Low Serum Uric Acid Predicts Risk of a Composite Disease Endpoint
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Measurement of Risk Factors
2.3. Blood Samples
2.4. Definitions
2.5. Outcomes
2.6. Data Analysis
3. Results
Dichotomized SUA in the Whole Sample as a Predictor of Outcomes
4. Discussion
4.1. Stratified Analysis and Covariates of SUA
4.2. Associations Interacted with Diabetic Status and Sex
4.3. Predictive Value for All-Cause Mortality Risk and Nonfatal Events, as Well as Explanation
4.4. Former Smoking Status, a Clear Contributor to Adverse Outcomes
4.5. Conceivability of Various Chronic Diseases Partially Rising from Autoimmune Activation
4.6. Implications
4.7. Limitations and Strength
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.; Iribarren, C.; Gross, M.D.; Comstock, G.W.; Cutler, R.G. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis 2000, 148, 131–139. [Google Scholar] [CrossRef]
- Kanellis, J.; Kang, D.-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 2005, 25, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Kanellis, J.; Han, L.; Feng, L.; Xia, Y.-Y.; Chen, Q.; Kang, D.-H.; Gordon, K.L.; Watanabe, S.; Nakagawa, T.; et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Physiol. 2002, 282, F991–F997. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Tunstall-Pedoe, H.; Woodward, M. Serum uric acid and the risk of mortality during 23 years follow-up in the Scottish Heart Health Extended Cohort Study. Atherosclerosis 2014, 233, 623–629. [Google Scholar] [CrossRef]
- Verdoia, M.; Barbieri, L.; Schaffer, A.; Cassetti, E.; Nardin, M.; Bellomo, G.; Aimaretti, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Impact of diabetes on uric acid and its relationship with the extent of coronary artery disease and platelet aggregation: A single-centre cohort study. Metabolism 2014, 63, 640–646. [Google Scholar] [CrossRef]
- Ong, G.; Davis, W.A.; Davis, T.M.E. Serum uric acid does not predict cardiovascular or all-cause mortality in type 2 diabetes: The Fremantle Diabetes Study. Diabetologia 2010, 53, 1288–1294. [Google Scholar] [CrossRef]
- Kuo, C.-F.; See, L.-C.; Yu, K.-H.; Chou, I.-J.; Chiou, M.-J.; Luo, S.-F. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology 2012, 52, 127–134. [Google Scholar] [CrossRef]
- Onat, A.; Can, G. Enhanced pro-inflammatory state and autoimmune activation: A breakthrough to understanding chronic diseases. Curr. Pharm. Des. 2014, 20, 575–584. [Google Scholar] [CrossRef]
- Onat, A.; Çakir, H.; Karadeniz, Y.; Dönmez, I.; Karagöz, A.; Yüksel, M.; Can, G. TEKHARF 2013 taramasıvediyabetprevalansındahızlıartış [Turkish Adult Risk Factor survey 2013: Rapid rise in the prevalence of diabetes]. Turk. Kardiyoldernars. 2014, 42, 511–516. [Google Scholar]
- American Diabetes Association. Classification and diagnosis of Diabetes. Diabetes 8 Care. 2021, 44 (Suppl. 1), 15–33. [Google Scholar]
- Hu, L.; Hu, G.; Xu, B.P.; Zhu, L.; Zhou, W.; Wang, T.; Bao, H.; Cheng, X. U-Shaped Association of Serum Uric Acid with All-Cause and Cause-Specific Mortality in US Adults: A Cohort Study. J. Clin. Endocrinol. Metab. 2019, 105, e597–e609. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Furuhashi, M.; Tanaka, M.; Numata, K.; Hisasue, T.; Hanawa, N.; Koyama, M.; Osanami, A.; Higashiura, Y.; Inyaku, M.; et al. U-shaped relationship between serum uric acid level and decline in renal function during a 10-year period in female subjects: BOREAS-CKD2. Hypertens. Res. 2021, 44, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Yüksel, H.; Can, G.; Koroglu, B.; Kaya, A.; Altay, S. Serum creatinine is associated with coronary disease risk even in the absence of metabolic disorders. Scand. J. Clin. Lab. Investig. 2013, 73, 569–575. [Google Scholar] [CrossRef]
- Altay, S.; Onat, A.; Özpamuk-Karadeniz, F.; Karadeniz, Y.; Kemaloğlu-Öz, T.; Can, G. Renal “hyperfiltrators” are at elevated risk of death and chronic diseases. BMC Nephrol. 2014, 15, 160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onat, A.; Cakmak, H.A.; Can, G.; Yuksel, M.; Koroglu, B.; Yüksel, H. Serum total and high-density lipoprotein phospholipids: Independent predictive value for cardiometabolic risk. Clin. Nutr. 2014, 33, 815–822. [Google Scholar] [CrossRef]
- Onat, A.; Can, G.; Örnek, E.; Altay, S.; Yüksel, M.; Ademoğlu, E. Elevated serum uric acid levels in non-diabetic people mark pro-inflammatory state and HDL dysfunction and predict coronary disease. Clin. Rheumatol. 2013, 32, 1767–1775. [Google Scholar] [CrossRef]
- Puddu, P.E.; Menotti, A. The U-shaped risk of estimated glomerular filtration rate for all-cause mortality and the role of serum uric acid. Int. J. Cardiol. 2014, 174, 737–738. [Google Scholar] [CrossRef]
- Puddu, P.E.; Bilancio, G.; TerraduraVagnarelli, O.; Lombardi, C.; Mancini, M.; Zanchetti, A.; Menotti, A. Serum uric acid and eGFR_CKD-EPI differently predict long-term cardiovascular events and all causes of deaths in a residential cohort. Int. J. Cardiol. 2014, 171, 361–367. [Google Scholar] [CrossRef]
- Huang, J.; Hu, D.; Wang, Y.; Zhang, N.; Qu, Y. Dose–response relationship of serum uric acid levels with risk of stroke mortality. Atherosclerosis 2014, 234, 1–3. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Kusunoki, T.; Abe, M.; Miki, T. Interaction between serum uric acid and triglycerides in relation to prehypertension in community-dwelling Japanese adults. Clin. Exp. Hypertens. 2013, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Baek, S.; Ahn, S.H.; Kim, S.H.; Jo, M.-W.; Bae, S.J.; Kim, H.-K.; Choe, J.; Park, G.-M.; Kim, Y.-H.; et al. Higher serum uric acid as a protective factor against incident osteoporotic fractures in Korean men: A longitudinal study using the National Claim Registry. Osteoporos. Int. 2014, 25, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann. Rheum. Dis. 2009, 69, 1310–1314. [Google Scholar] [CrossRef]
- Onat, A.; Dönmez, I.; Karadeniz, Y.; Çakır, H.; Kaya, A. Type-2 diabetes and coronary heart disease: Common physiopathology, viewed from autoimmunity. Expert Rev. Cardiovasc. Ther. 2014, 12, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Villines, T.C.; Stanek, V.J. Paradoxical progression of atherosclerosis related to low-density lipoprotein reduction and exposure to ezetemibe. Eur. Heart J. 2012, 33, 2939–2945. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onat, A.; Can, G.; Murat, S.; Cicek, G.; Ornek, E.; Yuksel, H. Aggregation of lipoprotein(a) to apolipoprotein A-I underlying HDL dysfunction as a major coronary risk factor. AnadoluKardiyol. Dergisi Anatol. J. Cardiol. 2013, 13, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Altay, S.; Onat, A.; Özpamuk-Karadeniz, F.; Karadeniz, Y.; Can, G. Prediction by Low Plasma HbA1c of Mortality, Cardiac and Noncardiac Disease Risk Modulation by Diabetic Status and Sex. J. Investig. Med. 2015, 63, 821–827. [Google Scholar] [CrossRef]
- Altay, S.; Onat, A.; Can, G.; Tusun, E.; Şimşek, B.; Kaya, A. High-normal thyroid-stimulating hormone in euthyroid subjects is associated with risk of mortality and composite disease endpoint only in women. Arch. Med. Sci. 2018, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, B.; Grill, V.; Midthjell, K.; Ahlbom, A.; Andersson, T.; Carlsson, S. Smoking Is Associated With Reduced Risk of Autoimmune Diabetes in Adults Contrasting With Increased Risk in Overweight Men with Type 2 Diabetes: A 22-year follow-up of the HUNT study. Diabetes Care 2012, 36, 604–610. [Google Scholar] [CrossRef]
- Kwaśniewska, M.; Pikala, M.; Kaczmarczyk-Chałas, K.; Piwońska, A.; Tykarski, A.; Kozakiewicz, K.; Pająk, A.; Zdrojewski, T.; Drygas, W. Smoking status, the menopausal transition, and metabolic syndrome in women. Menopause 2012, 19, 194–201. [Google Scholar] [CrossRef]
- Onat, A.; Özhan, H.; Esen, A.M.; Albayrak, S.; Karabulut, A.; Can, G.; Hergenç, G. Prospective epidemiologic evidence of a “protective” effect of smoking on metabolic syndrome and diabetes among Turkish women—Without associated overall health benefit. Atherosclerosis 2007, 193, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Jinjuvadia, R.; Lohia, P.; Jinjuvadia, C.; Montoya, S.; Liangpunsakul, S. The association between metabolic syndrome and colorectal neoplasm: Systemic review and meta-analysis. J. Clingastroenterol. 2013, 47, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Hasin, T.; Gerber, Y.; McNallan, S.M.; Weston, S.A.; Kushwaha, S.S.; Nelson, T.J.; Cerhan, J.R.; Roger, V.L. Patients with Heart Failure Have an Increased Risk of Incident Cancer. J. Am. Coll. Cardiol. 2013, 62, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Rhee, E.-J.; Sung, K.-C. Metabolic Syndrome, Insulin Resistance and Systemic Inflammation as Risk Factors for Reduced Lung Function in Korean Nonsmoking Males. J. Korean Med. Sci. 2010, 25, 1480–1486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colayco, D.C.; Niu, F.; McCombs, J.S.; Cheetham, T.C. A1C and Cardiovascular Outcomes in Type 2 Diabetes: A nested case-control study. Diabetes Care 2010, 34, 77–83. [Google Scholar] [CrossRef] [PubMed]

| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 453) | Women (n = 560) | |||||||
| Low Tertile1 | Mid Tertile2 | High Tertile3 | Low Tertile1 | Mid Tertile2 | High Tertile3 | |||
| 111 (n) (4.49 mg/dL) | 120 (n) (5.76 mg/dL) | 118 (n) (7.29 mg/dL) | p-Value | 165 (n) (3.34 mg/dL) | 154 (n) (4.54 mg/dL) | 125 (n) (6.08 mg/dL) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Men | Women | Mean ± SD | Mean ± SD | Mean ± SD | |
| Non-Diabetic Sample | n = 349 | n = 444 | ||||||
| Age, years | 56.4 ± 10.3 | 54 ± 9.5 | 55.7 ± 9 | 0.070 | 0.002 | 54.4 ± 10 | 56.7 ± 9 | 58.3 ± 8.7 |
| Body mass index (kg/m2) | 26.8 ± 3 | 26.9 ± 3 | 28.1 ± 3,7 | 0.008 | <0.001 | 26.1 ± 4 | 28 ± 5 | 28.2 ± 4 |
| Systolic BP, mmHg | 126 ± 14 | 128 ± 12 | 131 ± 13 | 0.027 | 0.032 | 125 ± 17 | 127 ± 17 | 130 ± 16 |
| Diastolic BP, mmHg | 78 ± 8.4 | 79 ± 8 | 81 ± 9 | 0.030 | 0.032 | 76.4 ± 10.5 | 78 ± 10.5 | 80 ± 10.5 |
| Total cholesterol, mg/dl | 209 ± 39 | 216 ± 44 | 217 ± 45 | 0.330 | 0.480 | 218 ± 43 | 224 ± 53 | 225 ± 49 |
| HDL-cholesterol, mg/dl | 48 ± 12 | 48 ± 12 | 45 ± 10 | 0.008 | 0.051 | 59 ± 14 | 57 ± 13 | 55 ± 16 |
| Fast triglycerides, mg/dl | 152.3 ± 92.3 | 164.1 ± 83.7 | 184.8 ± 103.8 | 0.030 | 0.009 | 132.2 ± 78.6 | 152.8 ± 82.9 | 162.5 ± 98.1 |
| LDL-cholesterol, mg/dl | 125 ± 36 | 127 ± 37 | 139 ± 40 | 0.160 | 0.670 | 135 ± 35 | 138 ± 41 | 139 ± 40 |
| HbA1c, % | 5.45 ± 0.34 | 5.5 ± 0.4 | 5.52 ± 0.4 | 0.320 | <0.001 | 5.41 ± 0.4 | 5.46 ± 0.4 | 5.59 ± 0.4 |
| Fasting glucose, mg/dl | 96.5 ± 11 | 97.3 ± 9 | 98.2 ± 11.4 | 0.120 | <0.001 | 93 ± 10 | 98 ± 10 | 100 ± 10 |
| Creatinine, mg/dl | 0.96 ± 0.18 | 1.05 ± 0.18 | 1.09 ± 0.15 | <0.001 | <0.001 | 0.78 ± 0.16 | 0.824 ± 0.17 | 0.88 ± 0.16 |
| C-reactive protein, mg/L | 2.86 ± 2.9 | 3.92 ± 10.1 | 4.02 ± 4.79 | 0.538 | 0.030 | 2.41 ± 2.46 | 3.48 ± 4.67 | 3.85 ± 3.49 |
| Current smoking, n (%) | 38 (34.2%) | 35 (29.1%) | 34 (28.8%) | 0.300 | 0.990 | 27 (16.4%) | 24 (15.6%) | 21 (16.8%) |
| LVEF, percent (%) | 62 ± 7 | 62 ± 8 | 61 ± 8 | 0.670 | 0.050 | 62 ± 6 | 62.5 ± 5 | 60.7 ± 9 |
| Overall mortality, n (%) | 7 (6.3%) | 2 (1.7%) | 9 (7.6%) | 0.073 | 0.820 | 5 (3%) | 3 (2%) | 3 (2.4%) |
| Composite endpoint, n (%) | 25 (22.5%) | 17 (14.2%) | 16 (13.6%) | 0.140 | 0.590 | 26 (15.8%) | 28 (18.2%) | 19 (15.2%) |
| (B) | ||||||||
| 42 (n) (4.15 mg/dl) | 34 (n) (5.77 mg/dL) | 28 (n) (7.43 mg/dL) | p-Value | 21 (n) (3.47 mg/dL) | 40 (n) (4.61 mg/dL) | 55 (n) (6.41 mg/dL) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Men | Women | Mean ± SD | Mean ± SD | Mean ± SD | |
| Diabetic Sample | n = 104 | n = 116 | ||||||
| Age, years | 57.3 ± 9 | 53.9 ± 8 | 58.2 ± 10.4 | 0.150 | 0.008 | 58.4 ± 8 | 56.8 ± 8.8 | 62.4 ± 8.9 |
| Body mass index, kg/m2 | 28 ± 4 | 28 ± 3 | 28.8 ± 3,6 | 0.510 | 0.390 | 28.5 ± 3.5 | 30.3 ± 4.5 | 29.6 ± 4.8 |
| Systolic BP, mmHg | 133 ± 15 | 128 ± 11 | 136.5 ± 12.6 | 0.062 | 0.340 | 128 ± 18 | 129 ± 17 | 134 ± 21 |
| Diastolic BP, mmHg | 83 ± 11 | 80 ± 9 | 85 ± 10.7 | 0.130 | 0.200 | 77 ± 13 | 80.6 ± 8.4 | 82 ± 9 |
| Total cholesterol, mg/dL | 212 ± 52 | 220 ± 104 | 220 ± 51 | 0.880 | 0.650 | 215 ± 35 | 225 ± 49 | 217 ± 46 |
| HDL-cholesterol, mg/dL | 46.5 ± 12.5 | 44 ± 9.5 | 47.5 ± 11 | 0.510 | 0.410 | 53.4 ± 14.5 | 53.6 ± 11.5 | 50.5 ± 11 |
| Fast triglycerides, mg/dL | 188.7 ± 114.1 | 238.1 ± 259.6 | 180.9 ± 70.2 | 0.334 | 0.910 | 169.7 ± 90.6 | 180.6 ± 102.5 | 176.1 ± 86.9 |
| LDL-cholesterol, mg/dL | 125 ± 46 | 121 ± 28 | 134 ± 39 | 0.430 | 0.970 | 130.6 ± 34 | 132.5 ± 41 | 133 ± 40 |
| HbA1c, % | 6.97 ± 1.0 | 6.82 ± 0.8 | 6.41 ± 0.7 | 0.030 | 0.084 | 6.94 ± 0.83 | 6.39 ± 0.74 | 6.60 ± 0.98 |
| Fasting glucose, mg/dL | 151 ± 46 | 136 ± 36 | 123 ± 25 | 0.010 | 0.074 | 159 ± 58 | 131 ± 34 | 140 ± 46 |
| Creatinine, mg/dL | 0.89 ± 0.20 | 1.03 ± 0.15 | 1.10 ± 0.17 | <0.001 | 0.079 | 0.75 ± 0.15 | 0.79 ± 0.18 | 0.85 ± 0.21 |
| C-reactive protein, mg/L | 3.81 ± 3.57 | 2.83 ± 3.76 | 4.91 ± 5.73 | 0.343 | 0.106 | 2.69 ± 1.51 | 4.48 ± 3.50 | 6.76 ± 8.40 |
| Current smoking, n (%) | 9 (24%) | 8 (24%) | 7 (27%) | 0.160 | 0.540 | 6 (28.6%) | 7 (18%) | 14 (26%) |
| LVEF, percent (%) | 59 ± 10 | 62 ± 3 | 61 ± 6.6 | 0.300 | 0.098 | 62 ± 3.3 | 61.5 ± 7 | 58 ± 8 |
| Overall mortality, n (%) | 3 (7%) | 1 (3%) | 2 (7.7%) | 0.690 | 0.400 | 1 (4.8%) | 1 (2.6%) | 5 (9.3%) |
| Composite endpoint, n (%) | 16 (41%) | 6 (18%) | 4 (15%) | 0.032 | 0.220 | 3 (14.3%) | 11 (33.3%) | 18 (33.3%) |
| Men, n = 319 * | Women, n = 408 * | |||||
|---|---|---|---|---|---|---|
| Non-Diabetic Subjects | ß coeff. | SE | p-Value | ß coeff. | SE | p-Value |
| Creatinine, 0.20 mg/dL | 0.43 | 0.08 | <0.001 | 0.43 | 0.07 | <0.001 |
| Body mass index,4 kg/m2 | 0.20 | 0.09 | 0.018 | 0.12 | 0.06 | 0.026 |
| Fasted glucose, 25 mg/dL | 0.40 | 0.17 | 0.028 | 0.60 | 0.16 | 0.002 |
| Fasted triglycerides, 30 mg/dL | 0.94 | 0.84 | 0.052 | 0.91 | 0.82 | 0.041 |
| HDL-cholesterol, 12 mg/dL | −0.04 | 0.08 | 0.640 | −0.07 | 0.06 | 0.210 |
| HbA1c, 0.8 % | 0.04 | 0.16 | 0.820 | 0.24 | 0.14 | 0.070 |
| Age, 11 years | −0.13 | 0.09 | 0.120 | 0.11 | 0.08 | 0.130 |
| Systolic BP, 25 mmHg | 0.15 | 0.13 | 0.270 | 0.25 | 0.1 | 0.710 |
| Constant | −0.93 | 1.68 | 0.580 | −3.35 | 1.22 | 0.006 |
| r2 | 15%, p < 0.001 | 19%, p < 0.001 | ||||
| Diabetic sample | n = 95 | n = 105 | ||||
| Creatinine, 0.20 mg/dl | 0.60 | 0.14 | <0.001 | 0.27 | 0.14 | 0.061 |
| Body mass index,4 kg/m2 | 0.28 | 0.15 | 0.084 | 0.07 | 0.11 | 0.530 |
| Fasted glucose, 25 mg/dl | −0.16 | 0.10 | 0.110 | −0.08 | 0.10 | 0.470 |
| Fasted triglycerides, 30 mg/dL | −0.60 | 0.93 | 0.950 | 0.43 | 0.93 | 0.990 |
| HDL-cholesterol, 12 mg/dL | −0.06 | 0.18 | 0.670 | −0.40 | 0.13 | 0.003 |
| HbA1c, 0.8% | −0.15 | 0.14 | 0.310 | −0.06 | 0.13 | 0.670 |
| Age, 11 years | 0.33 | 0.16 | 0.860 | 0.43 | 0.15 | 0.008 |
| Systolic BP, 25 mmHg | 0.10 | 0.25 | 0.670 | 0.05 | 0.18 | 0.770 |
| Constant | 2.64 | 2.7 | 0.330 | 3.51 | 2.26 | 0.120 |
| r2 | 28%, p < 0.001 | 18%, p = 0.002 | ||||
| Non-Diabetic | Diabetic Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 793 | Men n = 349 | Women n = 444 | Total n = 220 | Men n = 104 | Women n = 116 | |||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Composite endpoint (n) | 138 | 64 | 74 | 62 | 28 | 34 | ||||||
| Sex, male | 1.11 | 0.71–1.72 | 1.76 | 0.85–3.66 | ||||||||
| Age, 10 years | 2.10 | 1.66–2.67 | 2.32 | 1.58–3.36 | 1.89 | 1.38–2.62 | 1.63 | 1.10–2.43 | 1.74 | 0.98; 3.11 | 1.32 | 0.72–2.32 |
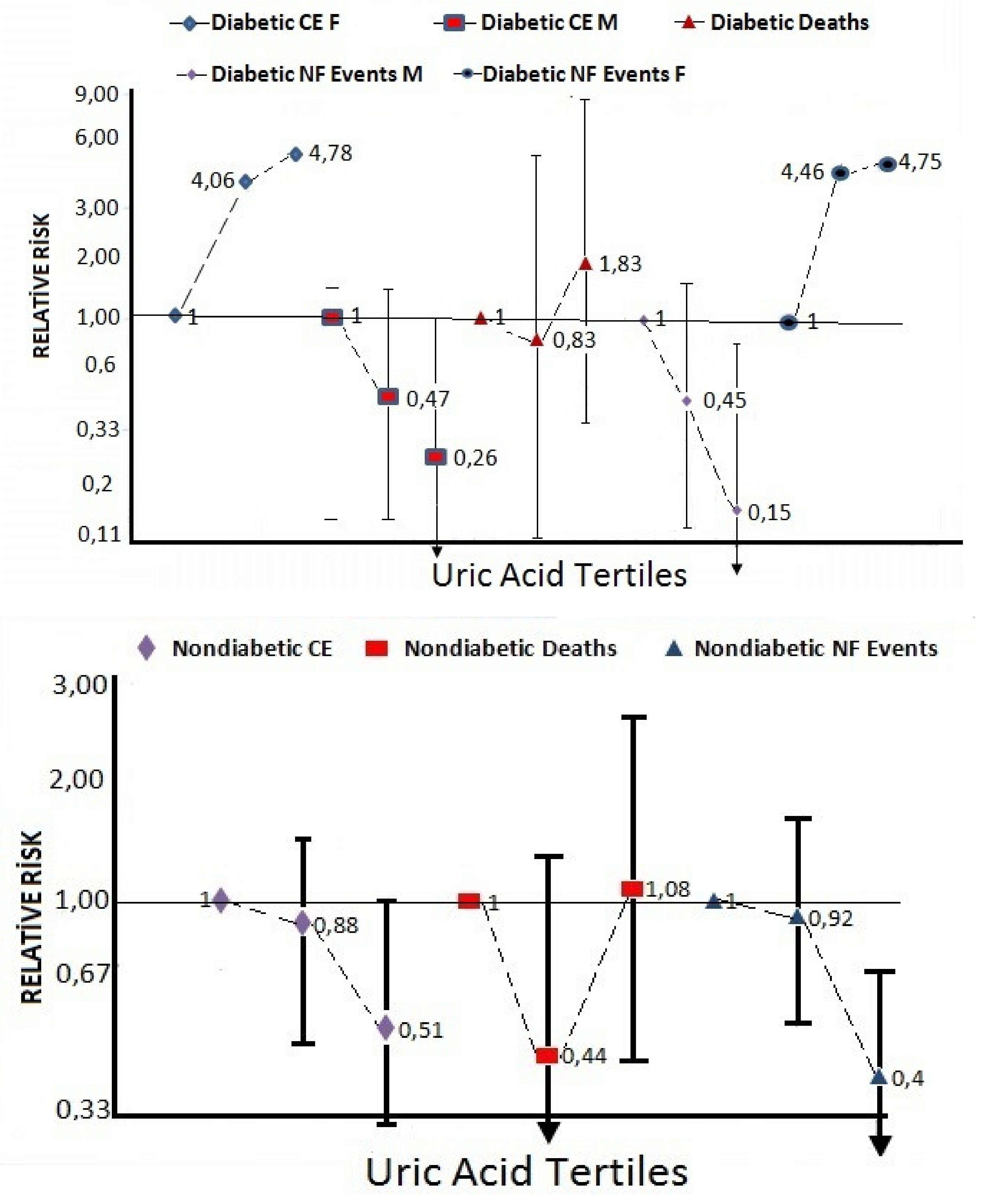
| Uric acid tertile 2 | 0.88 | 0.45–1.43 | 0.86 | 0.41–1.84 | 0.94 | 0.50–1.79 | 0.99 | 0.36–2.02 | 0.47 | 0.15; 1.52 | 4.06 | 0.66–24.9 |
| Uric acid tertile 3 | 0.51 | 0.31–0.86 | 0.47 | 0.22–1.01 | 0.57 | 0.28–1.17 | 0.85 | 0.37–2.19 | 0.26 | 0.06; 1.11 | 4.78 | 0.08–28.5 |
| Current vs. never smoking | 1.83 | 1.06–3.15 | 2.44 | 1.14–5.21 | 1.36 | 0.59–3.13 | 2.04 | 0.90–4.66 | 7.62 | 1.90; 30.6 | 0.67 | 0.19–2.32 |
| Former vs. never smoking | 3.32 | 1.81–6.09 | 3.58 | 1.57–8.19 | 3.57 | 1.32–9.66 | 7.11 | 2.91–17.4 | 8.37 | 2.26; 31.0 | 16.0 | 2.41–106 |
| Hypertension, yes/no | 3.00 | 1.94–4.65 | 2.23 | 1.17–4.27 | 3.75 | 2.04–6.88 | 1.88 | 0.93–3.78 | 1.03 | 0.35; 3.08 | 3.31 | 1.19–9.25 |
| Total cholesterol, 35 mg/dL | 0.93 | 0.78–1.07 | 0.78 | 0.61–1.01 | 1.00 | 0.81–1.19 | 0.93 | 0.78–1.11 | 1.04 | 0.84; 1.28 | 0.78 | 0.53–1.15 |
| All-cause mortality (n) | 30 | 19 | 11 | 13 | 6 | 7 | ||||||
| Sex, male | 0.76 | 0.33–1.75 | 1.23 | 0.29–5.19 | ||||||||
| Age,10 years | 1.69 | 1.07–2.67 | 1.99 | 1.04–3.81 | 1.33 | 0.67–2.62 | 1.95 | 1.06–4.23 | 2.43 | 0.63–9.39 | 1.61 | 0.51–5.10 |
| Uric acid tertile 2 | 0.44 | 0.15–1.31 | 0.41 | 0.19–11.1 | 0.50 | 0.11–2.28 | 0.83 | 0.13–5.36 | 0.61 | 0.03–11.1 | 0.57 | 0.03–11.2 |
| Uric acid tertile 3 | 1.08 | 0.46–2.53 | 1.52 | 0.52–4.47 | 0.62 | 0.14–2.75 | 1.83 | 0.36–9.27 | 2.97 | 0.17–51.4 | 2.01 | 0.19–21.2 |
| Current vs. never smoking | 2.79 | 0.99–7.81 | 8.23 | 1.55–43.8 | 0.74 | 0.61–3.69 | 1.46 | 0.24–9.02 | NS | Too few | prot. | Too few |
| Former vs. never smoking | 4.84 | 1.76–13.3 | 9.77 | 1.81–52.7 | 3.08 | 0.56–17.1 | 4.36 | 0.95–20.0 | NS | Too few | 3.59 | 0.54–24.1 |
| Hypertension, yes/no | 1.23 | 0.54–2.81 | 0.87 | 0.30–2.57 | 1.84 | 0.48–7.08 | 0.39 | 0.11–1.42 | 0.17 | 0.01–7.17 | 0.92 | 0.17–4.87 |
| Total cholesterol, 35 mg/dL | 0.70 | 0.51–0.93 | 0.61 | 0.38–0.99 | 0.75 | 0.49–1.15 | 0.68 | 0.41–1.15 | 0.21 | 0.07–1.37 | 1.04 | 0.55–2.00 |
| Non-fatal events * (n) | 108 | 45 | 63 | 49 | 22 | 27 | ||||||
| Age,10 years | 2.16 | 1.66–2.81 | 2.26 | 1.48–3.46 | 2.06 | 1.45–2.92 | 1.49 | 0.97–2.30 | 1.63 | 0.87–3.05 | 1.12 | 0.59–2.12 |
| Uric acid tertile 2 | 0.92 | 0.55–1.55 | 0.88 | 0.39–1.98 | 0.99 | 0.50–1.97 | 0.93 | 0.37–2.33 | 0.45 | 0.14–1.65 | 4.46 | 0.57–34.7 |
| Uric acid tertile 3 | 0.40 | 0.22–0.72 | 0.26 | 0.10–0.69 | 0.55 | 0.25–1.18 | 0.66 | 0.26–1.71 | 0.15 | 0.03–0.84 | 4.75 | 0.61–36.7 |
| Current vs. never smoking | 1.63 | 0.88–3.00 | 1.73 | 0.73–4.10 | 1.50 | 0.61–3.69 | 2.05 | 0.85–4.94 | 5.99 | 1.40–25.6 | 0.78 | 0.22–2.84 |
| Former vs. never smoking | 2.78 | 1.41–5.47 | 2.51 | 0.99–6.37 | 3.70 | 1.29–10.8 | 6.70 | 2.57–17.5 | 6.12 | 1.57–23.8 | 18.1 | 2.53–130 |
| Hypertension, yes/no | 3.65 | 2.22–5.99 | 3.03 | 1.42–6.48 | 4.25 | 2.19–8.28 | 2.68 | 1.19–6.03 | 1.59 | 0.47–5.31 | 5.47 | 1.55–19.3 |
| Total cholesterol, 35 mgdL | 1.00 | 0.84–1.15 | 0.90 | 0.66–1.19 | 1.04 | 0.84–1.28 | 0.97 | 0.81–1.15 | 1.07 | 0.87–1.37 | 0.73 | 0.48–1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özpamuk-Karadeniz, F.; Karadeniz, Y.; Kaya, A.; Altay, S.; Can, G.; Onat, A. Low Serum Uric Acid Predicts Risk of a Composite Disease Endpoint. Medicina 2021, 57, 361. https://doi.org/10.3390/medicina57040361
Özpamuk-Karadeniz F, Karadeniz Y, Kaya A, Altay S, Can G, Onat A. Low Serum Uric Acid Predicts Risk of a Composite Disease Endpoint. Medicina. 2021; 57(4):361. https://doi.org/10.3390/medicina57040361
Chicago/Turabian StyleÖzpamuk-Karadeniz, Fatma, Yusuf Karadeniz, Adnan Kaya, Servet Altay, Günay Can, and Altan Onat. 2021. "Low Serum Uric Acid Predicts Risk of a Composite Disease Endpoint" Medicina 57, no. 4: 361. https://doi.org/10.3390/medicina57040361
APA StyleÖzpamuk-Karadeniz, F., Karadeniz, Y., Kaya, A., Altay, S., Can, G., & Onat, A. (2021). Low Serum Uric Acid Predicts Risk of a Composite Disease Endpoint. Medicina, 57(4), 361. https://doi.org/10.3390/medicina57040361






