Population-Based COVID-19 Screening in Mexico: Assessment of Symptoms and Their Weighting in Predicting SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
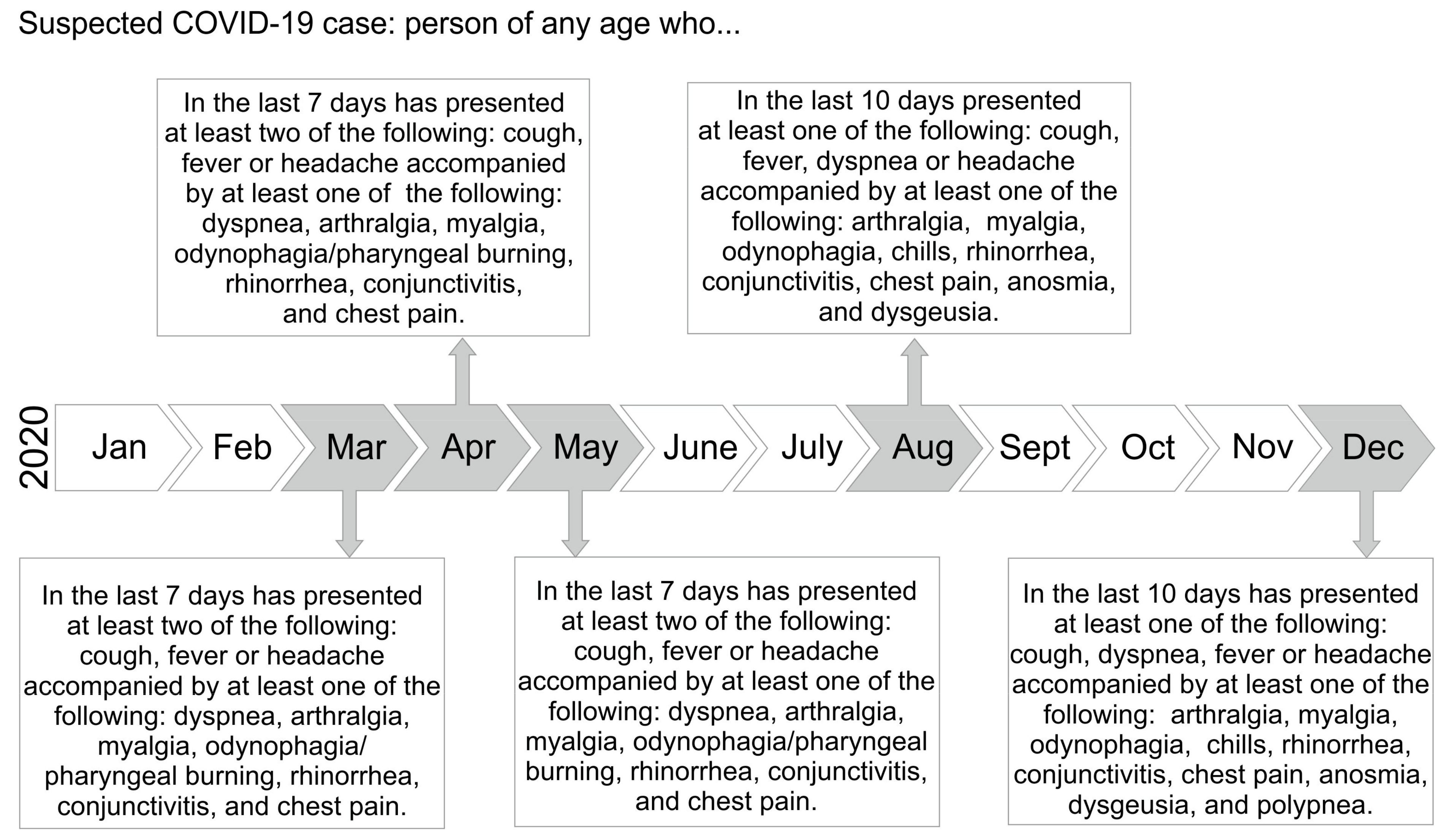
2.1. Patients and Study Definitions
2.2. Biological Samples and SARS-CoV-2 Screening
2.3. Data Analysis
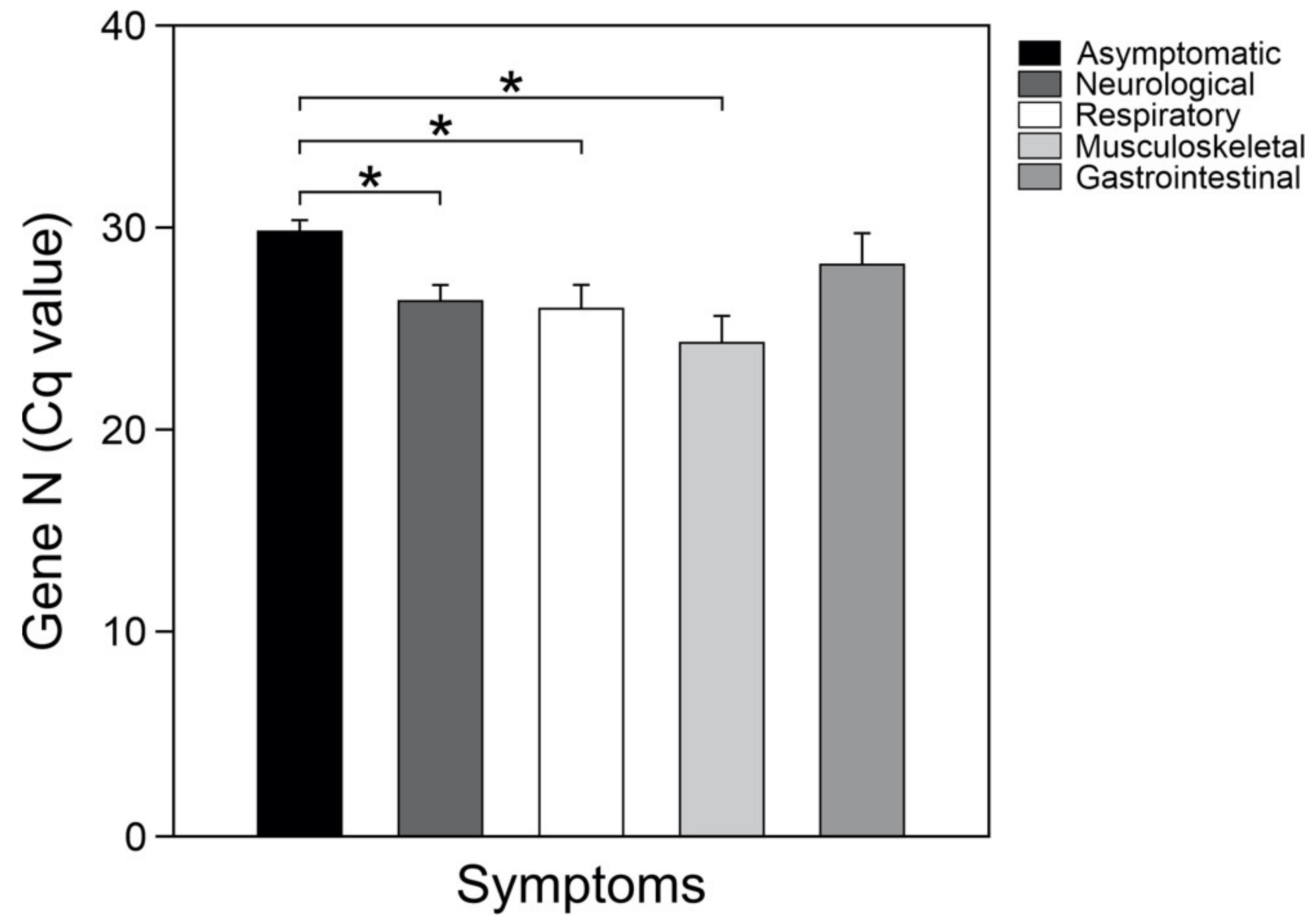
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ather, A.; Patel, B.; Ruparel, N.B.; Diogenes, A.; Hargreaves, K.M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J. Endod. 2020, 46, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet. Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Mendez-Dominguez, N.; Alvarez-Baeza, A.; Carrillo, G. Demographic and Health Indicators in Correlation to Interstate Variability of Incidence, Confirmation, Hospitalization, and Lethality in Mexico: Preliminary Analysis from Imported and Community Acquired Cases during COVID-19 Outbreak. Int. J. Environ. Res. Public Health 2020, 17, 4281. [Google Scholar] [CrossRef] [PubMed]
- Nazeam, J.; Mohammed, E.Z.; Raafat, M.; Houssein, M.; Elkafoury, A.; Hamdy, D.; Jamil, L. Based on Principles and Insights of COVID-19 Epidemiology, Genome Sequencing, and Pathogenesis: Retrospective Analysis of Sinigrin and Prolixin(RX) (Fluphenazine) Provides Off-Label Drug Candidates. SLAS Discov. 2020, 25, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Soleimani, J.; Herasevich, S.; Pinevich, Y.; Pennington, K.M.; Dong, Y.; Pickering, B.W.; Barwise, A.K. Clinical Characteristics, Treatment, and Outcomes of Critically Ill Patients With COVID-19: A Scoping Review. Mayo Clin. Proc. 2021, 96, 183–202. [Google Scholar] [CrossRef]
- Popov, G.T.; Baymakova, M.; Vaseva, V.; Kundurzhiev, T.; Mutafchiyski, V. Clinical Characteristics of Hospitalized Patients with COVID-19 in Sofia, Bulgaria. Vector Borne Zoonotic Dis. 2020, 20, 910–915. [Google Scholar] [CrossRef]
- Pongpirul, W.A.; Mott, J.A.; Woodring, J.V.; Uyeki, T.M.; MacArthur, J.R.; Vachiraphan, A.; Suwanvattana, P.; Uttayamakul, S.; Chunsuttiwat, S.; Chotpitayasunondh, T.; et al. Clinical Characteristics of Patients Hospitalized with Coronavirus Disease, Thailand. Emerg. Infect. Dis. 2020, 26, 1580–1585. [Google Scholar] [CrossRef]
- Al Nsour, M.; Bashier, H.; Al Serouri, A.; Malik, E.; Khader, Y.; Saeed, K.; Ikram, A.; Abdalla, A.M.; Belalia, A.; Assarag, B.; et al. The Role of the Global Health Development/Eastern Mediterranean Public Health Network and the Eastern Mediterranean Field Epidemiology Training Programs in Preparedness for COVID-19. JMIR Public Health Surveill. 2020, 6, e18503. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ibrahim, N.K. Epidemiologic surveillance for controlling Covid-19 pandemic: Types, challenges and implications. J. Infect. Public Health 2020, 13, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Epidemiología. Lineamiento Estandarizado Para la Vigilancia Epidemiológica y por Laboratorio de Enfermedad por 2019-nCoV; Secretaría de Salud México: Mexico City, Mexico, 2020. [Google Scholar]
- Mexico S.D.S.G.D. Información General COVID-19 México. Mexico 2020. [Google Scholar]
- Mexico S.D.S.G.D. Algoritmos Interinos Para la Atención del COVID-19. Gobierno de Mexico: Ciudad de Mexico. 2020. p. 33. Available online: http://educacionensalud.imss.gob.mx/es/system/files/Algoritmos_interinos_COVID19_CTEC.pdf (accessed on 1 August 2020).
- Martinez-Fierro, M.L.; Rios-Jasso, J.; Garza-Veloz, I.; Reyes-Veyna, L.; Cerda-Luna, R.M.; Duque-Jara, I.; Galvan-Jimenez, M.; Ramirez-Hernandez, L.A.; Morales-Esquivel, A.; Ortiz-Castro, Y.; et al. The role of close contacts of COVID-19 patients in the SARS-CoV-2 transmission: An emphasis on the percentage of nonevaluated positivity in Mexico. Am. J. Infect. Control. 2021, 49, 15–20. [Google Scholar] [CrossRef]
- Gao, M.; Yang, L.; Chen, X.; Deng, Y.; Yang, S.; Xu, H.; Chen, Z.; Gao, X.J. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir. Med. 2020, 169, 106026. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.A.; Maldonado, Y.A.; Tompkins, L.S.; Wald, S.H.; Chawla, A.; Hawn, M.T. Asymptomatic SARS-CoV-2 Transmission from Community Contacts in Healthcare Workers. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Just, J.; Puth, M.T.; Regenold, F.; Weckbecker, K.; Bleckwenn, M. Risk factors for a positive SARS-CoV-2 PCR in patients with common cold symptoms in a primary care setting—A retrospective analysis based on a joint documentation standard. BMC Fam. Pract. 2020, 21, 251. [Google Scholar] [CrossRef]
- Nokhodian, Z.; Ranjbar, M.M.; Nasri, P.; Kassaian, N.; Shoaei, P.; Vakili, B.; Rostami, S.; Ahangarzadeh, S.; Alibakhshi, A.; Yarian, F.; et al. Current status of COVID-19 pandemic; characteristics, diagnosis, prevention, and treatment. J. Res. Med. Sci 2020, 25, 101. [Google Scholar] [CrossRef]
- Meneses Calderon, J.; Figueroa Flores, M.D.R.; Paniagua Coria, L.; Briones Garduno, J.C.; Meneses Figueroa, J.; Vargas Contretas, M.J.; De la Cruz Avila, L.; Diaz Meza, S.; Ramirez Chacon, R.; Padmanabhan, S.; et al. Nitazoxanide against COVID-19 in three explorative scenarios. J. Infect. Dev. Ctries 2020, 14, 982–986. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.J.T. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.J.T.L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Fernández-Rojas, M.A.; Esparza, M.A.; Campos-Romero, A.; Calva-Espinosa, D.Y.; Moreno-Camacho, J.L.; Langle-Martínez, A.P.; García-Gil, A.; Solís-González, C.J.; Canizalez-Román, A.; León-Sicairos, N.; et al. Epidemiology of COVID-19 in Mexico: Symptomatic profiles and presymptomatic people. Int. J. Infect. Dis. 2021, 104, 572–579. [Google Scholar] [CrossRef]
- Ñamendys-Silva, S.A.; Alvarado-Ávila, P.E.; Domínguez-Cherit, G.; Rivero-Sigarroa, E.; Sánchez-Hurtado, L.A.; Gutiérrez-Villaseñor, A.; Romero-González, J.P.; Rodríguez-Bautista, H.; García-Briones, A.; Garnica-Camacho, C.E.J.H.; et al. Outcomes of patients with COVID-19 in the intensive care unit in Mexico: A multicenter observational study. Heart Lung 2021, 50, 28–32. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Place, S.; Van Laethem, Y.; Cabaraux, P.; Mat, Q.; Huet, K.; Plzak, J.; Horoi, M.; Hans, S.; et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020, 288, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Slomka, A.; Kowalewski, M.; Zekanowska, E. Coronavirus Disease 2019 (COVID-19): A Short Review on Hematological Manifestations. Pathogens 2020, 9, 493. [Google Scholar] [CrossRef]
- Cohen, S.L.; Gianos, E.; Barish, M.A.; Chatterjee, S.; Kohn, N.; Lesser, M.; Giannis, D.; Coppa, K.; Hirsch, J.S.; McGinn, T.G.; et al. Prevalence and Predictors of Venous Thromboembolism or Mortality in Hospitalized COVID-19 Patients. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
| Comorbidity/Risk Factor | COVID-19 Cases (n = 325) | Controls (n = 954) | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Type 2 diabetes mellitus | 25 (7.69) | 30 (3.17) | <0.001 * | 2.5 | 1.471–4.390 |
| Obesity | 91 (28) | 206 (21.79) | 0.028 * | 1.3 | 1.047–1.859 |
| Smoking | 60 (18.46) | 207 (21.90) | 0.217 | 0.8 | 0.586–1.111 |
| Hypertension | 35 (10.76) | 81 (8.57) | 0.282 | 1.3 | 0.847–1.956 |
| Endocrinological disorders 1 | 6 (1.84) | 29 (3.04) | 0.346 | 0.6 | 0.247–1.458 |
| Allergies | 5 (1.53) | 23 (2.41) | 0.478 | 0.6 | 0.238–1.677 |
| Rheumatic diseases | 4 (1.23) | 18 (1.88) | 0.59 | 0.6 | 0.218–1.929 |
| Asthma | 8 (2.46) | 17 (1.79) | 0.61 | 1.4 | 0.589–3.223 |
| COPD 2 | 2 (0.61) | 93 (0.31) | 0.821 | 1.9 | 0.323–11.688 |
| Cardiovascular disease | 3 (0.92) | 11 (1.16) | 0.959 | 0.7 | 0.219–2.853 |
| Chronic kidney disease | 1 (0.30) | 5 (0.52) | 0.973 | 0.5 | 0.0675–4.985 |
| Symptoms (n, %) | COVID-19 Cases (n = 325) | Controls (n = 954) | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| Fever | 98 (30.15) | 95 (10.05) | <0.001 * | 3.8 | 2.811–5.308 |
| Dry cough | 115 (35.38) | 119 (12.59) | <0.001 * | 3.8 | 2.822–5.119 |
| Headache | 107 (32.92) | 184 (19.47) | <0.001 * | 2.03 | 1.531–2.691 |
| Chills | 39 (12.0) | 46 (4.84) | <0.001 * | 2.67 | 1.712–4.185 |
| Odynophagia | 85 (26.15) | 146 (15.45) | <0.001 * | 1.9 | 1.430–2.626 |
| Myalgia | 68 (20.92) | 87 (9.20) | <0.001 * | 2.6 | 1.845–3.690 |
| Arthralgia | 55 (16.92) | 65 (6.87) | <0.001 * | 2.7 | 1.878–4.049 |
| Asthenia | 67 (20.61) | 90 (9.52) | <0.001 * | 2.4 | 1.746–3.485 |
| Anosmia | 59 (18.15) | 29 (3.05) | <0.001 * | 7.03 | 4.420–11.202 |
| Dysgeusia | 61 (18.76) | 25 (2.63) | <0.001 * | 8.5 | 5.257–13.872 |
| Chest pain | 35 (10.76) | 50 (5.29) | 0.001 * | 2.1 | 1.375–3.394 |
| Irritability | 14 (4.30) | 12 (1.26) | 0.002 * | 3.5 | 1.609–7.680 |
| Rhinorrhea | 38 (11.62) | 58 (6.11) | 0.002 * | 2.03 | 1.323–3.127 |
| Diarrhea | 35 (10.76) | 55 (5.67) | 0.004 * | 1.9 | 1.258–3.058 |
| Dyspnea | 29 (8.92) | 43 (4.45) | 0.005 * | 2.05 | 1.260–3.351 |
| Abdominal pain | 26 (8.0) | 37 (3.91) | 0.005 * | 2.1 | 1.271–3.583 |
| Cyanosis | 11 (3.38) | 10 (1.05) | 0.01 * | 3.2 | 1.378–7.786 |
| Polypnea | 6 (1.84) | 7 (0.73) | 0.163 | 2.5 | 0.844–7.587 |
| Vomit | 8 (2.46) | 14 (1.48) | 0.357 | 1.6 | 0.698–4.038 |
| Conjunctivitis | 14 (4.30) | 30 (3.16) | 0.423 | 1.3 | 0.722–2.634 |
| Convulsion | 2 (0.61) | 5 (0.52) | 0.8 | 1.1 | 0.225–6.029 |
| Variable | Coefficient | Standard Error | Wald Statistic | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Oxygen saturation | −0.0964 | 0.0323 | 8.938 | 0.003 * | 0.908 | 0.852–0.967 |
| Fever | 0.736 | 0.253 | 8.472 | 0.004 * | 2.087 | 1.272–3.424 |
| Dysgeusia | 1.605 | 0.566 | 8.033 | 0.005 * | 4.976 | 1.641–15.094 |
| Asthenia | 0.555 | 0.269 | 4.237 | 0.040 * | 1.741 | 1.027–2.952 |
| Irritability | 1.119 | 0.687 | 2.654 | 0.103 | 3.063 | 0.797–11.774 |
| Dyspnea | −0.636 | 0.397 | 2.569 | 0.109 | 0.529 | 0.243–1.152 |
| T2DM | 0.430 | 0.354 | 1.475 | 0.225 | 1.537 | 0.768–3.074 |
| Obesity | 0.181 | 0.171 | 1.119 | 0.290 | 1.198 | 0.857–1.676 |
| Sex | −0.156 | 0.164 | 0.900 | 0.343 | 0.856 | 0.620–1.181 |
| Age | 0.0055 | 0.00609 | 0.818 | 0.366 | 1.006 | 0.994–1.018 |
| Diarrhea | 0.234 | 0.330 | 0.502 | 0.479 | 1.264 | 0.661–2.415 |
| Arthralgia | 0.249 | 0.393 | 0.403 | 0.526 | 1.283 | 0.594–2.770 |
| Rhinorrhea | 0.204 | 0.323 | 0.401 | 0.527 | 1.227 | 0.652–2.309 |
| Abdominal pain | −0.197 | 0.394 | 0.250 | 0.617 | 0.821 | 0.379–1.778 |
| Headache | 0.110 | 0.235 | 0.219 | 0.640 | 1.116 | 0.705–1.768 |
| Chills | 0.153 | 0.349 | 0.191 | 0.662 | 1.165 | 0.588–2.308 |
| Myalgia | 0.104 | 0.362 | 0.0825 | 0.774 | 1.109 | 0.546–2.254 |
| Dry cough | −0.000002 | 0.000009 | 0.0472 | 0.828 | 1.000 | 1.000–1.000 |
| Anosmia | 0.0861 | 0.565 | 0.0232 | 0.879 | 1.090 | 0.360–3.301 |
| Odynophagia | 0.0220 | 0.221 | 0.0099 | 0.921 | 1.022 | 0.663–1.576 |
| Chest pain | 0.0250 | 0.340 | 0.005 | 0.941 | 1.025 | 0.527–1.995 |
| Cyanosis | −0.0195 | 0.640 | 0.0009 | 0.976 | 0.981 | 0.280–3.437 |
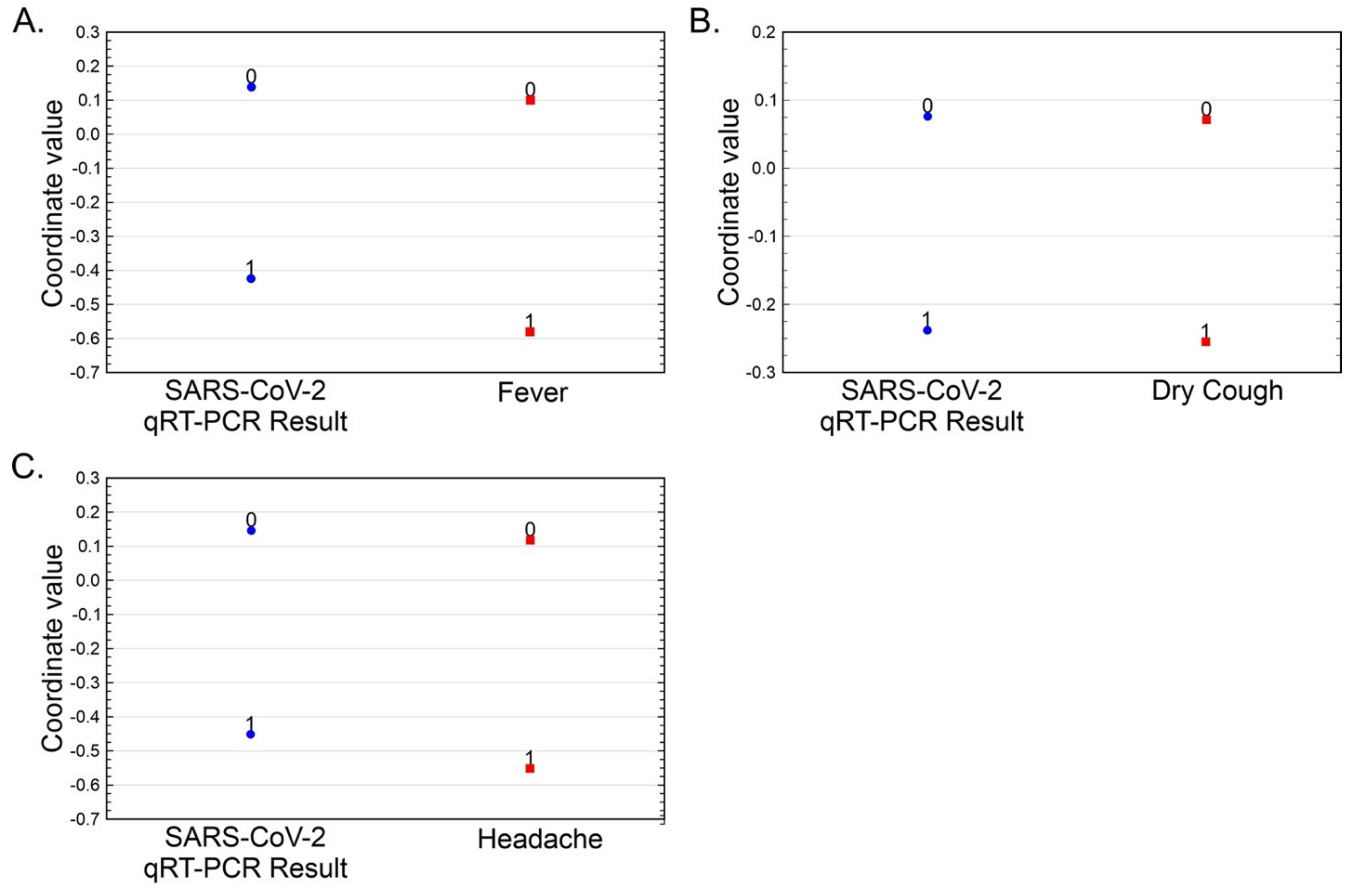
| Variable | qRT-PCR for SARS-CoV-2 | Eigen Value | Chi-Square | Degrees of Freedom | p-Value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Fever (%) | 7.72 | 17.89 | 0.05963 | 75.67 | 1 | <0.001 |
| Dry Cough (%) | 9.06 | 16.55 | 0.01945 | 83.41 | 1 | <0.001 |
| Headache (%) | 8.43 | 17.18 | 0.06573 | 24.68 | 1 | <0.001 |
| Variable/Study | MEX This Study | MEX [21] | CHN [22] | CHN [23] | CHN [24] | DEU [19] | MEX [16] | MEX [25] | MEX [26] | CHN [27] | BGR [8] | CHN [28] | BEL [29] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 cases | 325 | 16 | 41 | 1099 | 99 | 26 | 34 | 196,738 | 164 | 138 | 138 | 140 | 1420 |
| Age, mean (years) | 40.9 | 47.8 | 49.0 | 47.0 | 55.5 | 52.4 | - | 36 | 57.3 | 56 | 52.9 | 57 | 39.17 |
| Sex (male) | 215 (66.1) | 8 (50) | 30 (73) | 640 (58.1) | 67 (68) | 14 (35) | - | 244,171 | 114 (69.5) | 75 (54.3) | 87 (63) | 71(50.7) | 458 (32.3) |
| Fever | 98 (30.15) | 43.80 | 40 (98) | 473 (43.8) | 82 (83) | 9 (34.6) | 5 (14.7) | 60,209 (56.7) | 138 (84.1) | 136 (98.6) | 100 (138) | 110/120 (91.7) | 645 (45.4) |
| Dry cough | 115 (35.38) | 68.80 | 31 (76) | 745 (67.8) | 81 (82) | 19 (73.1) | 10 (29.4) | 60,720 (55.4) | 131 (79.8) | 82 (59.4) | 95 (68.8) | 90/120 (75.0) | 897 (63.2) |
| Headache | 107 (32.92) | 81.30 | 3 (8) | 150 (13.6) | 8 (8) | 3 (11.5) | 13 (38.2) | 106,103 (44.4) | 9 (6.5) | 95 (68.8) | - | 998 (70.3) | |
| Dyspnea | 29 (8.92) | 43.80 | 22 (55) | 205 (18.7) | 31 (31) | 4 (15.4) | 6 (17.6) | 34,588 (49.8) | 152 (92.6) | 43 (31.2) | 39 (28.52) | 44/120 (36.7) | 697 (49.1) |
| Irritability | 14 (4.30) | - | - | - | - | - | 6 (17.6) | - | - | - | - | ||
| Diarrhea | 35 (10.76) | - | 1 (3) | 42 (3.8) | 2 (2) | 1 (3.8) | 6 (17.6) | 32,843 (45.7) | 29 (17.6) | 14 (10.1) | 7 (5.0) | 18/139 (12.9) | 473 (38.1) |
| Chest pain | 35 (10.76) | 31.30 | - | - | 2 (2) | 9 (26.4) | - | - | - | - | - | 173 (27.2) * | |
| Chills | 286 (88) | - | - | 126 (11.5) | - | 5 (19.5) | 4 (11.7) | 39,003 (56.1) | - | - | - | - | - |
| Odynophagia | 85 (26.15) | - | - | - | - | 12 (35.2) | - | - | 24 (17.4) | 46 (33.3) | - | 274 (19.3) | |
| Myalgia | 68 (20.92) | 62.50 | 18 (44) | 164 (14.9) | 11 (11) | 7 (26.9) | 13 (38.2) | 93,926 (50.9) | 84 (51.2) | 48 (34.8) | 67 (48.5) | - | 887 (62.5) |
| Arthralgia | 55 (16.92) | 62.50 | - | with myalgia | - | 9 (26.4) | with myalgia | - | With myalgia | - | 519 (36.5) | ||
| Asthenia | 67 (20.61) | - | - | 419 (38.1) | - | 5 (19.2) | 7 (20.5) | - | - | 96 (69.6) | 124 (89.8) | 90/120 (75.0) | 514 (63.3) * |
| Rhinorrhea | 38 (11.62) | 50 | - | - | 4 (4) | 5 (19.2) | 10 (29.4) | 48,082 (51.4) | 31 (18.9) | - | - | - | 854 (60.1) |
| Polypnea | 6 (1.84) | - | - | - | - | 3 (8.8) | - | - | - | - | - | - | |
| Vomit | 8 (2.46) | - | - | 55 (5.0) | 1 (1) | 0 (0) | 0 (0.0) | 15,841 (49.8) | - | 5 (3.6) | - | 7/139 (5.0) | 272 (19.2) and nausea |
| Abdominal pain | 26 (8.0) | - | - | - | - | 0 (0) | 1 (2.9) | 17,515 (44.8) | - | 3 (2.2) | - | 8/139 (5.8) | 270 (19.1) |
| Conjunctivitis | 14 (4.30) | 6.30 | - | - | - | - | 5 (14.7) | 13,738 (46.6) | - | - | 12 (8.6) | - | 644 (45.4) |
| Anosmia | 59 (18.15) | 37.50 | - | 9 (0.8) | - | 7 (26.9) | 5 (14.7) | - | - | - | - | - | 997 (70.2) |
| Dysgeusia | 61 (18.76) | 37.50 | - | - | - | - | 3 (.8) | - | - | - | - | - | 770 (54.2) |
| Cyanosis | 11 (3.38) | - | - | - | - | - | 0 (0.0) | - | - | - | - | - | - |
| Convulsions | 2 (0.61) | - | - | - | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Fierro, M.L.; Diaz-Lozano, M.; Alvarez-Zuñiga, C.; Ramirez-Hernandez, L.A.; Araujo-Espino, R.; Trejo-Ortiz, P.M.; Mollinedo-Montaño, F.E.; Ortiz-Castro, Y.; Vazquez-Reyes, S.; Velasco-Elizondo, P.; et al. Population-Based COVID-19 Screening in Mexico: Assessment of Symptoms and Their Weighting in Predicting SARS-CoV-2 Infection. Medicina 2021, 57, 363. https://doi.org/10.3390/medicina57040363
Martinez-Fierro ML, Diaz-Lozano M, Alvarez-Zuñiga C, Ramirez-Hernandez LA, Araujo-Espino R, Trejo-Ortiz PM, Mollinedo-Montaño FE, Ortiz-Castro Y, Vazquez-Reyes S, Velasco-Elizondo P, et al. Population-Based COVID-19 Screening in Mexico: Assessment of Symptoms and Their Weighting in Predicting SARS-CoV-2 Infection. Medicina. 2021; 57(4):363. https://doi.org/10.3390/medicina57040363
Chicago/Turabian StyleMartinez-Fierro, Margarita L, Martha Diaz-Lozano, Claudia Alvarez-Zuñiga, Leticia A Ramirez-Hernandez, Roxana Araujo-Espino, Perla M Trejo-Ortiz, Fabiana E Mollinedo-Montaño, Yolanda Ortiz-Castro, Sodel Vazquez-Reyes, Perla Velasco-Elizondo, and et al. 2021. "Population-Based COVID-19 Screening in Mexico: Assessment of Symptoms and Their Weighting in Predicting SARS-CoV-2 Infection" Medicina 57, no. 4: 363. https://doi.org/10.3390/medicina57040363
APA StyleMartinez-Fierro, M. L., Diaz-Lozano, M., Alvarez-Zuñiga, C., Ramirez-Hernandez, L. A., Araujo-Espino, R., Trejo-Ortiz, P. M., Mollinedo-Montaño, F. E., Ortiz-Castro, Y., Vazquez-Reyes, S., Velasco-Elizondo, P., Garcia-Esquivel, L., Araujo-Conejo, A., & Garza-Veloz, I. (2021). Population-Based COVID-19 Screening in Mexico: Assessment of Symptoms and Their Weighting in Predicting SARS-CoV-2 Infection. Medicina, 57(4), 363. https://doi.org/10.3390/medicina57040363









