Aspirin Desensitization: Implications for Acetylsalicylic Acid-Sensitive Pregnant Women
Abstract
1. Introduction
2. Obstetric Complications Preventable by ASA
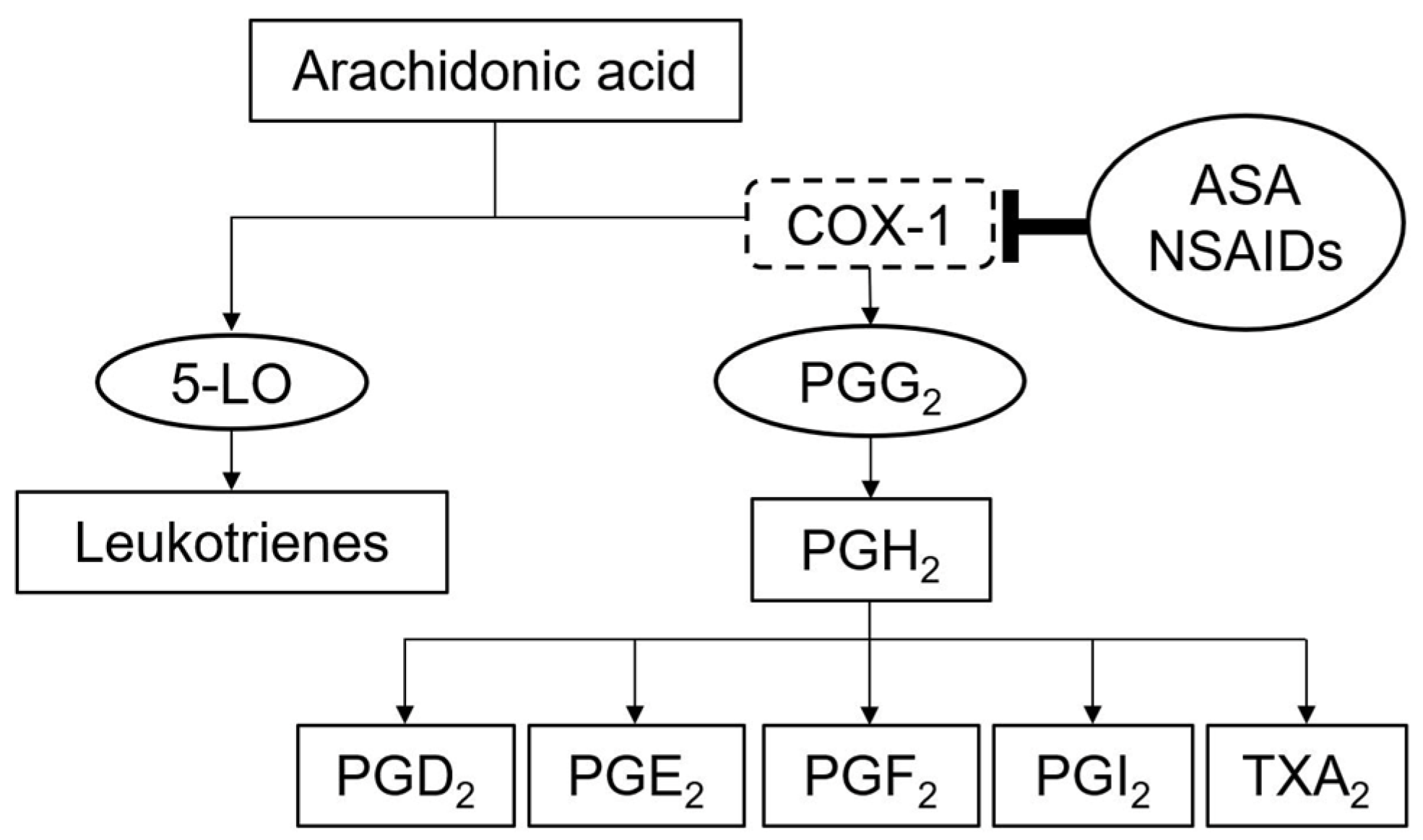
3. ASA Hypersensitivity
4. Diagnosis of ASA and NSAID Hypersensitivity Reactions
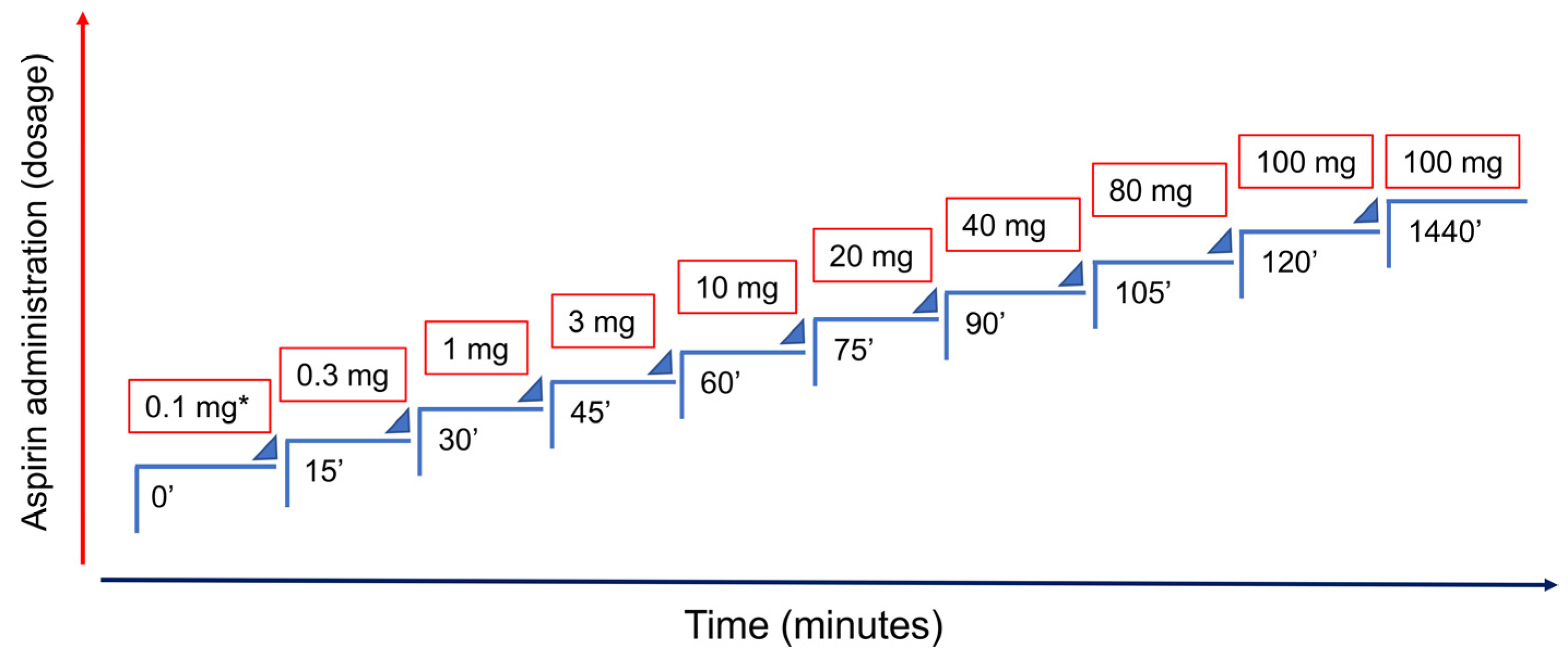
5. ASA Desensitization Therapy and Pregnancy
6. Practical Approach for ASA-Sensitive Pregnant Women
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: Low-dose aspirin use during pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar] [CrossRef]
- Settipane, R.A.; Constantine, H.P.; Settipane, G.A. Aspirin intolerance and recurrent urticaria in normal adults and children. Epidemiology and review. Allergy 1980, 35, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Cardoso, M.F.; Praca, F.; Gomes, L.; Marino, E.; Demoly, P. Self-reported drug allergy in a general adult Portuguese population. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Gomes, E.R.; Demoly, P. Epidemiology of hypersensitivity drug reactions. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Makowska, J.S.; Blanca, M.; Bavbek, S.; Bochenek, G.; Bousquet, J.; Bousquet, P.; Celik, G.; Demoly, P.; Gomes, E.R.; et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs)—classification, diagnosis and management: Review of the EAACI/ENDA(#) and GA2LEN/HANNA. Allergy 2011, 66, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Miguel-Moncin, M.S.; Cistero-Bahima, A. Aspirin desensitization in the treatment of antiphospholipid syndrome during pregnancy in ASA-sensitive patients. Am. J. Reprod. Immunol. 2006, 55, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cernadas, J.R.; Leblanc, A.; de Castro, E.D. Desensitisation to aspirin in antiphospholipid antibody syndrome. Allergol. Et Immunopathol. 2013, 41, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Gaspar, A.; Livramento, S.; Sampaio, G.; Morais-Almeida, M. Aspirin desensitization in a woman with inherited thrombophilia and recurrent miscarriage. Eur. Ann. Allergy Clin. Immunol. 2012, 44, 256–257. [Google Scholar]
- Atallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for prevention of preeclampsia. Drugs 2017, 77, 1819–1831. [Google Scholar] [CrossRef]
- Groom, K.M.; David, A.L. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S829–S840. [Google Scholar] [CrossRef]
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 80–83. [Google Scholar]
- Redman, C.W.G.; Jacobson, S.-L.; Russell, R. Hypertension in pregnancy. In de Swiet’s Medical Disorders in Obstetric Practice; Powrie, R., Greene, M., Camann, W., Eds.; Blackwell Publishing: Oxford, UK, 2010; pp. 153–181. [Google Scholar]
- Makino, S.; Takeda, J.; Takeda, S.; Watanabe, K.; Matsubara, K.; Nakamoto, O.; Ushijima, J.; Ohkuchi, A.; Koide, K.; Mimura, K.; et al. New definition and classification of “Hypertensive Disorders of Pregnancy (HDP). Hypertens. Res. Pregnancy 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Roberge, S.; Odibo, A.O.; Bujold, E. Aspirin for the prevention of preeclampsia and intrauterine growth restriction. Clin. Lab. Med. 2016, 36, 319–329. [Google Scholar] [CrossRef]
- Crandon, A.J.; Isherwood, D.M. Effect of aspirin on incidence of pre-eclampsia. Lancet 1979, 1, 1356. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120. [Google Scholar] [CrossRef]
- Meher, S.; Duley, L.; Hunter, K.; Askie, L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: An individual participant data meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; PG, D.E.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. JTH 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Garcia, D.; Erkan, D. Diagnosis and management of the antiphospholipid syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Di Prima, F.A.; Valenti, O.; Hyseni, E.; Giorgio, E.; Faraci, M.; Renda, E.; De Domenico, R.; Monte, S. Antiphospholipid syndrome during pregnancy: The state of the art. J. Prenat. Med. 2011, 5, 41–53. [Google Scholar]
- Schreiber, K.; Sciascia, S.; de Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Primers 2018, 4, 17103. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dorner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 92–128. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Cahill, K.N. Current knowledge and management of hypersensitivity to aspirin and NSAIDs. J. Allergy Clin. Immunol. Pract. 2017, 5, 537–545. [Google Scholar] [CrossRef]
- Stevenson, D.D.; Simon, R.A. Lack of cross-reactivity between rofecoxib and aspirin in aspirin-sensitive patients with asthma. J. Allergy Clin. Immunol. 2001, 108, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.V.; Blanca, M.; Garro, L.S.; Ribeiro, M.R.; Kalil, J.; Motta, A.A.; Castells, M.; Giavina-Bianchi, P. Nonsteroidal anti-inflammatory drugs are major causes of drug-induced anaphylaxis. J. Allergy Clin. Immunol. Pract. 2014, 2, 414–420. [Google Scholar] [CrossRef]
- Cabello, A.J.P.; Contreras, L.G.G.; Gamero, M.V.M.; Jimenez, F.J.G.; Canas, E.P. Prevalence of fatal adverse drug reactions in hospitalized patients. Int. J. Clin. Pharmacol. Ther. 2009, 47, 596–602. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Boyce, J.A. Aspirin-exacerbated respiratory disease--new prime suspects. N. Engl. J. Med. 2016, 374, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Zembowicz, A.; Mastalerz, L.; Setkowicz, M.; Radziszewski, W.; Szczeklik, A. Safety of cyclooxygenase 2 inhibitors and increased leukotriene synthesis in chronic idiopathic urticaria with sensitivity to nonsteroidal anti-inflammatory drugs. Arch. Dermatol. 2003, 139, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.; Nagy, C.S.; Krinzman, S.J.; Maclean, J.A.; Bloch, K.J. Rapid oral challenge-desensitization for patients with aspirin-related urticaria-angioedema. J. Allergy Clin. Immunol. 2000, 105, 997–1001. [Google Scholar] [CrossRef]
- Stevenson, D.D.; Simon, R.A. Selection of patients for aspirin desensitization treatment. J. Allergy Clin. Immunol. 2006, 118, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Aspirin desensitization as a treatment for aspirin-sensitive chronic spontaneous urticaria. Dermatol. Ther. 2015, 28, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.S.; Pichler, W.; et al. International consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Cernadas, J.R.; Brockow, K.; Romano, A.; Aberer, W.; Torres, M.J.; Bircher, A.; Campi, P.; Sanz, M.L.; Castells, M.; Demoly, P.; et al. General considerations on rapid desensitization for drug hypersensitivity—A consensus statement. Allergy 2010, 65, 1357–1366. [Google Scholar] [CrossRef]
- Widal, F.; Abrami, P.; Lermoyez, J. Anaphylaxie et idiosyncrasie. Allergy Proc. 1993, 14, 373–376. [Google Scholar]
- Stevenson, D.D.; Pleskow, W.W.; Simon, R.A.; Mathison, D.A.; Lumry, W.R.; Schatz, M.; Zeiger, R.S. Aspirin-sensitive rhinosinusitis asthma: A double-blind crossover study of treatment with aspirin. J. Allergy Clin. Immunol. 1984, 73, 500–507. [Google Scholar] [CrossRef]
- White, A.A.; Stevenson, D.D. Aspirin-exacerbated respiratory disease. N. Engl. J. Med. 2018, 379, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, L.P.; Galera, C.; Bousquet, P.J.; Piot, C.; Demoly, P. Protocole d’induction de tolérance pour l’hypersensibilité à l’aspirine. Rev. Fr. Allergol 2011, 51, 485–491. [Google Scholar] [CrossRef]
- Rossini, R.; Iorio, A.; Pozzi, R.; Bianco, M.; Musumeci, G.; Leonardi, S.; Lettieri, C.; Bossi, I.; Colombo, P.; Rigattieri, S.; et al. Aspirin desensitization in patients with coronary artery disease: Results of the multicenter adapted registry (aspirin desensitization in patients with coronary artery disease). Circulation. Cardiovasc. Interv. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Namazy, J.A.; Simon, R.A. Sensitivity to nonsteroidal anti-inflammatory drugs. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2002, 89, 542–550. [Google Scholar] [CrossRef]
- Gollapudi, R.R.; Teirstein, P.S.; Stevenson, D.D.; Simon, R.A. Aspirin sensitivity: Implications for patients with coronary artery disease. JAMA 2004, 292, 3017–3023. [Google Scholar] [CrossRef]
- Solensky, R. Drug desensitization. Immunol. Allergy Clin. N. Am. 2004, 24, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Desensitization for drug allergy. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Wendel, G.D.; Stark, B.J.; Jamison, R.B.; Molina, R.D.; Sullivan, T.J. Penicillin allergy and desensitization in serious infections during pregnancy. N. Engl. J. Med. 1985, 312, 1229–1232. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito-Garcia, F.; Pires, I.; Lima, J. Aspirin Desensitization: Implications for Acetylsalicylic Acid-Sensitive Pregnant Women. Medicina 2021, 57, 390. https://doi.org/10.3390/medicina57040390
Benito-Garcia F, Pires I, Lima J. Aspirin Desensitization: Implications for Acetylsalicylic Acid-Sensitive Pregnant Women. Medicina. 2021; 57(4):390. https://doi.org/10.3390/medicina57040390
Chicago/Turabian StyleBenito-Garcia, Filipe, Inês Pires, and Jorge Lima. 2021. "Aspirin Desensitization: Implications for Acetylsalicylic Acid-Sensitive Pregnant Women" Medicina 57, no. 4: 390. https://doi.org/10.3390/medicina57040390
APA StyleBenito-Garcia, F., Pires, I., & Lima, J. (2021). Aspirin Desensitization: Implications for Acetylsalicylic Acid-Sensitive Pregnant Women. Medicina, 57(4), 390. https://doi.org/10.3390/medicina57040390





