Percutaneous Left Atrial Appendage Occlusion: An Emerging Option in Patients with Atrial Fibrillation at High Risk of Bleeding
Abstract
1. Introduction
2. Pathophysiology of Thrombus Formation in LAA during AF
- (1)
- (2)
- (3)
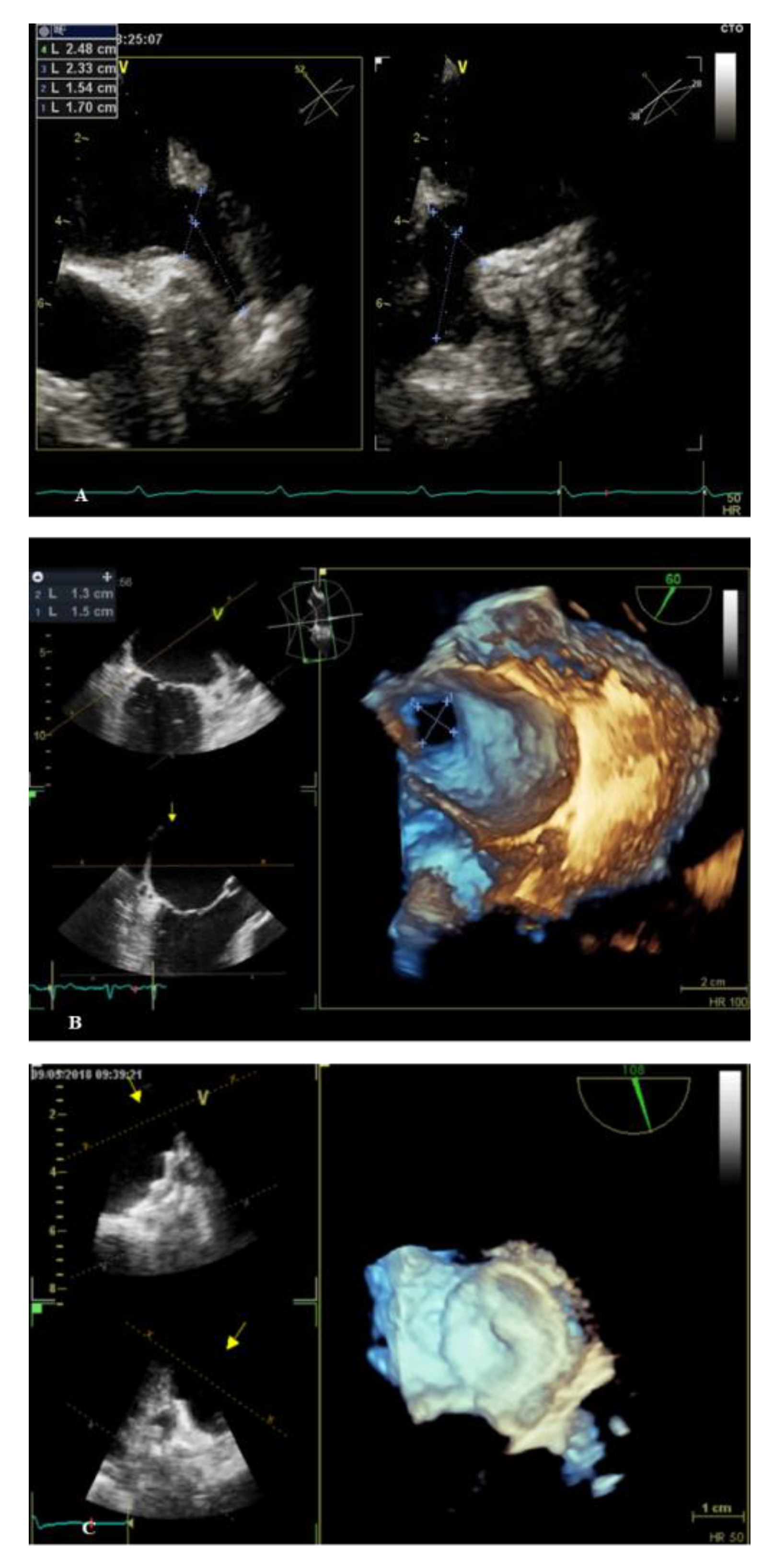
3. Percutaneous LAAO Procedure
4. Devices Characteristics
5. Randomized Clinical Trials Evaluating Safety and Efficacy of Percutaneous LAAO
6. Advanced Data Analysis Exploring the Available Data on LAAO
7. Ongoing Studies Looking to a Close Future
8. LAAO in Clinical Practice: Current Indications and Patients’ Selection
- Patients at high risk of bleeding under chronic anticoagulant therapy. This category includes: patients with HAS-BLED ≥3, patients whose bleeding risk is underestimated by the HAS-BLED (e.g., tumors, thrombocytopenia), patients with prolonged triple antithrombotic therapy. Patients with previous major bleeding from gastrointestinal tract, with a source who cannot be eliminated (e.g., diffuse intestinal angiodysplasia) [72].
- Patients with end-stage CKD or on hemodialysis treatment. DOACs are contraindicated when creatinine clearance is <15 mL/min, while warfarin could cause tissue calcifications in these patients. A meta-analysis [73] highlighted that in AF patients with end-stage CKD, warfarin could raise the risk of major bleedings. Thus, patients with end-stage CKD would be suitable candidates for LAAO. However, no data from RCTs are available for this specific population.
- Non-compliance to long-term medical therapy (dementia, patients who discontinue drug after a minor bleeding event) or patients with difficulties in managing oral therapies (e.g., visual impairment, psychiatric diseases). In this category, LAAO could be considered only after attempting to improve patient’s compliance.
- Patients in whom OACs were not able to prevent cerebral ischemic events likely related to thrombus-embolism from LAA. This group includes patients developing an ischemic stroke event despite adequate OAC therapy. A number of studies (Table 2) have evaluated the rate of ischemic stroke on OACs varying from 1.69%, 2.2%, and 1.05% using warfarin, to 1.53% with dabigatran, 1.7% with rivaroxaban and 1.27% with apixaban [54,55,56]. In this context, percutaneous LAAO could represent a potential alternative to medical therapy. However, data are insufficient to provide specific recommendations [26].
- Patients who underwent electrical isolation of LAA as part of AF ablation procedure due to an increased risk of stroke. Evidence is still scarce and there are no trials comparing DOACs with percutaneous LAAO in these patients [74].
- Combination of AF ablation and LAAO. AF patients that undergo ablation with a high risk of bleeding may benefit from combining these two procedures. Ablation requires a transeptal approach that allows percutaneous LAAO using the same access. Small cohorts have demonstrated feasibility, but there are no trials comparing a combined procedure versus a two-step procedure [75].
- LAAO for “primary prevention”. In patients with ASD and at high risk to develop AF, LAAO could be used before closing septal defects due to future technical problems related to the presence of septal devices [76]. More data are needed to better evaluate the adequacy of this indication.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Gallinoro, E.; D’Elia, S.; Prozzo, D.; Lioncino, M.; Natale, F.; Golino, P.; Cimmino, G. Cognitive Function and Atrial Fibrillation: From the Strength of Relationship to the Dark Side of Prevention. Is There a Contribution from Sinus Rhythm Restoration and Maintenance? Medicina 2019, 55, 587. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.W.; Kim, S.; Piccini, J.P.; Sr Ansell, J.E.; Fonarow, G.C.; Hylek, E.M.; Singer, D.E.; Mahaffey, K.W.; Kowey, P.R.; Tomas, L.; et al. Risks and benefits of anticoagulation in atrial fibrillation: Insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 461–469. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, C.V.; Prakriti, B.G.; Tri, J.; Syed, F.; Noheria, S.M.A.; Asirvatham, S.J. A Review of The Relevant Embryology, Pathohistology, and Anatomy of The Left Atrial Appendage for The Invasive Cardiac Electrophysiologist. J. Atr. Fibrillation 2015, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Aberg, H. Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med. Scand. 1969, 185, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Abrich, V.A.; Narichania, A.D.; Love, W.T.; Lanza, L.A.; Shen, W.K.; Soraja, D. Left atrial appendage exclusion during mitral valve surgery and stroke in atrial fibrillation. J. Interv. Card. Electrophysiol. 2018, 53, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Sakellaridis, T.; Argiriou, M.; Charitos, C.; Tsakiridis, K.; Zarogoulidis, P.; Katsikogiannis, N.; Kougioumtzi, I.; Machairiotis, N.; Tsiouda, T.; Arikas, S.; et al. Left atrial appendage exclusion—Where do we stand? J. Thorac. Dis. 2014, 6, S70–S77. [Google Scholar] [CrossRef]
- Yerasi, C.; Lazkani, M.; Kolluru, P.; Miryala, V.; Kim, J.; Moole, H.; Sawant, A.C.; Morris, M.; Pershad, A. An updated systematic review and meta-analysis of early outcomes after left atrial appendage occlusion. J. Interv. Cardiol. 2018, 31, 197–206. [Google Scholar] [CrossRef]
- Topcuoglu, M.A.; Liu, L.; Kim, D.E.; Gurol, M.E. Updates on Prevention of Cardioembolic Strokes. J. Stroke 2018, 20, 180–196. [Google Scholar] [CrossRef]
- Wunderlich, N.C.; Beigel, R.; Swaans, M.J.; Ho, S.Y.; Siegel, R.J. Percutaneous interventions for left atrial appendage exclusion: Options, assessment, and imaging using 2D and 3D echocardiography. JACC Cardiovasc. Imaging 2015, 8, 472–488. [Google Scholar] [CrossRef]
- Davis, C.A., 3rd; Rembert, J.C.; Greenfield, J.C., Jr. Compliance of left atrium with and without left atrium appendage. Am. J. Physiol. 1990, 259, H1006–H1008. [Google Scholar] [CrossRef] [PubMed]
- Al-Saady, N.M.; Obel, O.A.; Camm, A.J. Left atrial appendage: Structure, function, and role in thromboembolism. Heart 1999, 82, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Granier, M.; Laugadin, G.; Massin, F.; Cade, S.; Winum, P.F.; Freitag, C.; Pasquie, J.C. Occurrence of incomplete endothelialization causing residual permeability after left atrial appendage closure. J. Invasive Cardiol. 2018, 30, 245–250. [Google Scholar]
- Schellinger, P.D.; Tsivgoulis, G.; Steiner, T.; Kohrmann, M. Percutaneous Left Atrial Appendage Occlusion for the Prevention of Stroke in Patients with Atrial Fibrillation: Review and Critical Appraisal. J. Stroke 2018, 20, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Ranka, S.; Reddy, M. Percutaneous left atrial appendage occlusion. Curr. Opin. Cardiol. 2021, 36, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Lip, G.Y.H. The prothrombotic state in atrial fibrillation: Pathophysiological and management implications. Cardiovasc. Res. 2019, 115, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.; Shantsila, E.; Lip, G.Y. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef]
- Seko, Y.; Kato, T.; Haruna, T.; Izumi, I.; Miyamoto, S.; Eisaku, N.; Inoko, M. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci. Rep. 2018, 8, 6366. [Google Scholar] [CrossRef]
- Mahajan, R.; Brooks, A.G.; Sullivan, T.; Lim, H.S.; Alasady, M.; Abed, H.S.; Ganesan, A.N.; Nayyar, S.; Lau, D.H.; Roberts-Thomson, K.C.; et al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: Implications for left atrial appendage closure. Heart 2012, 98, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Okin, P.M.; Elkind, M.S.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef]
- Lau, D.H.; Schotten, U.; Mahajan, R.; Antic, N.A.; Hatem, S.N.; Pathak, R.K.; Hendriks, J.M.L.; Kalman, J.M.; Sanders, P. Novel mechanisms in the pathogenesis of atrial fibrillation: Practical applications. Eur. Heart J. 2016, 37, 1573–1581. [Google Scholar] [CrossRef]
- Thakkar, J.; Vasdeki, D.; Tzikas, A.; Meier, B.; Saw, J. Incidence, Prevention, and Management of Periprocedural Complications of Left Atrial Appendage Occlusion. Interv. Cardiol. Clin. 2018, 7, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Faletra, F.F.; Regoli, F.; Pasotti, E.; Pedrazzini, G.; Moccetti, T.; Auricchio, A. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: Implications for catheter-based left atrial appendage closure. Circ. Cardiovasc. Imaging 2011, 4, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef] [PubMed]
- Goitein, O.; Fink, N.; Guetta, V.; Beinart, R.; Brodov, Y.; Konen, E.; Goitein, D.; Di Segni, E.; Grupper, A.; Glikson, M. Printed MDCT 3D models for prediction of left atrial appendage (LAA) occluder device size: A feasibility study. EuroIntervention 2017, 13, e1076–e1079. [Google Scholar] [CrossRef]
- Obasare, E.; Mainigi, S.K.; Morris, D.L.; Slipczuk, L.; Goykhman, I.; Friend, E.; Ziccardi, M.R.; Pressman, G.S. CT based 3D printing is superior to transesophageal echocardiography for pre-procedure planning in left atrial appendage device closure. Int. J. Cardiovasc. Imaging 2018, 34, 821–831. [Google Scholar] [CrossRef]
- Eng, M.H.; Wang, D.D.; Greenbaum, A.B.; Gheewala, N.; Kupsky, D.; Aka, T.; Song, T.; Kendall, B.J.; Wyman, J.; Myers, E.; et al. Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter. Cardiovasc. Interv. 2018, 92, 401–407. [Google Scholar] [CrossRef]
- Vivoli, G.; Gasparotti, E.; Rezzaghi, M.; Cerone, E.; Mariani, M.; Landini, L.; Berti, S.; Positano, V.; Celi, S. Simultaneous Functional and Morphological Assessment of Left Atrial Appendage by 3D Virtual Models. J. Healthc. Eng. 2019, 2019. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, C.Y.; Huang, D.Q.; Liu, Y.Y.; Qin, Y.Y.; Zhang, L.Z.; Liu, L. Evaluation of the left atrial appendage by real time three-dimensional transesophageal echocardiography online. Echocardiography 2018, 35, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, X.; Wu, H.; Xie, J.; Dai, Q.; Wang, L.; Xu, B. A meta-analysis for efficacy and safety evaluation of transcatheter left atrial appendage occlusion in patients with nonvalvular atrial fibrillation. Medicine (Baltimore) 2016, 95, e4382. [Google Scholar] [CrossRef]
- Fauchier, L.; Cinaud, A.; Brigadeau, F.; Lepillier, A.; Pierre, B.; Abbey, S.; Fatemi, M.; Franceschi, F.; Guedeney, P.; Jacon, P.; et al. Device-Related Thrombosis after Percutaneous Left Atrial Appendage Occlusion for Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Boersma, L.V.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Samaras, A.; Saw, J.; Berti, S.; Tzikas, A.; Nielsen-Kudsk, J.E. Peridevice Leak Following Amplatzer Left Atrial Appendage Occlusion: Cardiac Computed Tomography Classification and Clinical Outcomes. JACC Cardiovasc. Interv. 2021, 14, 83–93. [Google Scholar] [CrossRef]
- Saw, J.; Fahmy, P.; DeJong, P.; Lempereur, M.; Spencer, R.; Tsang, M.; Gin, K.; Jue, J.; Mayo, J.; McLaughlin, P.; et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur. Heart J. Cardiovasc. Imaging. 2015, 16, 1198–1206. [Google Scholar] [CrossRef]
- Saw, J.; Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Nielsen-Kudsk, J.E.; Kefer, J.; Aminian, A.; Berti, S.; Santoro, G.; et al. Incidence and Clinical Impact of Device-Associated Thrombus and Peri-Device Leak Following Left Atrial Appendage Closure with the Amplatzer Cardiac Plug. JACC Cardiovasc. Interv. 2017, 10, 391–399. [Google Scholar] [CrossRef]
- Safavi-Naeini, P.; Rasekh, A. Closure of Left Atrial Appendage to Prevent Stroke: Devices and Status. Tex. Heart Inst. J. 2018, 45, 172–174. [Google Scholar] [CrossRef]
- Chen, S.; Weise, F.K.; Chun, K.R.J.; Schmidt, B. Antithrombotic strategies after interventional left atrial appendage closure: An update. Expert Rev. Cardiovasc. Ther. 2018, 16, 675–678. [Google Scholar] [CrossRef]
- Sievert, H.; Lesh, M.D.; Trepels, T.; Omran, H.; Bartorelli, A.; Della Bella, P.; Nakai, T.; Reisman, M.; DiMario, C.; Block, P.; et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: Early clinical experience. Circulation 2002, 105, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Danna, P.; Sagone, A.; Proietti, R.; Arensi, A.; Viecca, M.; Santangeli, P.; Di Biase, L.; Natale, A. New technology for prevention of embolic events in atrial fibrillation: A systematic review on percutaneous endovascular left atrial appendage closure. G. Ital. Cardiol. 2012, 13, 571–582. [Google Scholar] [CrossRef]
- Bergmann, M.W. European registry data on LAA closure: Advancing the field of interventional stroke prevention. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, 135–138. [Google Scholar] [CrossRef]
- KhodjaevMd, S.; Le Md, M.D.; RaoMd, W.; MorelliMdFacc, R. Retrospective Evaluation of Novel Percutaneous Left Atrial Appendage Ligation Using The LARIAT Suturing Device: Single Center Initial Experience. J. Atr. Fibrillation 2014, 7, 1106. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Firstenberg, M.S.; Martella, A.T.; Lau, L.; Zlock, S.; Mohan, A.; Spangler, T.; Currie, S.; Salzberg, S.P.; Caliskan, E. Epicardial left atrial appendage occlusion with a new medical device: Assessment of procedural feasibility, safety and efficacy in a large animal model. J. Cardiothorac. Surg. 2020, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Hildick-Smith, D. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, e590–e597. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Shakir, S.; Fankhauser, M.; Nietlispach, F.; Attinger-Toller, A.; Moschovitis, A.; Wenaweser, P.; Pilgrim, T.; Stortecky, S.; Praz, F.; et al. Predictors of Early (1-Week) Outcomes Following Left Atrial Appendage Closure With Amplatzer Devices. JACC Cardiovasc. Interv. 2016, 9, 1374–1383. [Google Scholar] [CrossRef]
- Gloekler, S.; Shakir, S.; Doblies, J.; Khattab, A.A.; Praz, F.; Guerios, E.; Koermendy, D.; Stortecky, S.; Pilgrim, T.; Buellesfeld, L.; et al. Early results of first versus second generation Amplatzeroccluders for left atrial appendage closure in patients with atrial fibrillation. Clin. Res. Cardiol. 2015, 104, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Schmidt, B.; Nielsen-Kudsk, J.E.; Lam, S.C.C.; Park, J.W.; Tarantini, G.; Cruz-Gonzalez, I.; Geist, V.; Della Bella, P.; Colombo, A.; et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: Periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention 2017, 13, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Gafoor, S.; Meerkin, D.; Freixa, X.; Cruz-Gonzalez, I.; Lewalter, T.; Saw, J.; Berti, S.; Nielsen-Kudsk, J.E.; Ibrahim, R.; et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: An expert consensus step-by-step approach. EuroIntervention 2016, 11, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Kleinecke, C.; Park, J.W.; Godde, M.; Zintl, K.; Schnupp, S.; Brachmann, J. Twelve-month follow-up of left atrial appendage occlusion with Amplatzer Amulet. Cardiol. J. 2017, 24, 131–138. [Google Scholar] [CrossRef]
- Bartus, K.; Han, F.T.; Bednarek, J.; Myc, J.; Kapelak, B.; Sadowski, J.; Lelakowski, J.; Bartus, S.; Yakubov, S.J.; Lee, R.J. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: Initial clinical experience. J. Am. Coll. Cardiol. 2013, 62, 108–118. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Ruzyllo, W.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Kar, S., Jr.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef]
- Russo, V.; Rago, A.; Proietti, R.; Di Meo, F.; Papa, A.A.; Calabrò, P.; D’Onofrio, A.; Nigro, G.; AlTurki, A. Efficacy and safety of the target-specific oral anticoagulants for stroke prevention in atrial fibrillation: The real-life evidence. Ther. Adv. Drug Saf. 2017, 8, 67–75. [Google Scholar] [CrossRef]
- Bertaglia, E.; Anselmino, M.; Zorzi, A.; Russo, V.; Toso, E.; Peruzza, F.; Rapacciuolo, A.; Migliore, F.; Gaita, F.; Cucchini, U.; et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int. J. Cardiol. 2017, 249, 179–183. [Google Scholar] [CrossRef]
- Wassef, A.; Butcher, K. Novel oral anticoagulant management issues for the stroke clinician. Int. J. Stroke 2016, 11, 759–767. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y., Jr.; Gordon, N.T.; Delurgio, D.; Doshi, S.K.; Desai, A.J.; Stone, J.E., Jr.; Kar, S. Long-Term Safety and Efficacy in Continued Access Left Atrial Appendage Closure Registries. J. Am. Coll. Cardiol. 2019, 74, 2878–2889. [Google Scholar] [CrossRef]
- Holmes, D.R.; Doshi, S.K., Jr.; Kar, S.; Price, M.J.; Sanchez, J.M.; Sievert, H.; Valderrabano, M.; Reddy, V.Y. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J. Am. Coll. Cardiol. 2015, 65, 2614–2623. [Google Scholar] [CrossRef]
- Price, M.J.; Reddy, V.Y.; Valderrabano, M.; Halperin, J.L.; Gibson, D.N.; Gordon, N.; Huber, K.C.; Holmes, D.R., Jr. Bleeding Outcomes After Left Atrial Appendage Closure Compared With Long-Term Warfarin: A Pooled, Patient-Level Analysis of the WATCHMAN Randomized Trial Experience. JACC Cardiovasc. Interv. 2015, 8, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Tousek, P.; Herman, D.; Neuzil, P.; Hala, P.; Stasek, J.; Haman, L.; Kala, P.; Poloczek, M.; Branny, M.; et al. Interventional left atrial appendage closure vs novel anticoagulation agents in patients with atrial fibrillation indicated for long-term anticoagulation (PRAGUE-17 study). Am. Heart J. 2017, 183, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, S.N.; Li, S.N.; Bai, R.; Liu, N.; Feng, L.; Ruan, Y.F.; Du, X.; Dong, J.Z.; Ma, C.S. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL triales. Heart Rhythm 2016, 13, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Left Atrial Appendage Closure Device with Delivery System: A Health Technology Assessment. Ont. Health Technol. Assess. Ser 2017, 17, 1–106. [Google Scholar]
- Bode, W.D.; Patel, N.; Gehi, A.K. Left atrial appendage occlusion for prevention of stroke in nonvalvular atrial fibrillation: A meta-analysis. J. Interv. Card. Electrophysiol. 2015, 43, 79–89. [Google Scholar] [CrossRef]
- Sahay, S.; Nombela-Franco, L.; Rodes-Cabau, J.; Jimenez-Quevedo, P.; Salinas, P.; Biagioni, C.; Nunez-Gil, I.; Gonzalo, N.; de Augustin, J.A.; Del Trigo, M. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: A network meta-analysis from randomised trials. Heart 2017, 103, 139–147. [Google Scholar] [CrossRef]
- Skurk, C.; Landmesser, U. Left atrial appendage occlusion for stroke prevention—State of the art as provided in an updated EHRA/EAPCI consensus statement and future perspectives. EuroIntervention 2020, 15, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Lempereur, M.; Aminian, A.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left Atrial Appendage Occlusion in Patients With Atrial Fibrillation and Previous Major Gastrointestinal Bleeding (from the Amplatzer Cardiac Plug Multicenter Registry). Am. J. Cardiol. 2017, 120, 414–420. [Google Scholar] [CrossRef]
- Dahal, K.; Kunwar, S.; Rijal, J.; Schulman, P.; Lee, J. Stroke, Major Bleeding, and Mortality Outcomes in Warfarin Users with Atrial Fibrillation and Chronic Kidney Disease: A Meta-Analysis of Observational Studies. Chest 2016, 149, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Shim, J.; Oh, S.K.; Lee, K.N.; Choi, J.I.; Kim, Y.H. Electrical isolation of the left atrial appendage increases the risk of ischemic stroke and transient ischemic attack regardless of postisolation flow velocity. Heart Rhythm 2018, 15, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Fassini, G.; Conti, S.; Moltrasio, M.; Maltagliati, A.; Tundo, F.; Riva, S.; Dello Russo, A.; Casella, M.; Majocchi, B.; Zucchetti, M.; et al. Concomitant cryoballoon ablation and percutaneous closure of left atrial appendage in patients with atrial fibrillation. Europace 2016, 18, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Wintgens, L.; Romanov, A.; Phillips, K.; Ballesteros, G.; Swaans, M.; Folkeringa, R.; Garcia-Bolao, I.; Pokushalov, E.; Boersma, L. Combined atrial fibrillation ablation and left atrial appendage closure: Long-term follow-up from a large multicentre registry. Europace 2018, 20, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
| Devices | Manufacturer | Type | Size Range | Approval Status |
|---|---|---|---|---|
| Watchman | Boston Scientific | Endocardial | 21–33 | Y(CE/FDA) |
| ACP 1 | Abbott Vascular | Endocardial | 16–30 | Y(CE) |
| Amulet | Abbott Vascular | Endocardial | 16–34 | Y(CE) |
| WaveCrest | Biosense Webster | Endocardial | 22–32 | Y(CE) |
| LAmbre LAAO | Lifetech Scientific | Endocardial | 16–26/36 | Y(CE) |
| Occlutech | Occlutech | Endocardial | 15–39 | Y(CE) |
| Ultraseal | Cardia | Endocardial | 16–32 | N |
| Sideris Patch | Custom Medical Devices | Endocardial | <25 | N |
| Pfm | Pfm Medical | Endocardial | 15–25 | N |
| Lariat | SentreHEART | Epicardial | 40 | Y(CE/FDA) |
| Sierra Ligation System | Aegis Medical Innovation | Epicardial | One size | N |
| RCT | DOACs ** | Warfarin | Median Follow-Up (Years) | Efficacy *,† | Hemorrhagic Stroke * | ||||
|---|---|---|---|---|---|---|---|---|---|
| DOACs | DOACs | Warfarin | DOACs | DOACs | Warfarin | ||||
| High-Dose | Low-Dose | High-dose | Low-Dose | ||||||
| ARISTOTLE | 9.120 | 9.081 | 1.8 | 1.27 | NA | 1.60 | 0.24 | NA | 0.47 |
| ENGAGE AF-TIMI 48 | 14.069 | 7.036 | 2.8 | 1.18 | 1.07 | 1.50 | 0.26 | 0.16 | 0.47 |
| RELY | 12.091 | 6.022 | 2 | 1.53 | 1.11 | 1.69 | 0.10 | 0.12 | 0.38 |
| ROCKET-AF | 7.131 | 7.133 | 1.6 | 1.7 | NA | 2.2 | 0.5 *** | NA | 0.7 *** |
| RCT | Device | Control | Mean Follow-Up (Months) | Efficacy *,† | Safety *,ɬ | Implant Success | ||
|---|---|---|---|---|---|---|---|---|
| Device | Control | Device | Control | |||||
| PROTECT-AF | 463 | 244 | 45 ± 20 | 2.3 | 3.8 | 3.6 | 3.1 | 90.9% |
| PREVAIL | 269 | 138 | 11.8 ± 5.8 | 6.4 | 6.3 | 2.2 | NA | 95.1% |
| PRAGUE-17 | 201 | 201 | 20.8 ± 10.8 | 10.99 | 13.42 | NA | NA | 95.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimmino, G.; Loffredo, F.S.; Gallinoro, E.; Prozzo, D.; Fabiani, D.; Cante, L.; Salerno, G.; Cappelli Bigazzi, M.; Golino, P. Percutaneous Left Atrial Appendage Occlusion: An Emerging Option in Patients with Atrial Fibrillation at High Risk of Bleeding. Medicina 2021, 57, 444. https://doi.org/10.3390/medicina57050444
Cimmino G, Loffredo FS, Gallinoro E, Prozzo D, Fabiani D, Cante L, Salerno G, Cappelli Bigazzi M, Golino P. Percutaneous Left Atrial Appendage Occlusion: An Emerging Option in Patients with Atrial Fibrillation at High Risk of Bleeding. Medicina. 2021; 57(5):444. https://doi.org/10.3390/medicina57050444
Chicago/Turabian StyleCimmino, Giovanni, Francesco S. Loffredo, Emanuele Gallinoro, Dario Prozzo, Dario Fabiani, Luigi Cante, Gemma Salerno, Maurizio Cappelli Bigazzi, and Paolo Golino. 2021. "Percutaneous Left Atrial Appendage Occlusion: An Emerging Option in Patients with Atrial Fibrillation at High Risk of Bleeding" Medicina 57, no. 5: 444. https://doi.org/10.3390/medicina57050444
APA StyleCimmino, G., Loffredo, F. S., Gallinoro, E., Prozzo, D., Fabiani, D., Cante, L., Salerno, G., Cappelli Bigazzi, M., & Golino, P. (2021). Percutaneous Left Atrial Appendage Occlusion: An Emerging Option in Patients with Atrial Fibrillation at High Risk of Bleeding. Medicina, 57(5), 444. https://doi.org/10.3390/medicina57050444






