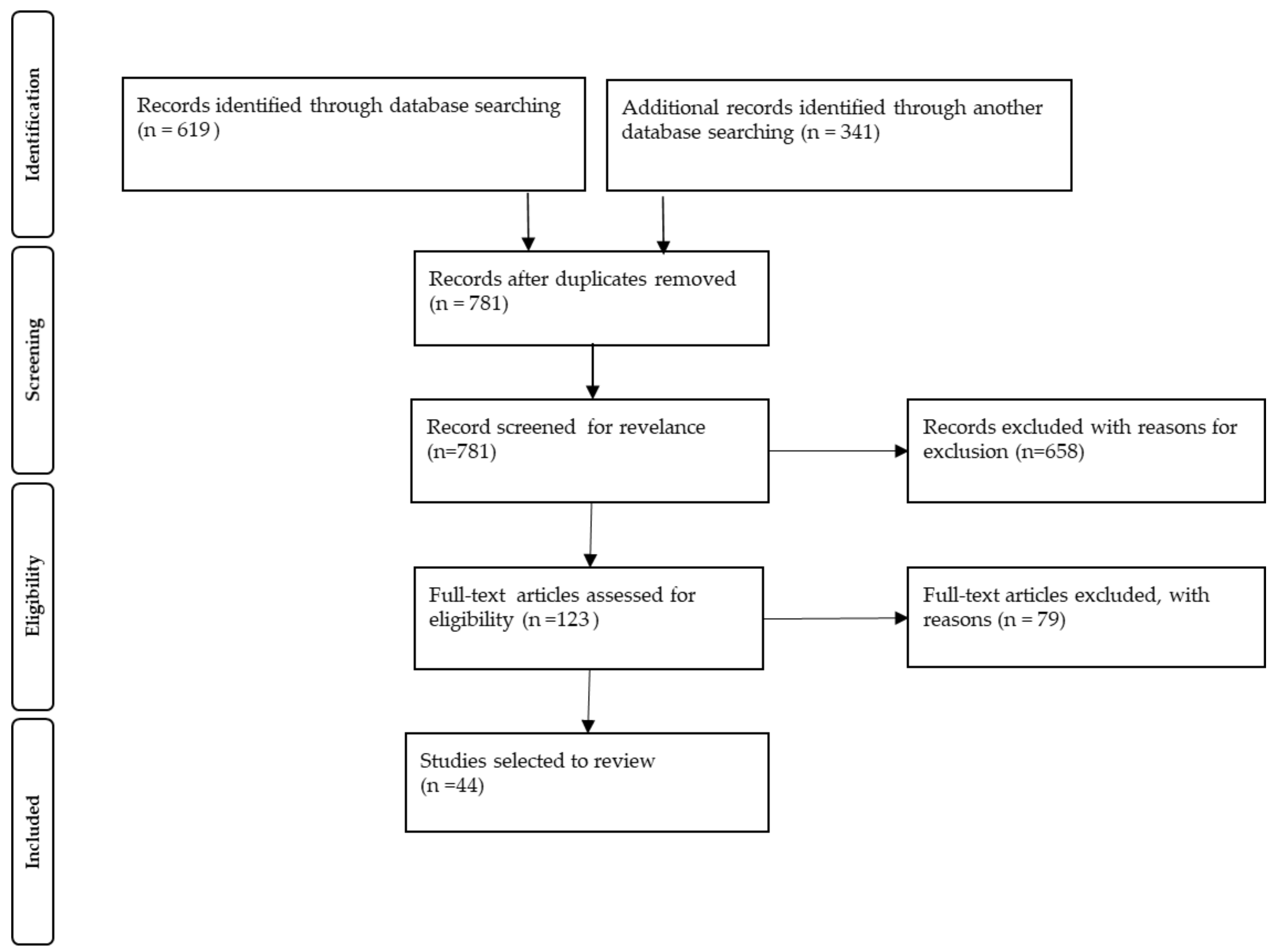
2. Materials and Methods
3. Results
3.1. Studies on Acute Pancreatitis (AP)
3.2. Studies on Chronic Pancreatitis (CP)
3.3. Studies Related to Asymptomatic Pancreatic Hyperenzynemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jose, F.A.; Garnett, E.A.; Vittinghoff, E.; Ferry, G.D.; Winter, H.S.; Baldassano, R.N.; Kirschner, B.S.; Cohen, S.A.; Gold, B.D.; Abramson, O.; et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Dotson, J.L.; Hyams, J.S.; Markowitz, J.; LeLeiko, N.S.; Mack, D.R.; Evans, J.S.; Pfefferkorn, M.D.; Griffiths, A.M.; Otley, A.R.; Bousvaros, A.; et al. Extraintestinal manifestations of pediatric inflammatory bowel disease and their relation to disease type and severity. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, C.E.; Heyman, M.B.; Lowe, M.E.; Pohl, J.F.; Werlin, S.L.; Wilschanski, M.; Barth, B.; Fishman, D.S.; Freedman, S.D.; Giefer, M.J.; et al. Causal Evaluation of Acute Recurrent and Chronic Pancreatitis in Children: Consensus From the INSPPIRE Group. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Párniczky, A.; Abu-El-Haija, M.; Husain, S.; Lowe, M.; Oracz, G.; Sahin-Tóth, M.; Szabó, F.K.; Uc, A.; Wilschanski, M.; Witt, H.; et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology 2018, 18, 146–160. [Google Scholar] [CrossRef]
- Antonini, F.; Pezzilli, R.; Angelelli, L.; Macarri, G. Pancreatic disorders in inflammatory bowel disease. World J. Gastrointest. Pathophysiol. 2016, 7, 276–282. [Google Scholar] [CrossRef]
- Stawarski, A.; Iwańczak, F. Incidence of acute pancreatitis in children with inflammatory bowel disease. Pol. Merkur. Lekarski. 2004, 17, 33–36. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Martinelli, M.; Strisciuglio, C.; Illiceto, M.T.; Cardile, S.; Guariso, G.; Vignola, S.; Aloi, M.; D’Altilia, M.R.; Alvisi, P.; Salvatore, S.; et al. Natural history of pancreatic involvement in paediatric inflammatory bowel disease. Dig. Liver Dis. 2015, 47, 384–389. [Google Scholar] [CrossRef]
- Weber, P.; Seibold, F.; Jenss, H. Acute pancreatitis in Crohn’s disease. J. Clin. Gastroenterol. 1993, 17, 286–291. [Google Scholar] [CrossRef]
- Broide, E.; Dotan, I.; Weiss, B.; Wilschanski, M.; Yerushalmi, B.; Klar, A.; Levine, A. Idiopathic pancreatitis preceding the diagnosis of inflammatory bowel disease is more frequent in pediatric patients. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 714–717. [Google Scholar] [CrossRef]
- Wintzell, V.; Svanström, H.; Olén, O.; Melbye, M.; Ludvigsson, J.F.; Pasternak, B. Association between use of azathioprine and risk of acute pancreatitis in children with inflammatory bowel disease: A Swedish-Danish nationwide cohort study. Lancet Child Adolesc. Health 2019, 3, 158–165. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Lamothe, S.; Yang, H.Y.; Targan, S.R.; Sinnett, D.; Théorêt, Y.; Seidman, E.G. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000, 118, 705–713. [Google Scholar] [CrossRef]
- Hindorf, U.; Lindqvist, M.; Hildebrand, H.; Fagerberg, U.; Almer, S. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 24, 331–342. [Google Scholar] [CrossRef]
- De Ridder, L.; Van Dieren, J.M.; Van Deventer, H.J.; Stokkers, P.C.; Van der Woude, J.C.; Van Vuuren, A.J.; Benninga, M.A.; Escher, J.C.; Hommes, D.W. Pharmacogenetics of thiopurine therapy in paediatric IBD patients. Aliment. Pharmacol. Ther. 2006, 23, 1137–1141. [Google Scholar] [CrossRef]
- Tajiri, H.; Tomomasa, T.; Yoden, A.; Konno, M.; Sasaki, M.; Maisawa, S.; Sumazaki, R.; Shimizu, T.; Toyoda, S.; Etani, Y.; et al. Efficacy and safety of azathioprine and 6-mercaptopurine in Japanese pediatric patients with ulcerative colitis: A survey of the Japanese Society for Pediatric Inflammatory Bowel Disease. Digestion 2008, 77, 150–154. [Google Scholar] [CrossRef]
- Cuffari, C.; Théorêt, Y.; Latour, S.; Seidman, G. 6-Mercaptopurine metabolism in Crohn’s disease: Correlation with efficacy and toxicity. Gut 1996, 39, 401–406. [Google Scholar] [CrossRef]
- Kirschner, B.S. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology 1998, 115, 813–821. [Google Scholar] [CrossRef]
- Keljo, D.J.; Sugerman, K.S. Pancreatitis in patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 108–112. [Google Scholar] [CrossRef]
- Bai, H.X.; Ma, M.H.; Orabi, A.I.; Park, A.; Latif, S.U.; Bhandari, V.; Husain, S.Z. Novel characterization of drug-associated pancreatitis in children. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 423–428. [Google Scholar] [CrossRef]
- Scheers, I.; Palermo, J.J.; Freedman, S.; Wilschanski, M.; Shah, U.; Abu-El-Haija, M.; Barth, B.; Fishman, D.S.; Gariepy, C.; Giefer, M.J.; et al. Autoimmune Pancreatitis in Children: Characteristic Features, Diagnosis, and Management. Am. J. Gastroenterol. 2017, 112, 1604–1611. [Google Scholar] [CrossRef]
- Ghersin, I.; Khateeb, N.; Katz, L.H.; Daher, S.; Shamir, R.; Assa, A. Comorbidities in adolescents with inflammatory bowel disease: Findings from a population-based cohort study. Pediatr. Res. 2020, 87, 1256–1262. [Google Scholar] [CrossRef]
- Gallego-Gutiérrez, S.; Navas-López, V.M.; Kolorz, M.; Bartosova, L.; Lukac, K.; Luque-Pérez, S.; Núñez-Caro, L.; García-Galán, P.; Fernández-Crehuet, F.G.; Blasco-Alonso, J.; et al. Successful Mercaptopurine Usage despite Azathioprine-Induced Pancreatitis in Paediatric Crohn’s Disease. J. Crohns Colitis 2015, 9, 676–679. [Google Scholar] [CrossRef]
- Yi, G.C.; Yoon, K.H.; Hwang, J.B. Acute Pancreatitis Induced by Azathioprine and 6-mercaptopurine Proven by Single and Low Dose Challenge Testing in a Child with Crohn Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2012, 15, 272–275. [Google Scholar] [CrossRef]
- Ledder, O.D.; Lemberg, D.A.; Ooi, C.Y.; Day, A.S. Are thiopurines always contraindicated after thiopurine-induced pancreatitis in inflammatory bowel disease? J. Pediatr. Gastroenterol. Nutr. 2013, 57, 583–586. [Google Scholar] [CrossRef]
- Mishra, S.; Garg, S.; Mahajan, R.; Patil, A.; Bhatia, P.; Sharma, V. Azathioprine induced pancreatitis, polyarthritis and panniculitis (PPP) syndrome in a patient with Crohn’s disease. Acta Gastroenterol. Belg. 2020, 83, 87–89. [Google Scholar]
- Abdullah, A.M.; Scott, R.B.; Martin, S.R. Acute pancreatitis secondary to 5-aminosalicylic acid in a child with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 441–444. [Google Scholar] [CrossRef]
- Paul, A.C.; Oommen, S.P.; Angami, S.; Moses, P.D. Acute pancreatitis in a child with idiopathic ulcerative colitis on long-term 5-aminosalicylic acid therapy. Indian J. Gastroenterol. 2000, 19, 195–196. [Google Scholar]
- Radke, M.; Bartolomaeus, G.; Müller, M.; Richter, I. Acute pancreatitis in Crohn’s disease due to 5-ASA therapy. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 337–339. [Google Scholar] [CrossRef]
- Garau, P.; Orenstein, S.R.; Neigut, D.A.; Kocoshis, S.A. Pancreatitis associated with olsalazine and sulfasalazine in children with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 481–485. [Google Scholar] [CrossRef]
- Paerregaard, A.; Krasilnikoff, P.A. Pancreatitis in a child after rectal administration of 5-aminosalicylic Acid. Inflamm. Bowel Dis. 1997, 3, 20–21. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, J.H.; Moon, K.R. Mesalizine-Induced Acute Pancreatitis and Interstitial Pneumonitis in a Patient with Ulcerative Colitis. Pediatr. Gastroenterol. Hepatol. Nutr. 2015, 18, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.N.; Gupta, N.; Lemberg, D.A. Vedolizumab-Associated Pancreatitis in Paediatric Ulcerative Colitis: Functional Selectivity of the α4β7integrin and MAdCAM-1 Pathway? J. Crohns Colitis 2018, 12, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, J.; Colodny, A.H.; Eraklis, A.J. Pancreatitis and intravenous fat: An association in patients with inflammatory bowel disease. J. Pediatr. Surg. 1983, 18, 269–272. [Google Scholar] [CrossRef]
- Lashner, B.A.; Kirsner, J.B.; Hanauer, S.B. Acute pancreatitis associated with high-concentration lipid emulsion during total parenteral nutrition therapy for Crohn’s disease. Gastroenterology 1986, 90, 1039–1041. [Google Scholar] [CrossRef]
- Gouveia, C.I.; Oliveira, L.; Campos, A.P.; Cabral, J. Autoimmune pancreatitis with associated ulcerative colitis in a teenager. BMJ Case Rep. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Cousin, E.; Cousin, I.; Aziz, K.; Chailloux, P.; Breton, E. Autoimmune Pancreatitis and Ulcerative Rectocolitis in an Adolescent. Pediatrics 2018, 141, S456–S461. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, N.T.; Johannsen, M.T.; Hollon, J.R. Fifteen-Year-Old Male with Type 2 Autoimmune Pancreatitis: An Argument for Endoscopy. Case Rep. Gastroenterol. 2017, 11, 329–334. [Google Scholar] [CrossRef]
- Dogan, G.; Akgun, O.; Ozdemir, S.; Uzuner, E.G.; Poturoglu, S. The Coexistence of Autoimmune Pancreatitis and Crohn’s Disease in an Adolescent Case. Medeni. Med. J. 2020, 35, 62–66. [Google Scholar] [CrossRef]
- Kugathasan, S.; Halabi, I.; Telega, G.; Werlin, S.L. Pancreatitis as a presenting manifestation of pediatric Crohn’s disease: A report of three cases. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 96–98. [Google Scholar] [CrossRef]
- Endo, K.; Hirota, M.; Sasaki, Y.; Koiwai, A.; Nihei, K.; Takasu, A.; Kawamura, K.; Murakami, K.; Kogure, T.; Meguro, T.; et al. Presymptomatic Crohn’s Disease in a Young Patient Diagnosed Just After the Onset of Idiopathic Acute Pancreatitis. Intern. Med. 2021, 60, 1205–1210. [Google Scholar] [CrossRef]
- Watanabe, T. Parotitis and acute pancreatitis in a patient with ulcerative colitis. Eur. J. Pediatr. 2008, 167, 945. [Google Scholar] [CrossRef]
- Knafelz, D.; Panetta, F.; Monti, L.; Bracci, F.; Papadatou, B.; Torre, G.; Dall’Oglio, L.; Diamanti, A. Chronic pancreatitis as presentation of Crohn’s disease in a child. World J. Gastroenterol. 2013, 19, 5204–5206. [Google Scholar] [CrossRef]
- Evans, J.S.; George, D.E.; Barwick, K.W.; Lafer, D.J. Crohn’s disease presenting as chronic pancreatitis with biliary tract obstruction. J. Pediatr. Gastroenterol. Nutr. 1996, 22, 384–388. [Google Scholar] [CrossRef]
- Potamianos, S.; Koutroubakis, I.E.; Chatzicostas, C.; Rolles, K.; Burroughs, A.K.; Kouroumalis, E.A. Idiopathic fibrosing pancreatitis and Crohn’s disease: An interesting association. Eur J. Gastroenterol. Hepatol. 2000, 12, 1021–1024. [Google Scholar] [CrossRef]
- Silbermintz, A.; Krishnan, S.; Banquet, A.; Markowitz, J. Granulomatous pneumonitis, sclerosing cholangitis, and pancreatitis in a child with Crohn disease: Response to infliximab. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 324–326. [Google Scholar] [CrossRef]
- Kim, H.A.; Suh, H.R.; Kang, B.; Choe, B.H. Acute pancreatitis associated with indigo naturalis in pediatric severe Crohn’s disease. Intest. Res. 2019, 17, 144–148. [Google Scholar] [CrossRef]
- Briem-Richter, A.; Grabhorn, E.; Wenke, K.; Ganschow, R. Hemorrhagic necrotizing pancreatitis with a huge pseudocyst in a child with Crohn’s disease. Eur J. Gastroenterol. Hepatol. 2010, 22, 234–236. [Google Scholar] [CrossRef]
- Venkataraman, D.; Howarth, L.; Beattie, R.M.; Afzal, N.A. A very high amylase can be benign in paediatric Crohn’s disease. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- Ray, P.; Van Arsdall, M.R. Elevated Lipase during Initial Presentation of Ulcerative Colitis in a Pediatric Patient: Do We Check for It. Case Rep. Gastroenterol. 2016, 10, 568–573. [Google Scholar] [CrossRef]
- Michaels, M.A.; Jendrek, S.T.; Korf, T.; Nitzsche, T.; Teegen, B.; Komorowski, L.; Derer, S.; Schröder, T.; Baer, F.; Lehnert, H.; et al. Pancreatic Autoantibodies Against CUZD1 and GP2 Are Associated with Distinct Clinical Phenotypes of Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2864–2872. [Google Scholar] [CrossRef]
- Kurashima, Y.; Kigoshi, T.; Murasaki, S.; Arai, F.; Shimada, K.; Seki, N.; Kim, Y.-G.; Hase, K.; Ohno, H.; Kawano, K.; et al. Pancreatic glycoprotein 2 is a first line of defense for mucosal protection in intestinal inflammation. Nat. Commun. 2021, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, J.; Feng, N.; Lowe, A.W. Determination of plasma glycoprotein 2 levels in patients with pancreatic disease. Arch. Pathol. Lab. Med. 2004, 128, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Huang, J.H.; Zhang, X.W.; Ahmed, R.; Xie, Q.L.; Li, B.; Zhu, Y.; Cai, X.; Peng, Q.; Qin, Y.; et al. Identification of potential diagnostic biomarkers of acute pancreatitis by serum metabolomic profiles. Pancreatology 2017, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Filimoniuk, A.; Daniluk, U.; Samczuk, P.; Wasilewska, N.; Jakimiec, P.; Kucharska, M.; Lebensztejn, D.M.; Ciborowski, M. Metabolomic profiling in children with inflammatory bowel disease. Adv. Med. Sci. 2020, 65, 65–70. [Google Scholar] [CrossRef]
- Martín-de-Carpi, J.; Moriczi, M.; Pujol-Muncunill, G.; Navas-López, V.M. Pancreatic Involvement in Pediatric Inflammatory Bowel Disease. Front. Pediatr. 2017, 5, 218. [Google Scholar] [CrossRef]
- Rasmussen, H.H.; Fonager, K.; Sørensen, H.T.; Pedersen, L.; Dahlerup, J.F.; Steffensen, F.H. Risk of acute pancreatitis in patients with chronic inflammatory bowel disease. A Danish 16-year nationwide follow-up study. Scand. J. Gastroenterol. 1999, 34, 199–201. [Google Scholar] [CrossRef]
- Bermejo, F.; Lopez-Sanroman, A.; Taxonera, C.; Gisbert, J.P.; Pérez-Calle, J.L.; Vera, I.; Menchén, L.; Martín-Arranz, M.D.; Opio, V.; Carneros, J.A.; et al. Acute pancreatitis in inflammatory bowel disease, with special reference to azathioprine-induced pancreatitis. Aliment. Pharmacol. Ther. 2008, 28, 623–628. [Google Scholar] [CrossRef]
- Moolsintong, P.; Loftus, E.V.; Chari, S.T.; Egan, L.J.; Tremaine, W.J.; Sandborn, W.J. Acute pancreatitis in patients with Crohn’s disease: Clinical features and outcomes. Inflamm. Bowel Dis. 2005, 11, 1080–1084. [Google Scholar] [CrossRef]
- Pezzilli, R.; Barassi, A.; Morselli-Labate, A.M.; Fantini, L.; Tomassetti, P.; Campana, D.; Casadei, R.; Finazzi, S.; d’Eril, G.M.; Corinaldesi, R. Fecal calprotectin and elastase 1 determinations in patients with pancreatic diseases: A possible link between pancreatic insufficiency and intestinal inflammation. J. Gastroenterol. 2007, 42, 754–760. [Google Scholar] [CrossRef]
- Daniluk, U.; Daniluk, J.; Krasnodebska, M.; Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Lebensztejn, D.M. The combination of fecal calprotectin with ESR, CRP and albumin discriminates more accurately children with Crohn’s disease. Adv. Med. Sci. 2019, 64, 9–14. [Google Scholar] [CrossRef]
- Fiorentini, M.T.; Fracchia, M.; Galatola, G.; Barlotta, A.; de la Pierre, M. Acute pancreatitis during oral 5-aminosalicylic acid therapy. Dig. Dis. Sci. 1990, 35, 1180–1182. [Google Scholar] [CrossRef]
- Van Geenen, E.J.; de Boer, N.K.; Stassen, P.; Linskens, R.K.; Bruno, M.J.; Mulder, C.J.; Stegeman, C.A.; van Bodegraven, A.A. Azathioprine or mercaptopurine-induced acute pancreatitis is not a disease-specific phenomenon. Aliment. Pharmacol. Ther. 2010, 31, 1322–1329. [Google Scholar] [CrossRef]
- Ledder, O.; Lemberg, D.A.; Day, A.S. Thiopurine-induced pancreatitis in inflammatory bowel diseases. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 399–403. [Google Scholar] [CrossRef]
- Sandborn, W.J. A review of immune modifier therapy for inflammatory bowel disease: Azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am. J. Gastroenterol. 1996, 91, 423–433. [Google Scholar]
- Pitchumoni, C.S.; Rubin, A.; Das, K. Pancreatitis in inflammatory bowel diseases. J. Clin. Gastroenterol. 2010, 44, 246–253. [Google Scholar] [CrossRef]
- Gearry, R.B.; Barclay, M.L.; Burt, M.J.; Collett, J.A.; Chapman, B.A. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol. Drug Saf. 2004, 13, 563–567. [Google Scholar] [CrossRef]
- Marinaki, A.M.; Duley, J.A.; Arenas, M.; Ansari, A.; Sumi, S.; Lewis, C.M.; Shobowale-Bakre, M.; Fairbanks, L.D.; Sanderson, J. Mutation in the ITPA gene predicts intolerance to azathioprine. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1393–1397. [Google Scholar] [CrossRef]
- Layer, P.H.; Goebell, H.; Keller, J.; Dignass, A.; Klotz, U. Delivery and fate of oral mesalamine microgranules within the human small intestine. Gastroenterology 1995, 108, 1427–1433. [Google Scholar] [CrossRef]
- Bokemeyer, B. Asymptomatic elevation of serum lipase and amylase in conjunction with Crohn’s disease and ulcerative colitis. Z. Gastroenterol. 2002, 40, 5–10. [Google Scholar] [CrossRef]
- Tromm, A.; Höltmann, B.; Hüppe, D.; Kuntz, H.D.; Schwegler, U.; May, B. Hyperamylasemia, hyperlipasemia and acute pancreatitis in chronic inflammatory bowel diseases. Leber Magen Darm. 1991, 21, 15–16, 19–22. [Google Scholar]
| Authors, Year of the Publication | Pancreatic Disorder | Number of Affected Children with IBD | Etiology of Pancreatic Disease | Severity of Pancreatic Disease | Comments Analyzed Population; Mean Age (Ranges) at the Time of Pancreatic Involvement | ||
|---|---|---|---|---|---|---|---|
| CD n (%) | UC n (%) | IBD-U | |||||
| Martinelli et al., 2015 [8] | AP HA/HL | 6/284 (2%) 7/284 (2%) | 4/290 (1.4%) 8/290 (2.8%) | 1/48 (2%) 1/48 (2%) | No data | Mild | IBD with pancreatic involvement; age 12.3 (5.4–25.9); active IBD in 85.1% of both AP and HA/HL group; in 18.5% of cases pancreatic involvement at the time of IBD diagnosis |
| Weber et al., 1993 [9] | AP | 1/12 (8.3%) 1/12 (8.3%) | NA | NA | Sulfasalazine Pancreas divisum | No data | CD; age of study group: 10–50 yr; only two children (10 yr and 18 yr) in the study group; 10-yr-old boy developed AP induced by sulfasalazine at the time of CD diagnosis, symptoms resolved after drug discontinuation 18-yr-old girl developed AP one year after CD, AP due to pancreas divisum |
| Broide et al., 2011 [10] | AP | 6/460 (1.3%) | 4/460 (0.9%) | No data | Idiopathic | Mild | AP preceded the IBD diagnosis in 12/460 patients including two adults; mean age of children was 13 ± 4.8 (range 3–19) yr, the description of the study group includes adults; nine patients had moderate to severe IBD |
| Wintzell et al., 2019 [11] | AP | 21/1923 (1.1%) | 19/1451 (1.3%) | No data | AZA | No data | IBD treated with AZA; age at the time of pancreatic involvement—no data; similar rate of AP in boys and girls and UC and CD |
| Dubinsky et al., 2000 [12] | AP HA/HL | 1/92 (1%) children with IBD6/92 (6.5%) children with IBD | AZA/6MP | No data | IBD treated with AZA/6MP; age at the time of pancreatic involvement—no data; IBD type - no data; normal TMPT genotype, no correlation between 6-MP dose or metabolite levels and pancreatic toxicity | ||
| Hindorf et al., 2006 [13] | AP | 2/79 (2.5%) children with IBD | No data | AZA | No data | IBD treated with AZA; age of the study group: 17–51 yr; age at the time of pancreatic involvement—no data; there was no difference in TPMT activity between patients with pancreatitis and patients without adverse event | |
| De Ridder et al., 2006 [14] | AP | 4/72 (5.6%) children with IBD | No data | AZA | No data | IBD treated with AZA; age at the time of pancreatic involvement—no data; IBD type not specified; normal TMPT genotype | |
| Tajiri et al., 2008 [15] | AP | NA | 1/35 | No data | AZA/6MP | No data | UC treated with AZA/6MP; age at the time of pancreatic involvement—no data. |
| Cuffari et al., 1996 [16] | AP | 4/15 (16%) | NA | No data | 6MP | No data | CD treated with 6MP; age at the time of pancreatic involvement—no data |
| Kirschner et al., 1998 [17] | AP | 2/95 (2%) | 2/95 (2%) | 0/95 | AZA/6MP | No data | IBD treated with AZA/6MP; age 11.5–16.2 yr; drug discontinued |
| Keljo et al., 1997 [18] | AP AP AP AP | 1/10 2/10 2/10 2/10 | 0/10 0/10 1/10 2/10 | No data | 5-ASA AZA Biliary pancreatitis Idiopathic | No data No data No data Mild | 8.6-yr-old girl, AP symptoms resolved after 5-ASA discontinuation 17.9-yr-old girl; 12.3-yr-old girl; in both cases symptoms resolved 17.2-yr-old girl with CD; 13.7-yr-old girl with CD; 12.8-yr-old girl with UC 12.7-yr-old boy and 10.8-yr-old girl with CD; 9.9-yr-old and 14.9-yr-old girls with UC. |
| Bai et al., 2011 [19] | AP | 6/51 (10.9%) | 5/51 (9.1%) | No data | Drug | No data | Drug-induced pancreatitis; no demographic data on patients with CD and UC |
| Scheers et al., 2017 [20] | AIP | 1/16 (6.3%) | 3/16 (18.8%) | No data | AIP | No data | AIP; no demographic data on patients with CD and UC |
| Jose et.al., 2009 [1] | Pancreatitis | 37/387 (9.6%) children with IBD | No data | No data | No data | IBD and EIMs; age at the time of pancreatic involvement—no data; IBD type not specified, pancreatitis type not specified | |
| Dotson et al., 2010 [2] | Pancreatitis | 5/728 (0.7%) | 4/281 (1.4%) | No data | No data | No data | IBD and EIMs; age at the time of pancreatic involvement—no data; pancreatitis type not specified |
| Ghersin et al., 2020 [21] | Pancreatitis | 5/231 (0.8%) | 3/231 (1%) | No data | No data | No data | Jewish adolescents with IBD; age at the time of pancreatic involvement—no data; IBD type—no data, pancreatitis type—no data |
| Authors, Year of the Publication | Pancreatic Disorder; AP Severity | Etiology of Pancreatic Disease | CD n (%) | UC n (%) | Comment |
|---|---|---|---|---|---|
| Gallego-Gutierrez et al., 2015 [22] | AP; mild, moderate | AZA | 2 cases (10- and 13-yr-old) | NA | AP symptoms resolved after AZA discontinuation; normal TMPT genotype |
| Yi et al., 2012 [23] | AP; severity-no data | AZA/6MP | 1 case (14-yr-old) | NA | AP symptoms resolved after AZA/6MP discontinuation |
| Ledder et al., 2013 [24] | AP; mild | AZA | 4 cases (11-, 13-, 13-, 14-yr-old) | NA | AP symptoms resolved after AZA discontinuation; 6MP was successfully used |
| Mishra et al., 2020 [25] | AP; mild | AZA | 1 case (16-yr-old) | NA | AP symptoms resolved after AZA discontinuation; normal TMPT genotype |
| Abdullah et al., 1993 [26] | AP; severity-no data | ASA | NA | 1 case (12-yr-old) | Sulfasalazine/mesalamine-induced AP; AP symptoms resolved after drug discontinuation |
| Paul et al., 2000 [27] | AP; severity-no data | ASA | NA | 1 case (10-yr-old) | AP symptoms resolved after drug discontinuation |
| Radke et al., 1993 [28] | AP; moderate | ASA | 1 case (12-yr-old) | NA | AP symptoms resolved after drug discontinuation |
| Garau et al., 1994 [29] | AP; severity-no data | ASA | NA | 3 cases (12, 12 and 13-yr-old) | AP symptoms resolved after drug discontinuation in all cases, but in one case intractable severe colitis unresponsive to intensive therapy led to subtotal colectomy |
| Paerregaard et al., 1997 [30] | AP; severity-no data | ASA | NA | 1 case (7-yr-old) | AP induced by oral or rectal administration of 5-ASA; AP symptoms resolved after drug discontinuation |
| Chung et al., 2015 [31] | AP; severity-no data | ASA | NA | 1 case (11-yr-old) | AP coexisting with pneumonitis induced by mesalazine; AP symptoms resolved after drug discontinuation |
| Lopez et al., 2018 [32] | AP; severity-no data | Vedolizumab | NA | 1 case (14-yr-old) | AP symptoms resolved after drug discontinuation, but refractory colitis led to subtotal colectomy |
| Noseworthy et al., 1983 [33] | AP, severe | Intralipid-supplemented TPN | 2 cases with IBD (not specified type of IBD) | AP developed after 7 weeks of 20% Intralipid-supplemented TPN combined with high dose of steroid | |
| Lashner et al., 1986 [34] | AP, mild | Intralipid-supplemented TPN | 1 case (17-yr-old) | NA | AP developed after 6 weeks of 20% Intralipid-supplemented TPN combined with steroid and oral foods (small amount) |
| Gouveia et al., 2018 [35] | AP; severity-no data | AIP | NA | 1 case (13-yr-old) | AIP preceded UC diagnosis; AIP therapy with an endoscopic retrograde cholangiopancreatography (ERCP) with stent placement induced sustain AIP remission |
| Cousin et al., 2018 [36] | AP; severe | AIP type 2 | NA | 1 case (16-yr-old) | AP with elevated IgG4, cholestasis with cirrhosis and UC |
| Kolasinski et al., 2017 [37] | AP; severity-no data | AIP type 2 | NA | 1 case (15-yr-old) | AIP with elevated IgG4 and coexisted with UC with no intestinal complaints |
| Dogan et al., 2020 [38] | AP; severity-no data | AIP | 1 case (16-yr-old) | NA | AIP with elevated IgG4 preceded CD diagnosis |
| Kugathasan at al., 2002 [39] | AP; severity-no data | Idiopathic | 3 cases (12, 13 and 16-yr-old) | NA | AP preceded CD development |
| Endo et al., 2021 [40] | AP; severity-no data | Idiopathic | 1 case (16-yr-old) | NA | AP preceded CD diagnosis |
| Watanabe 2008 [41] | AP, mild | idiopathic | NA | 1 case (15-yr-old) | AP coexisted with parotitis |
| Knafelz et al., 2013 [42] | CP | CFTR mutation | 1 case (4-yr-old) | NA | CP preceded CD development |
| Evans et al., 1996 [43] | CP | Biliary tract obstruction | 1 case (13-yr-old) | NA | CP with biliary tract obstruction preceded CD development |
| Potamianos et al., 2000 [44] | CP | Idiopathic | 1 case (16-yr-old) | NA | Fibrosing pancreatitis preceded CD development |
| Silbermintz et al., 2006 [45] | Pancreatitis | Idiopathic | 1 case (10-yr-old) | NA | Coexistence of CD, granulomatous pneumonitis, and PCS |
| Kim et al., 2019 [46] | AP; severity-no data | Indigo-naturalis | 1 case (11-yr-old) | NA | Boy with severe CD |
| Briem-Richter et al., 2010 [47] | AP; severity-no data | Hemorrhagic necrotizing pancreatitis | 1 case (6-yr-old) | NA | CD and familial hyperparathyreoidism |
| Venkataraman et al., 2012 [48] | HA | Idiopathic | 1 case (13-yr-old) | NA | CD and macroamylasemia |
| Ray et al., 2016 [49] | HL | Idiopathic | NA | 1 case (13-yr-old) | HL correlated with severity of UC and lipase activity decreased when remission of UC was achieved |
| Drug | Potential Mechanism of Pancreatitis |
|---|---|
| 5-ASA | Hypersensitivity reaction [46] Increased pancreatic duct permeability [61] |
| AZA | Direct toxic reaction [62] Genetic predisposition [63] Immunological reaction [24,64] Idiosyncratic reaction [24,65,66] Conflicting data: inosine triphosphate pyrophosphatase (ITPase) deficiency [14,67] |
| 6-MP | Direct toxic reaction [62] Genetic predisposition [63] |
| Vedolizumab | Dysregulation of immune response [32] |
| Intralipid-supplemented total parental nutrition (TPN) | Hyperlipidemia in combination with high dose of steroid [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakimiec, P.; Zdanowicz, K.; Kwiatek-Sredzinska, K.; Filimoniuk, A.; Lebensztejn, D.; Daniluk, U. Pancreatic Disorders in Children with Inflammatory Bowel Disease. Medicina 2021, 57, 473. https://doi.org/10.3390/medicina57050473
Jakimiec P, Zdanowicz K, Kwiatek-Sredzinska K, Filimoniuk A, Lebensztejn D, Daniluk U. Pancreatic Disorders in Children with Inflammatory Bowel Disease. Medicina. 2021; 57(5):473. https://doi.org/10.3390/medicina57050473
Chicago/Turabian StyleJakimiec, Piotr, Katarzyna Zdanowicz, Kamila Kwiatek-Sredzinska, Aleksandra Filimoniuk, Dariusz Lebensztejn, and Urszula Daniluk. 2021. "Pancreatic Disorders in Children with Inflammatory Bowel Disease" Medicina 57, no. 5: 473. https://doi.org/10.3390/medicina57050473
APA StyleJakimiec, P., Zdanowicz, K., Kwiatek-Sredzinska, K., Filimoniuk, A., Lebensztejn, D., & Daniluk, U. (2021). Pancreatic Disorders in Children with Inflammatory Bowel Disease. Medicina, 57(5), 473. https://doi.org/10.3390/medicina57050473






