Challenges of Diagnosing Antibody-Mediated Rejection: The Role of Invasive and Non-Invasive Biomarkers
Abstract
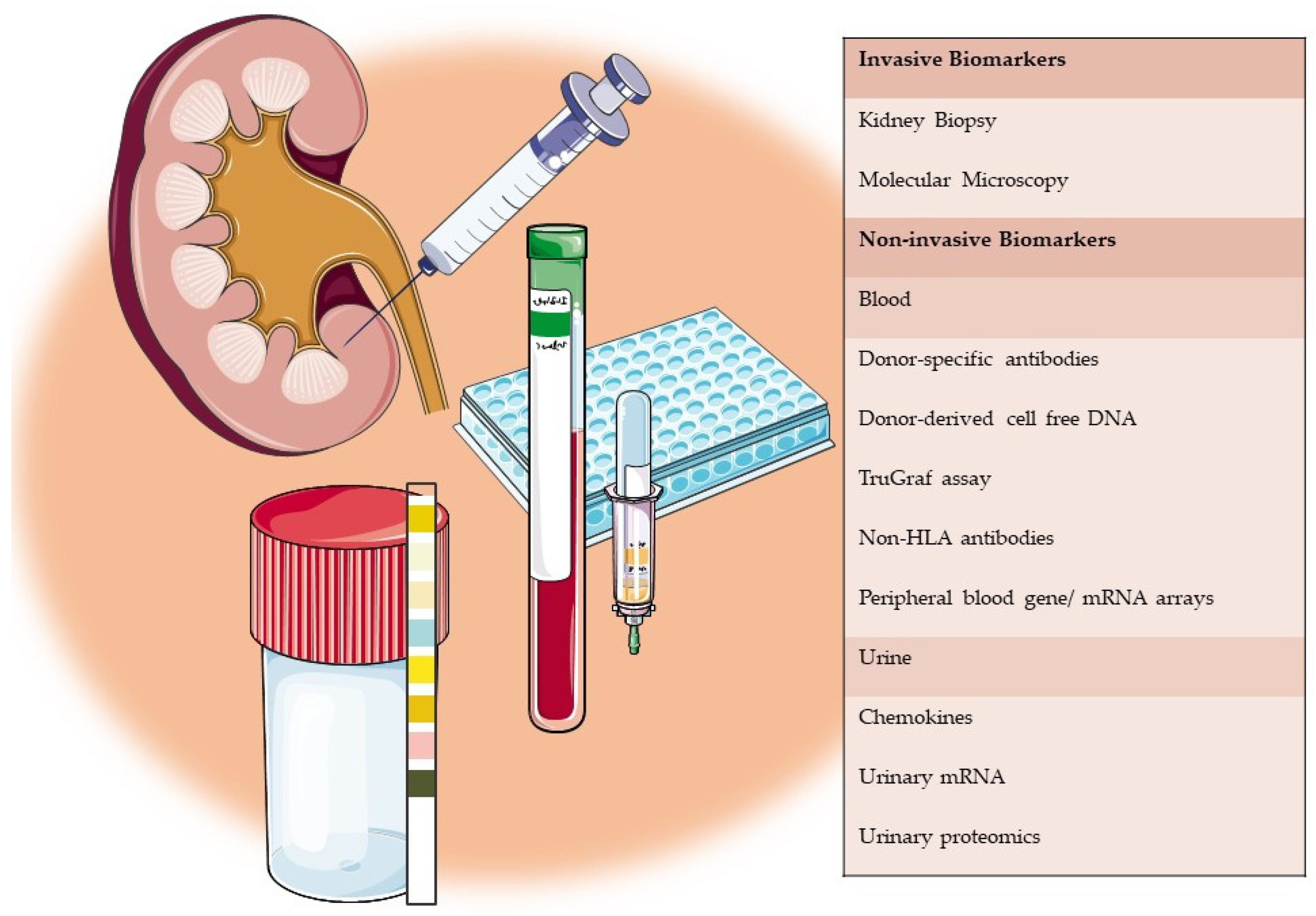
1. Introduction
2. Invasive Biomarkers
2.1. Kidney Biopsy—Banff Classification
2.2. Molecular Microscopy Diagnostic System
3. Non-Invasive Biomarkers
3.1. Blood
3.1.1. Donor-Specific Antibodies
3.1.2. Donor-Derived Cell Free DNA
3.1.3. Peripheral Blood Gene Expression Profiling
3.1.4. Non-HLA Antibodies
3.1.5. Peripheral Blood Gene/mRNA Arrays
3.2. Urine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OPTN Kidney Kaplan Meier Patient Survival Rates for Transplants Performed: 2008-2015: OPTN/ HRSA; 2021 [Updated Based on OPTN data as of 26 February 2021]. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (accessed on 26 February 2021).
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long Term Kidney Transplant Graft Survival–Making Progress When Most Needed. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Clayton, P.A.; McDonald, S.P.; Russ, G.R.; Chadban, S.J. Long-Term Outcomes after Acute Rejection in Kidney Transplant Recipients: An ANZDATA Analysis. J. Am. Soc. Nephrol. 2019, 30, 1697–1707. [Google Scholar] [CrossRef]
- Halloran, P.F.; Chang, J.; Famulski, K.; Hidalgo, L.G.; Salazar, I.D.; Lopez, M.M.; Matas, A.; Picton, M.; De Freitas, D.; Bromberg, J.; et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2014, 26, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Sellarés, J.; De Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am. J. Transplant. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Aubert, O.; Loupy, A.; Hidalgo, L.; Van Huyen, J.-P.D.; Higgins, S.; Viglietti, D.; Jouven, X.; Glotz, D.; Legendre, C.; Lefaucheur, C.; et al. Antibody-Mediated Rejection Due to Preexisting versusDe NovoDonor-Specific Antibodies in Kidney Allograft Recipients. J. Am. Soc. Nephrol. 2017, 28, 1912–1923. [Google Scholar] [CrossRef]
- El-Zoghby, Z.M.; Stegall, M.D.; Lager, D.J.; Kremers, W.K.; Amer, H.; Gloor, J.M.; Cosio, F.G. Identifying Specific Causes of Kidney Allograft Loss. Am. J. Transplant. 2009, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Einecke, G.; Sis, B.; Reeve, J.; Mengel, M.; Campbell, P.M.; Hidalgo, L.G.; Kaplan, B.; Halloran, P.F. Antibody-Mediated Microcirculation Injury Is the Major Cause of Late Kidney Transplant Failure. Am. J. Transplant. 2009, 9, 2520–2531. [Google Scholar] [CrossRef]
- Gaston, R.S.; Cecka, J.M.; Kasiske, B.L.; Fieberg, A.M.; LeDuc, R.; Cosio, F.C.; Gourishankar, S.; Grande, J.; Halloran, P.; Hunsicker, L.; et al. Evidence for Antibody-Mediated Injury as a Major Determinant of Late Kidney Allograft Failure. Transplantation 2010, 90, 68–74. [Google Scholar] [CrossRef]
- Hidalgo, L.; Campbell, P.; Sis, B.; Einecke, G.; Mengel, M.; Chang, J.; Sellares, J.; Reeve, J.; Halloran, P. De novo donor—Specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am. J. Transplant. 2009, 9, 2532–2541. [Google Scholar] [CrossRef]
- Loupy, A.; Hill, G.S.; Jordan, S.C. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat. Rev. Nephrol. 2012, 8, 348–357. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C. Antibody-Mediated Rejection of Solid-Organ Allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Suberbielle—Boissel, C.; Hill, G.; Lefaucheur, C.; Anglicheau, D.; Zuber, J.; Martinez, F.; Thervet, E.; Méjean, A.; Charron, D. Outcome of subclinical antibody—Mediated rejection in kidney transplant recipients with preformed donor—Specific antibodies. Am. J. Transplant. 2009, 9, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Hill, G.; Suberbielle, C.; Charron, D.; Anglicheau, D.; Zuber, J.; Timsit, M.; Duong, J.; Bruneval, P.; Vernerey, D. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor—Specific antibodies (DSA). Am. J. Transplant. 2011, 11, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Furness, P.N.; Taub, N.; Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project. International variation in the interpretation of renal transplant biopsies: Report of the CERTPAP Project. Kidney Int. 2001, 60, 1998–2012. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Gelpi, R.; Helanterä, I.; Melilli, E.; Honkanen, E.; Bestard, O.; Grinyo, J.M.; Cruzado, J.M. Decreased Kidney Graft Survival in Low Immunological Risk Patients Showing Inflammation in Normal Protocol Biopsies. PLoS ONE 2016, 11, e0159717. [Google Scholar] [CrossRef] [PubMed]
- Erpicum, P.; Hanssen, O.; Weekers, L.; Lovinfosse, P.; Meunier, P.; Tshibanda, L.; Krzesinski, J.-M.; Hustinx, R.; Jouret, F. Non-invasive approaches in the diagnosis of acute rejection in kidney transplant recipients, part II: Omics analyses of urine and blood samples. Clin. Kidney J. 2016, 10, 106–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anglicheau, D.; Naesens, M.; Essig, M.; Gwinner, W.; Marquet, P. Establishing biomarkers in transplant medicine: A critical review of current approaches. Transplantation 2016, 100, 2024–2038. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Malone, A. Biomarkers to detect rejection after kidney transplantation. Pediatr. Nephrol. 2017, 33, 1113–1122. [Google Scholar] [CrossRef]
- Solez, K.; Axelsen, R.A.; Benediktsson, H.; Burdick, J.F.; Cohen, A.H.; Colvin, R.B.; Croker, B.P.; Droz, D.; Dunnill, M.S.; Halloran, P.F.; et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int. 1993, 44, 411–422. [Google Scholar] [CrossRef]
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.; Halloran, P.; Colvin, R.; Akalin, E. The Banff 2017 Kidney Meeting Report: Revised Diagnostic Criteria for Chronic Active T Cell—Mediated Rejection, Antibody—Mediated Rejection, and Prospects for Integrative Endpoints for Next—Generation Clinical Trials; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Halloran, P.F.; Famulski, K.S.; Reeve, P.F.H.K.S.F.J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat. Rev. Nephrol. 2016, 12, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.B.; Suthanthiran, M. Transcriptional profiling to assess the clinical status of kidney transplants. Nat. Clin. Pract. Nephrol. 2006, 2, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J.; Sellarés, J.; Mengel, M.; Sis, B.; Skene, A.; Hidalgo, L.; De Freitas, D.; Famulski, K.; Halloran, P. Molecular diagnosis of T cell—Mediated rejection in human kidney transplant biopsies. Am. J. Transplant. 2013, 13, 645–655. [Google Scholar] [CrossRef]
- Venner, J.; Famulski, K.; Badr, D.; Hidalgo, L.; Chang, J.; Halloran, P. Molecular Landscape of T Cell–Mediated Rejection in Human Kidney Transplants: Prominence of CTLA4 and PD Ligands. Am. J. Transplant. 2014, 14, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Sellarés, J.; Reeve, J.; Loupy, A.; Mengel, M.; Sis, B.; Skene, A.; De Freitas, D.; Kreepala, C.; Hidalgo, L.; Famulski, K. Molecular diagnosis of antibody—Mediated rejection in human kidney transplants. Am. J. Transplant. 2013, 13, 971–983. [Google Scholar] [CrossRef]
- Halloran, P.; Pereira, A.; Chang, J.; Matas, A.; Picton, M.; De Freitas, D.; Bromberg, J.; Serón, D.; Sellarés, J.; Einecke, G. Potential impact of microarray diagnosis of T cell–mediated rejection in kidney transplants: The INTERCOM study. Am. J. Transplant. 2013, 13, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Willicombe, M.; Brookes, P.; Sergeant, R.; Santos-Nunez, E.; Steggar, C.; Galliford, J.; Mclean, A.; Cook, T.H.; Cairns, T.; Roufosse, C. De novo DQ donor—Specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation 2012, 94, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ginevri, F.; Nocera, A.; Comoli, P.; Innocente, A.; Cioni, M.; Parodi, A.; Fontana, I.; Magnasco, A.; Nocco, A.; Tagliamacco, A. Posttransplant de novo donor—Specific HLA antibodies identify pediatric kidney recipients at risk for late antibody—Mediated rejection. Am. J. Transplant. 2012, 12, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Palanisamy, A.; Tsapepas, D.; Tanriover, B.; Crew, R.J.; Dube, G.; Ratner, L.E.; Cohen, D.J.; Radhakrishnan, J. Donor-Specific Antibodies Adversely Affect Kidney Allograft Outcomes. J. Am. Soc. Nephrol. 2012, 23, 2061–2071. [Google Scholar] [CrossRef]
- Wiebe, C.; Gibson, I.; Blydt—Hansen, T.; Karpinski, M.; Ho, J.; Storsley, L.; Goldberg, A.; Birk, P.; Rush, D.; Nickerson, P. Evolution and clinical pathologic correlations of de novo donor—Specific HLA antibody post kidney transplant. Am. J. Transplant. 2012, 12, 1157–1167. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; Van Huyen, J.-P.D.; Mooney, N.; Suberbielle, C.; Frémeaux-Bacchi, V.; Méjean, A.; Desgrandchamps, F.; et al. Complement-Binding Anti-HLA Antibodies and Kidney-Allograft Survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar] [CrossRef]
- Lee, H.; Han, E.; Choi, A.-R.; Ban, T.H.; Chung, B.H.; Yang, C.W.; Choi, Y.J.; Oh, E.-J. Clinical impact of complement (C1q, C3d) binding De Novo donor-specific HLA antibody in kidney transplant recipients. PLoS ONE 2018, 13, e0207434. [Google Scholar] [CrossRef]
- Bailly, E.; Anglicheau, D.; Blancho, G.; Gatault, P.; Vuiblet, V.; Chatelet, V.; Morelon, E.; Malvezzi, P.; Parissiadis, A.; Tourret, J. Prognostic value of the persistence of C1q-binding anti-HLA antibodies in acute antibody-mediated rejection in kidney transplantation. Transplantation 2018, 102, 688–698. [Google Scholar] [CrossRef]
- Lee, D.; Kim, B.; Kim, J.; Kim, I.; Jeon, M. (Eds.) C3d-binding donor-specific hla antibody is associated with a high risk of antibody-mediated rejection and graft loss in stable kidney transplant recipients: A single-center cohort study. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hayde, N.; Solomon, S.; Caglar, E.; Ge, J.; Qama, E.; Colovai, A. C1q—Binding DSA and allograft outcomes in pediatric kidney transplant recipients. Pediatr. Transplant. 2021, 25, e13885. [Google Scholar] [CrossRef]
- Gebel, H.M.; Bray, R.A. A diagnostic ‘C’ saw: The ups and downs of C1q testing. Curr. Opin. Organ Transplant. 2019, 24, 402–410. [Google Scholar] [CrossRef]
- Courant, M.; Visentin, J.; Linares, G.; Dubois, V.; Lepreux, S.; Guidicelli, G.; Thaunat, O.; Merville, P.; Couzi, L.; Taupin, J.-L. The disappointing contribution of anti-human leukocyte antigen donor-specific antibodies characteristics for predicting allograft loss. Nephrol. Dial. Transplant. 2018, 33, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.G.; Olagne, J.; Parissiadis, A.; Joly, M.; Cognard, N.; Perrin, P.; Froelich, N.; Guntz, P.; Gachet, C.; Moulin, B. Does a Useful Test Exist to Properly Evaluate the Pathogenicity of Donor-specific Antibodies? Lessons from a Comprehensive Analysis in a Well-studied Single-center Kidney Transplant Cohort. Transplantation 2019, 104, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Eskandary, F.; Bond, G.; Kozakowski, N.; Regele, H.; Marinova, L.; Wahrmann, M.; Kikic, Ž.; Haslacher, H.; Rasoul-Rockenschaub, S.; Kaltenecker, C.C. Diagnostic contribution of donor-specific antibody characteristics to uncover late silent antibody-mediated rejection—Results of a cross-sectional screening study. Transplantation 2017, 101, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Nocera, A.; Tagliamacco, A.; Cioni, M.; Innocente, A.; Fontana, I.; Barbano, G.; Carrea, A.; Ramondetta, M.; Sementa, A.; Basso, S. Kidney intragraft homing of de novo donor—Specific HLA antibodies is an essential step of antibody—Mediated damage but not per se predictive of graft loss. Am. J. Transplant. 2017, 17, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, T.; Couzi, L.; Lepreux, S.; Legeret, M.; Pariscoat, G.; Guidicelli, G.; Merville, P.; Taupin, J.L. Kidney intragraft donor—Specific antibodies as determinant of antibody—Mediated lesions and poor graft outcome. Am. J. Transplant. 2013, 13, 2855–2864. [Google Scholar] [CrossRef]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Oellerich, M.; Schulz, U.; Schauerte, V.; Reinhard, L.; Fuchs, U.; Knabbe, C.; Zittermann, A.; Olbricht, C.; Gummert, J.; et al. Donor-Derived Cell-Free DNA Is a Novel Universal Biomarker for Allograft Rejection in Solid Organ Transplantation. Transplant. Proc. 2015, 47, 2400–2403. [Google Scholar] [CrossRef]
- Stites, E.; Kumar, D.; Olaitan, O.; John Swanson, S.; Leca, N.; Weir, M.; Bromberg, J.; Melancon, J.; Agha, I.; Fattah, H.; et al. High levels of dd-cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am. J. Transplant. 2020, 20, 2491–2498. [Google Scholar] [CrossRef]
- Oellerich, M.; Shipkova, M.; Asendorf, T.; Walson, P.D.; Schauerte, V.; Mettenmeyer, N.; Kabakchiev, M.; Hasche, G.; Grone, H.J.; Friede, T.; et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am. J. Transplant. 2019, 19, 3087–3099. [Google Scholar] [CrossRef]
- Djamali, A.; Kaufman, D.B.; Ellis, T.M.; Zhong, W.; Matas, A.; Samaniego, M. Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am. J. Transplant. 2014, 14, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.G.; García, B.P.; Martín, J.M.B.; Suárez, F.O.; Alvarez, F.V. Cell-Free DNA as a Noninvasive Acute Rejection Marker in Renal Transplantation. Clin. Chem. 2009, 55, 1958–1966. [Google Scholar] [CrossRef]
- LO, Y.D.; TEIN, M.C.; PANG, C.P.; Yeung, C.K.; Tong, K.-L. Presence of donor-specific DNA in plasma of kidney and liver-transplant recipients. Lancet 1998, 351, 1329–1330. [Google Scholar] [CrossRef]
- Beck, J.; Bierau, S.; Balzer, S.; Andag, R.; Kanzow, P.; Schmitz, J.; Gaedcke, J.; Moerer, O.; Slotta, J.E.; Walson, P.; et al. Digital Droplet PCR for Rapid Quantification of Donor DNA in the Circulation of Transplant Recipients as a Potential Universal Biomarker of Graft Injury. Clin. Chem. 2013, 59, 1732–1741. [Google Scholar] [CrossRef]
- Loupy, A.; Vernerey, D.; Tinel, C.; Aubert, O.; Van Huyen, J.-P.D.; Rabant, M.; Verine, J.; Nochy, D.; Empana, J.-P.; Martinez, F.; et al. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allografts. J. Am. Soc. Nephrol. 2015, 26, 1721–1731. [Google Scholar] [CrossRef]
- Marsh, C.; Kurian, S.; Rice, J.; Whisenant, T.; David, J.; Rose, S.; Schieve, C.; Lee, D.; Case, J.; Barrick, B. (Eds.) Application of TruGraf v1: A novel molecular biomarker for managing kidney transplant recipients with stable renal function. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Lefaucheur, C.; Viglietti, D.; Bouatou, Y.; Philippe, A.; Pievani, D.; Aubert, O.; Van Huyen, J.-P.D.; Taupin, J.-L.; Glotz, D.; Legendre, C.; et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019, 96, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Sas-Strózik, A.; Donizy, P.; Kościelska-Kasprzak, K.; Kamińska, D.; Gawlik, K.; Mazanowska, O.; Madziarska, K.; Hałoń, A.; Krajewska, M.; Banasik, M. (Eds.) Angiotensin II type 1 receptor expression in renal transplant biopsies and anti-AT1R antibodies in serum indicates the risk of transplant loss. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fichtner, A.; Süsal, C.; Schröder, C.; Höcker, B.; Rieger, S.; Waldherr, R.; Westhoff, J.H.; Sander, A.; Dragun, D.; Tönshoff, B. Association of angiotensin II type 1 receptor antibodies with graft histology, function and survival in paediatric renal transplant recipients. Nephrol. Dial. Transplant. 2018, 33, 1065–1072. [Google Scholar] [CrossRef]
- Banasik, M.; Boratyńska, M.; Kościelska-Kasprzak, K.; Kamińska, D.; Bartoszek, D.; Żabińska, M.; Myszka, M.; Zmonarski, S.; Protasiewicz, M.; Nowakowska, B.; et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl. Int. 2014, 27, 1029–1038. [Google Scholar] [CrossRef]
- Giral, M.; Foucher, Y.; Dufay, A.; Van Huyen, J.D.; Renaudin, K.; Moreau, A.; Philippe, A.; Hegner, B.; Dechend, R.; Heidecke, H. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am. J. Transplant. 2013, 13, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Huh, K.H.; Park, Y.; Park, B.G.; Yang, J.; Jeong, J.C.; Lee, J.; Park, J.B.; Cho, J.-H.; Lee, S.; et al. The clinicopathological relevance of pretransplant anti-angiotensin II type 1 receptor antibodies in renal transplantation. Nephrol. Dial. Transplant. 2015, 32, 1244–1250. [Google Scholar] [CrossRef]
- Taniguchi, M.; Rebellato, L.; Cai, J.; Hopfield, J.; Briley, K.; Haisch, C.; Catrou, P.; Bolin, P.; Parker, K.; Kendrick, W. Higher risk of kidney graft failure in the presence of anti—Angiotensin II type—1 receptor antibodies. Am. J. Transplant. 2013, 13, 2577–2589. [Google Scholar] [CrossRef]
- Banasik, M.; Boratyńska, M.; Kościelska-Kasprzak, K.; Krajewska, M.; Mazanowska, O.; Kamińska, D.; Bartoszek, D.; Żabińska, M.; Myszka, M.; Nowakowska, B.; et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transpl. Immunol. 2014, 30, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Delville, M.; Lamarthée, B.; Anglicheau, D. Sensitization to endothelial cell antigens: Unraveling the cause or effect paradox. Hum. Immunol. 2019, 80, 614–620. [Google Scholar] [CrossRef]
- Riesco, L.; Irure, J.; Rodrigo, E.; Guiral, S.; Ruiz, J.C.; Gómez, J.; López-Hoyos, M.; Segundo, D.S. Anti-perlecan antibodies and acute humoral rejection in hypersensitized patients without forbidden HLA specificities after kidney transplantation. Transpl. Immunol. 2019, 52, 53–56. [Google Scholar] [CrossRef]
- Dieudé, M.; Cardinal, H.; Hébert, M.-J. Injury derived autoimmunity: Anti-perlecan/LG3 antibodies in transplantation. Hum. Immunol. 2019, 80, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Catar, R.; Philippe, A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016, 90, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Wiebe, C.; Hickey, M.J. The role of non-HLA antibodies in solid organ transplantation: A complex deliberation. Curr. Opin. Organ Transplant. 2020, 25, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Delville, M.; Lamarthée, B.; Pagie, S.; See, S.B.; Rabant, M.; Burger, C.; Gatault, P.; Giral, M.; Thaunat, O.; Arzouk, N. Early acute microvascular kidney transplant rejection in the absence of anti-HLA antibodies is associated with preformed IgG antibodies against diverse glomerular endothelial cell antigens. J. Am. Soc. Nephrol. 2019, 30, 692–709. [Google Scholar] [CrossRef]
- Van Loon, E.; Gazut, S.; Yazdani, S.; Lerut, E.; De Loor, H.; Coemans, M.; Noël, L.-H.; Thorrez, L.; Van Lommel, L.; Schuit, F.; et al. Development and validation of a peripheral blood mRNA assay for the assessment of antibody-mediated kidney allograft rejection: A multicentre, prospective study. EBioMedicine 2019, 46, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Roedder, S.; Sigdel, T.; Salomonis, N.; Hsieh, S.; Dai, H.; Bestard, O.; Metes, D.; Zeevi, A.; Gritsch, A.; Cheeseman, J.; et al. The kSORT Assay to Detect Renal Transplant Patients at High Risk for Acute Rejection: Results of the Multicenter AART Study. PLoS Med. 2014, 11, e1001759. [Google Scholar] [CrossRef]
- Van Loon, E.; Giral, M.; Anglicheau, D.; Lerut, E.; Dubois, V.; Rabeyrin, M.; Brouard, S.; Roedder, S.; Spigarelli, M.G.; Rabant, M. Diagnostic performance of kSORT, a blood—Based mRNA assay for noninvasive detection of rejection after kidney transplantation: A retrospective multicenter cohort study. Am. J. Transplant. 2021, 21, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Rabant, M.; Amrouche, L.; Morin, L.; Bonifay, R.; Lebreton, X.; Aouni, L.; Benon, A.; Sauvaget, V.; Le Vaillant, L.; Aulagnon, F. Early Low Urinary CXCL 9 and CXCL 10 Might Predict Immunological Quiescence in Clinically and Histologically Stable Kidney Recipients. Am. J. Transplant. 2016, 16, 1868–1881. [Google Scholar] [CrossRef]
- Rabant, M.; Amrouche, L.; Lebreton, X.; Aulagnon, F.; Benon, A.; Sauvaget, V.; Bonifay, R.; Morin, L.; Scemla, A.; Delville, M.; et al. Urinary C-X-C Motif Chemokine 10 Independently Improves the Noninvasive Diagnosis of Antibody–Mediated Kidney Allograft Rejection. J. Am. Soc. Nephrol. 2015, 26, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Van de Vrie, M.; Deegens, J.; Eikmans, M.; van der Vlag, J.; Hilbrands, L. Urinary microRNA as biomarker in renal transplantation. Am. J. Transplant. 2017, 17, 1160–1166. [Google Scholar] [CrossRef]
- El Fekih, R.; Hurley, J.; Tadigotla, V.; Alghamdi, A.; Srivastava, A.; Coticchia, C.; Choi, J.; Allos, H.; Yatim, K.; Alhaddad, J.; et al. Discovery and Validation of a Urinary Exosome mRNA Signature for the Diagnosis of Human Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Kanzelmeyer, N.K.; Zürbig, P.; Mischak, H.; Metzger, J.; Fichtner, A.; Ruszai, K.H.; Seemann, T.; Hansen, M.; Wygoda, S.; Krupka, K. Urinary proteomics to diagnose chronic active antibody—Mediated rejection in pediatric kidney transplantation—A pilot study. Transpl. Int. 2019, 32, 28–37. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnamoorthy, S.; Kyeso, Y. Challenges of Diagnosing Antibody-Mediated Rejection: The Role of Invasive and Non-Invasive Biomarkers. Medicina 2021, 57, 439. https://doi.org/10.3390/medicina57050439
Krishnamoorthy S, Kyeso Y. Challenges of Diagnosing Antibody-Mediated Rejection: The Role of Invasive and Non-Invasive Biomarkers. Medicina. 2021; 57(5):439. https://doi.org/10.3390/medicina57050439
Chicago/Turabian StyleKrishnamoorthy, Sambhavi, and Yousuf Kyeso. 2021. "Challenges of Diagnosing Antibody-Mediated Rejection: The Role of Invasive and Non-Invasive Biomarkers" Medicina 57, no. 5: 439. https://doi.org/10.3390/medicina57050439
APA StyleKrishnamoorthy, S., & Kyeso, Y. (2021). Challenges of Diagnosing Antibody-Mediated Rejection: The Role of Invasive and Non-Invasive Biomarkers. Medicina, 57(5), 439. https://doi.org/10.3390/medicina57050439




