Effect of Synergistic Action of Bovine Lactoferrin with Antibiotics on Drug Resistant Bacterial Pathogens
Abstract
1. Introduction
2. Materials and Methods
Isolation & Identification of Bacterial Pathogens
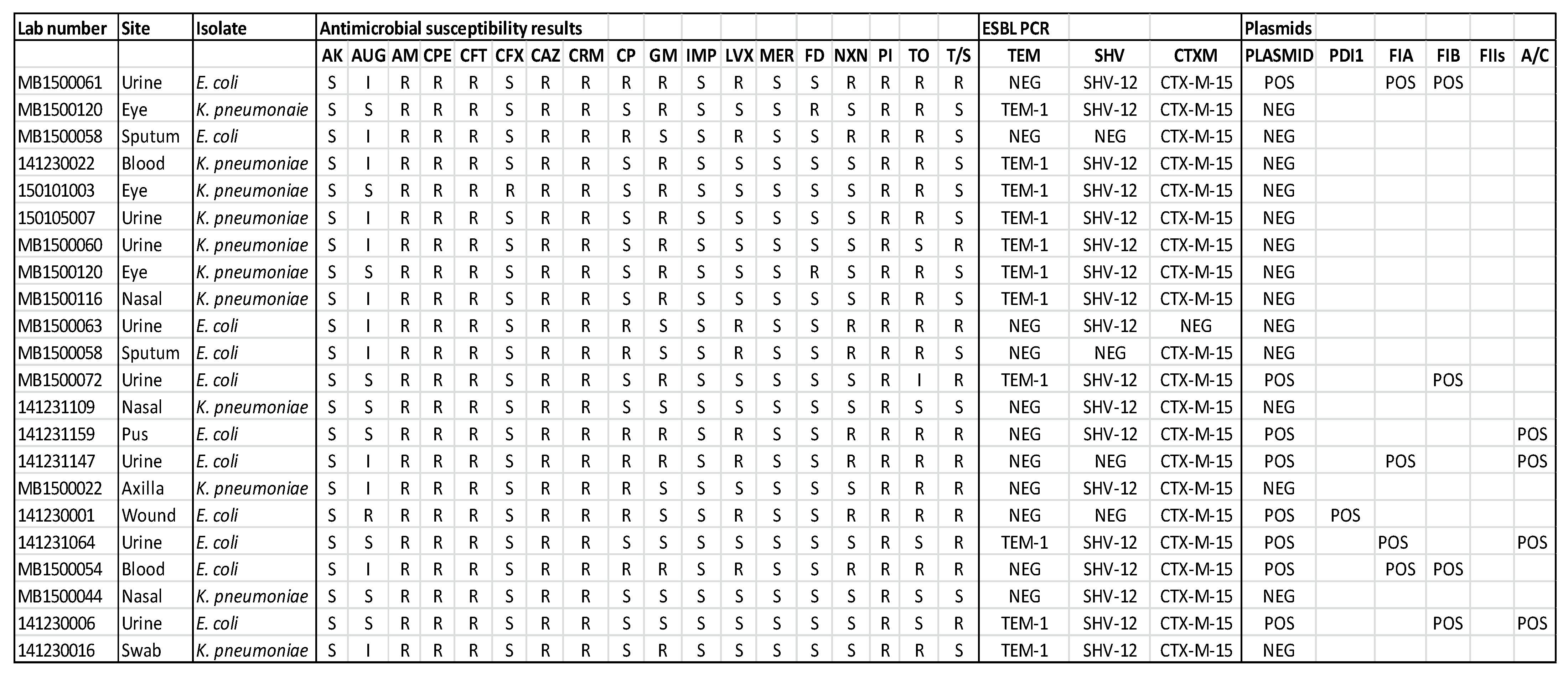
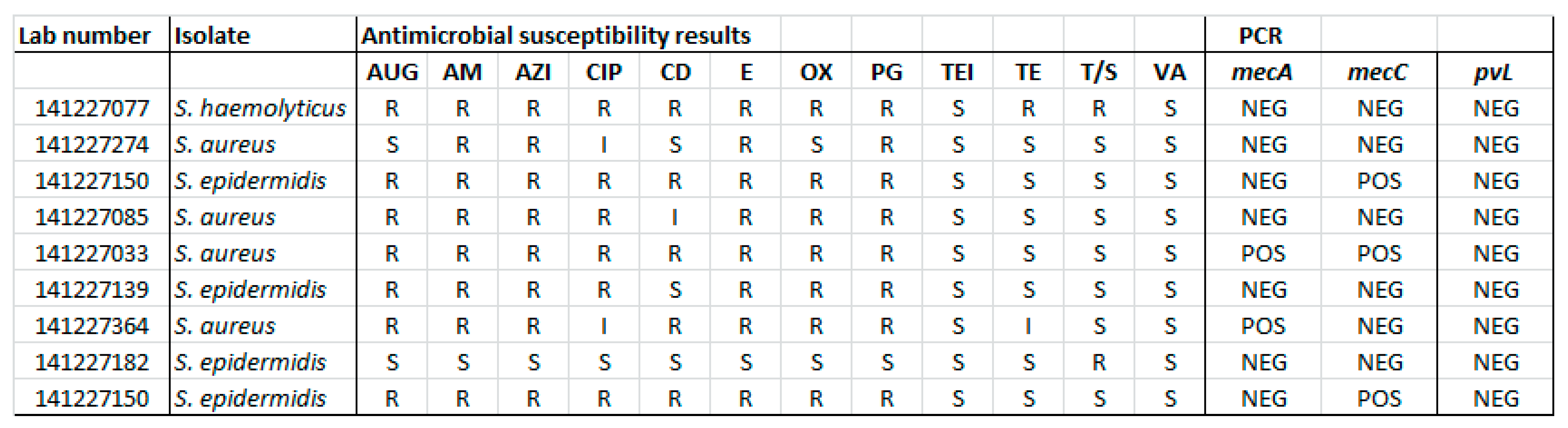
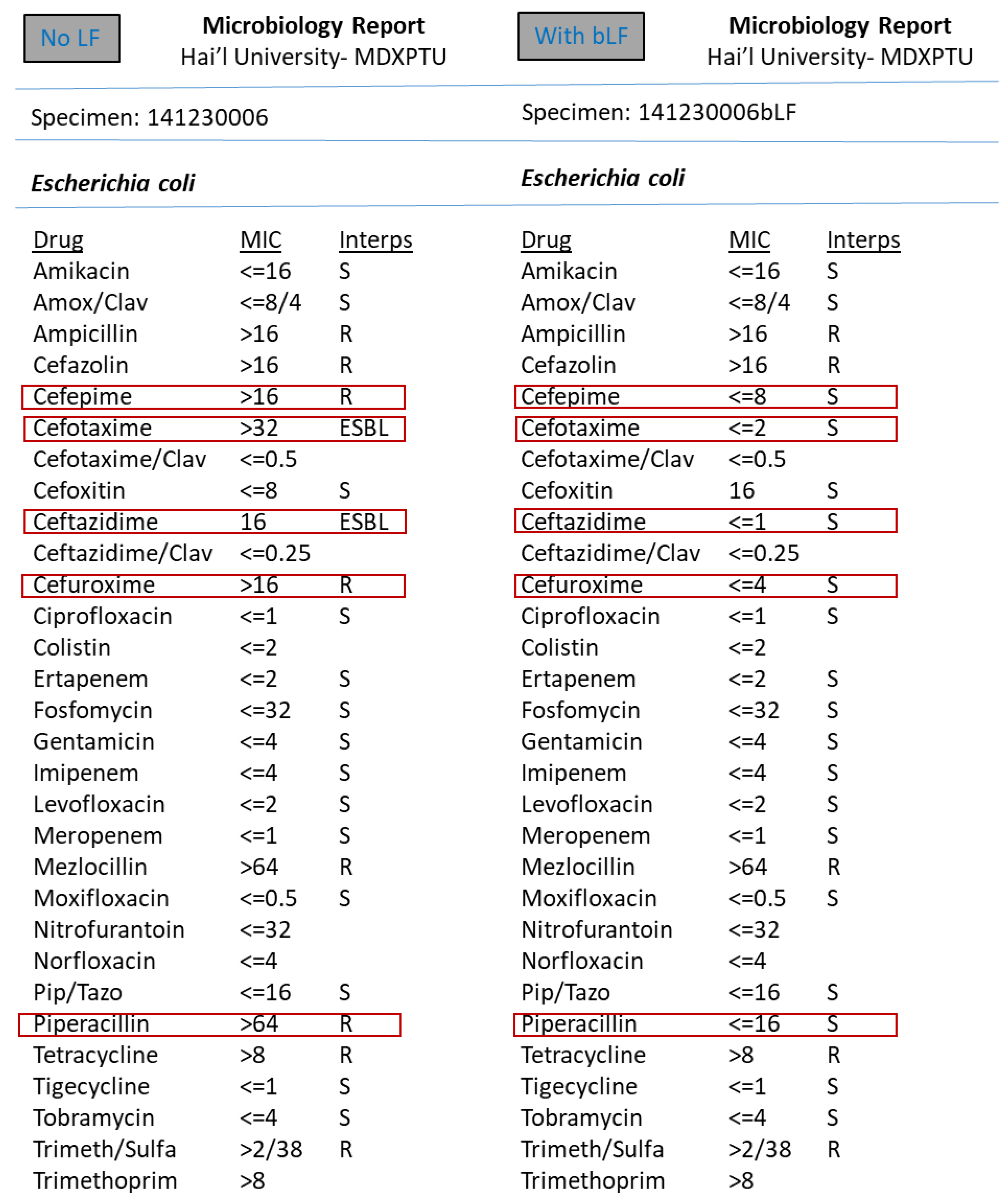
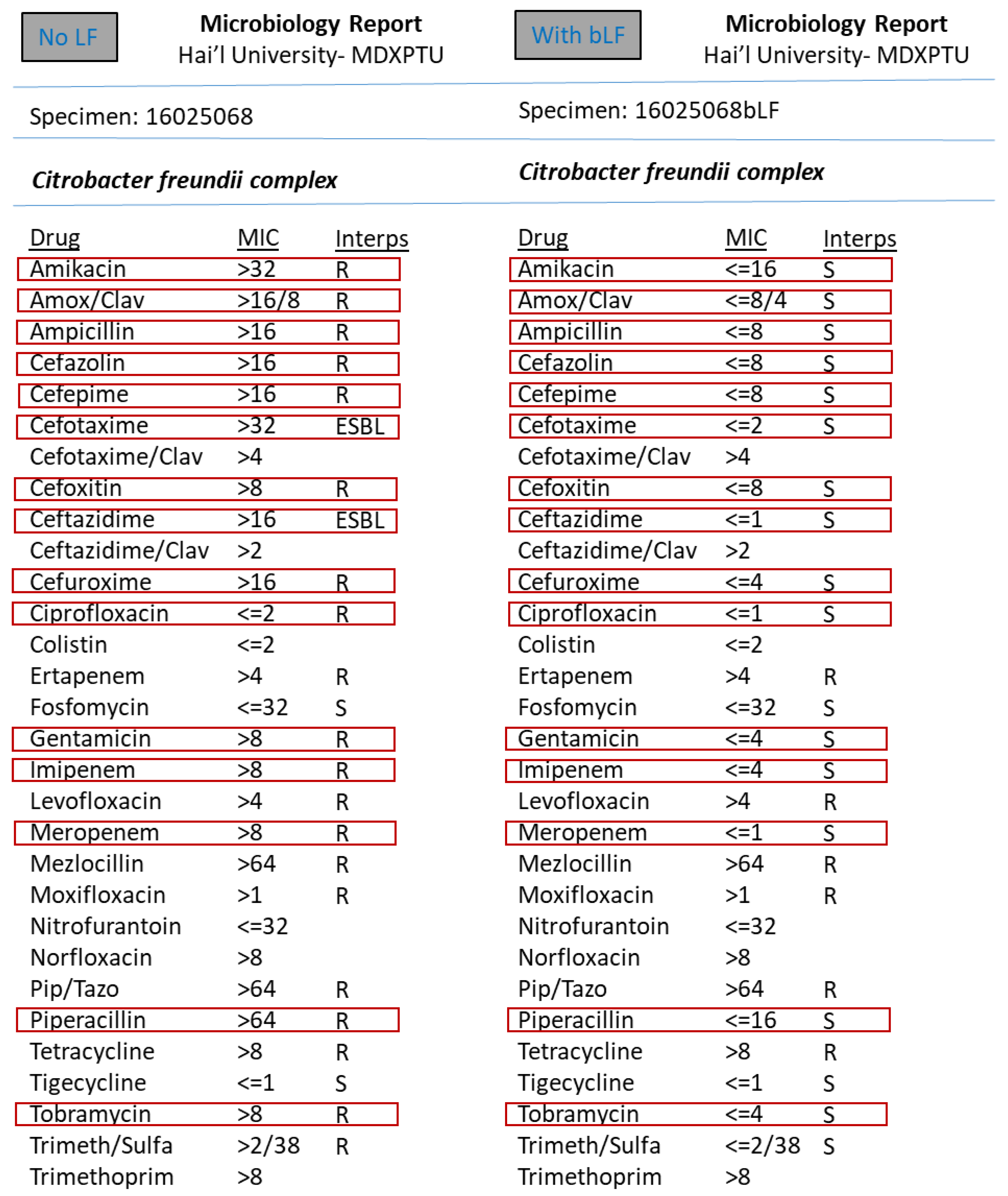
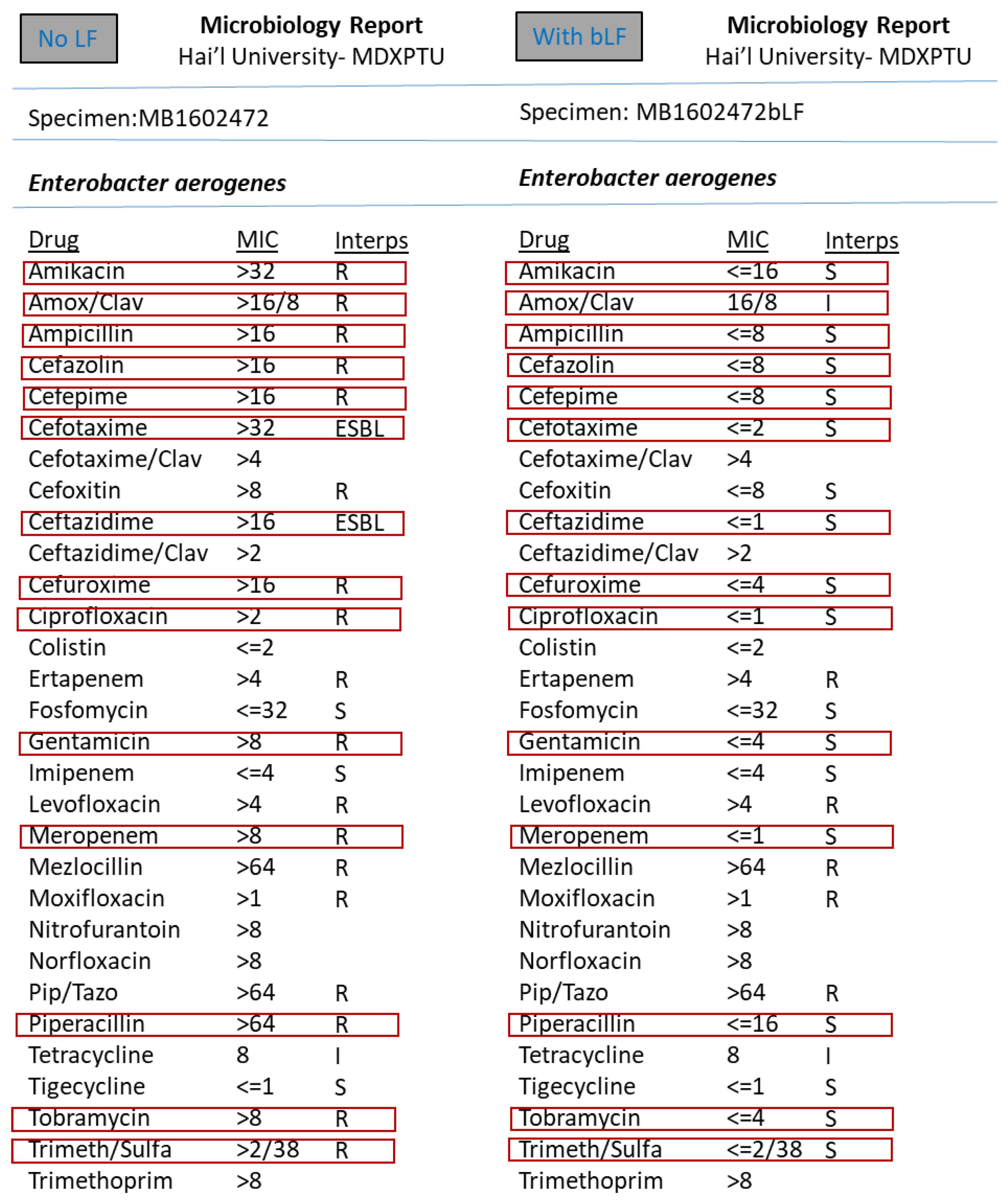
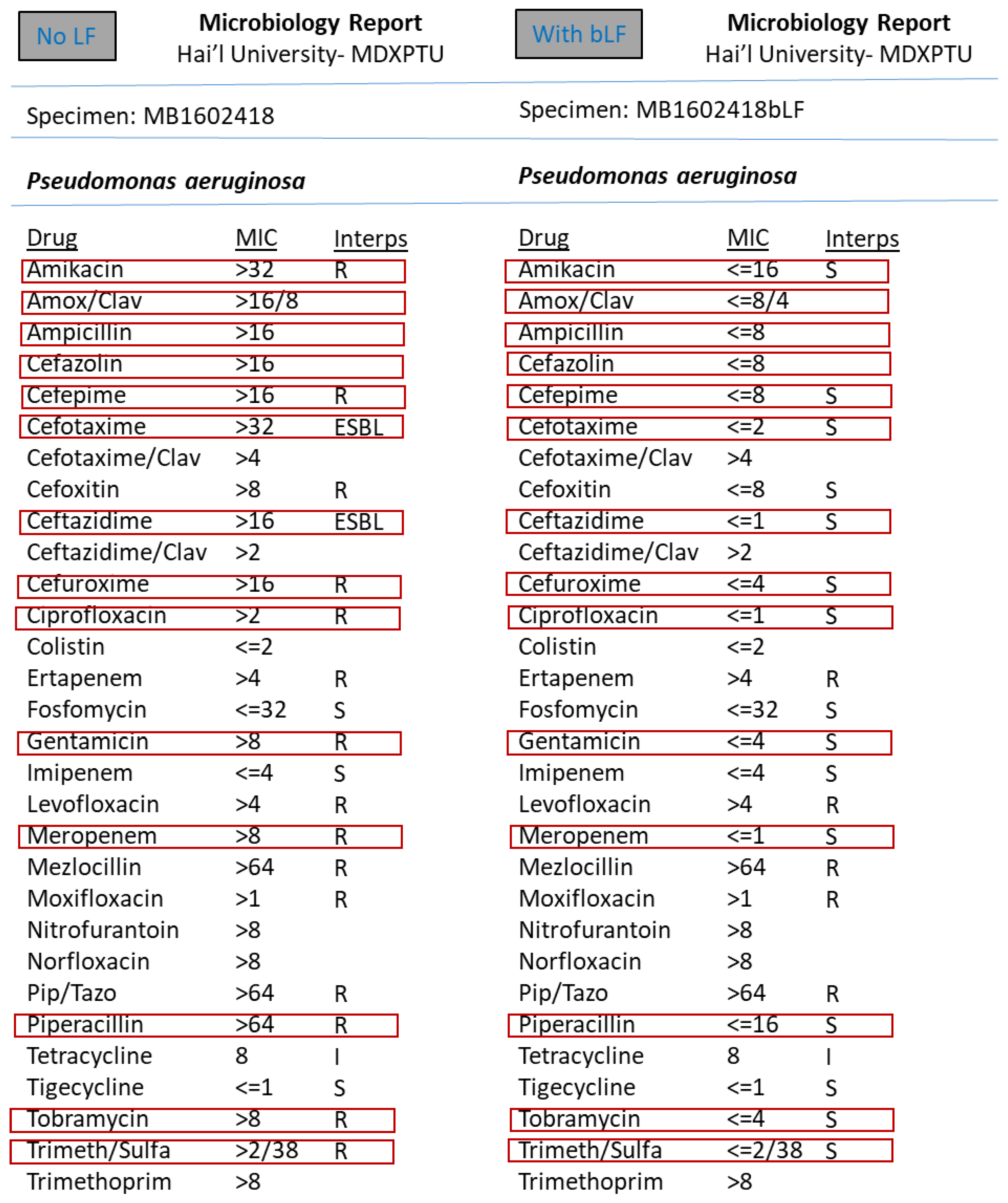
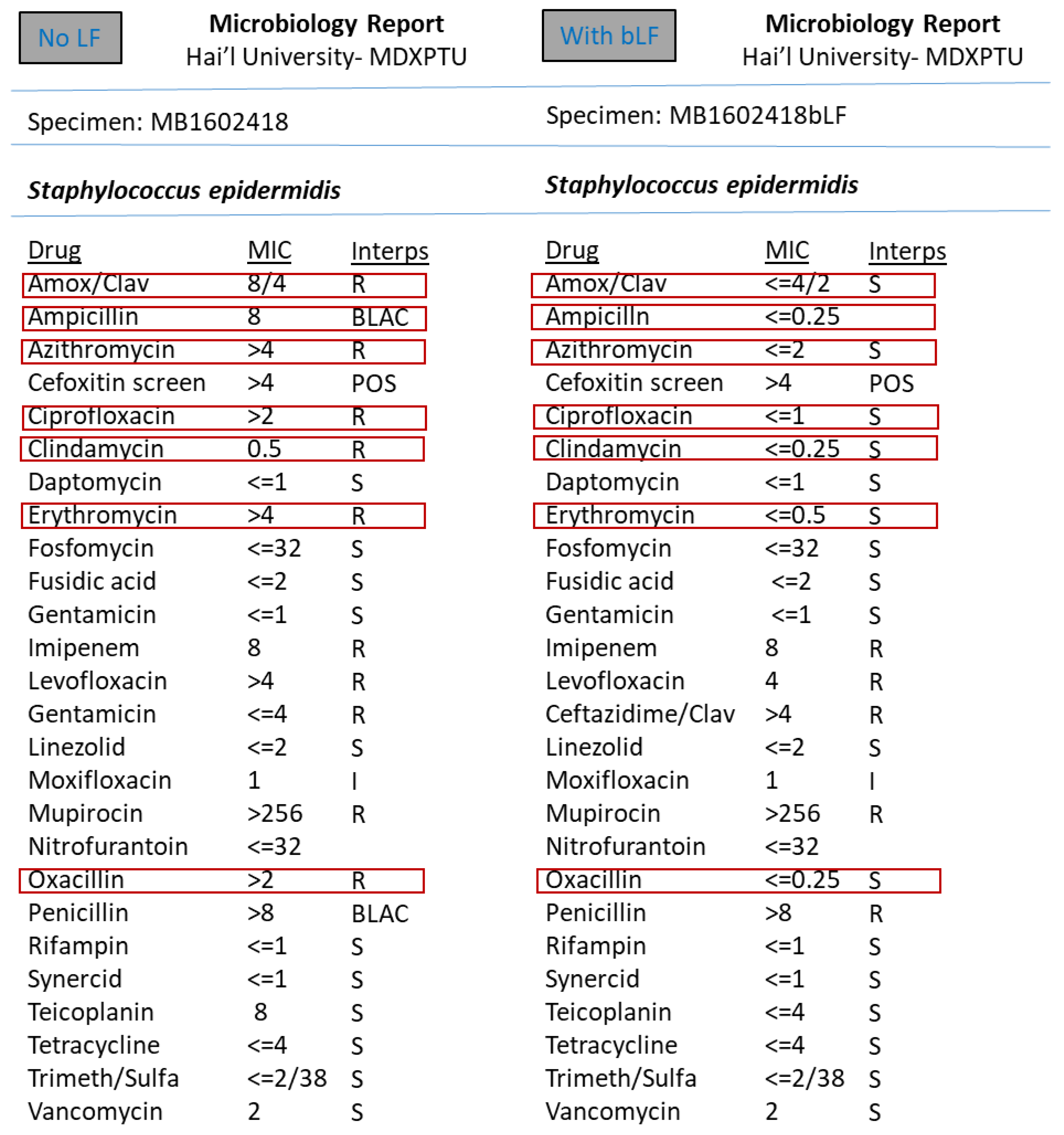
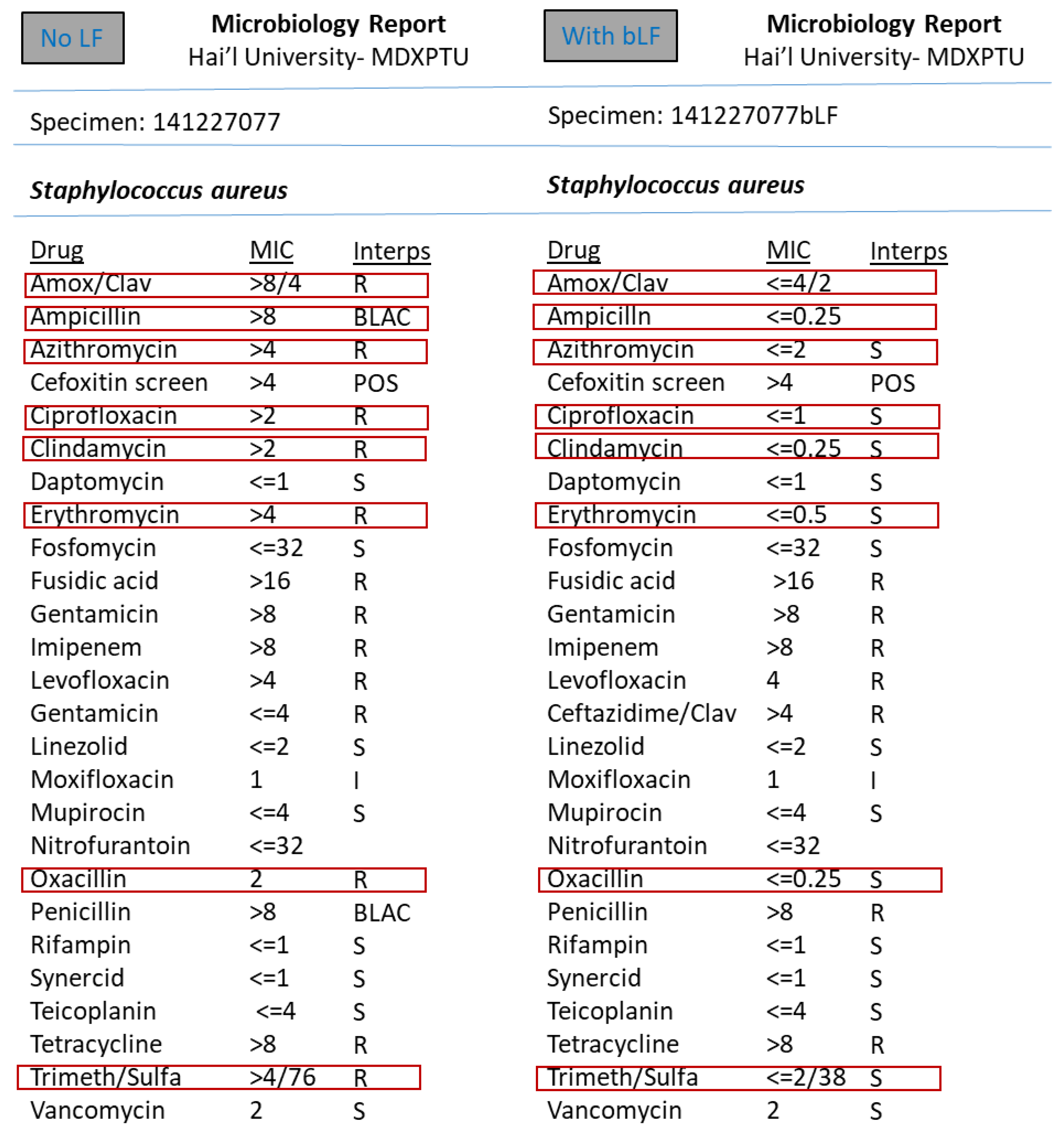
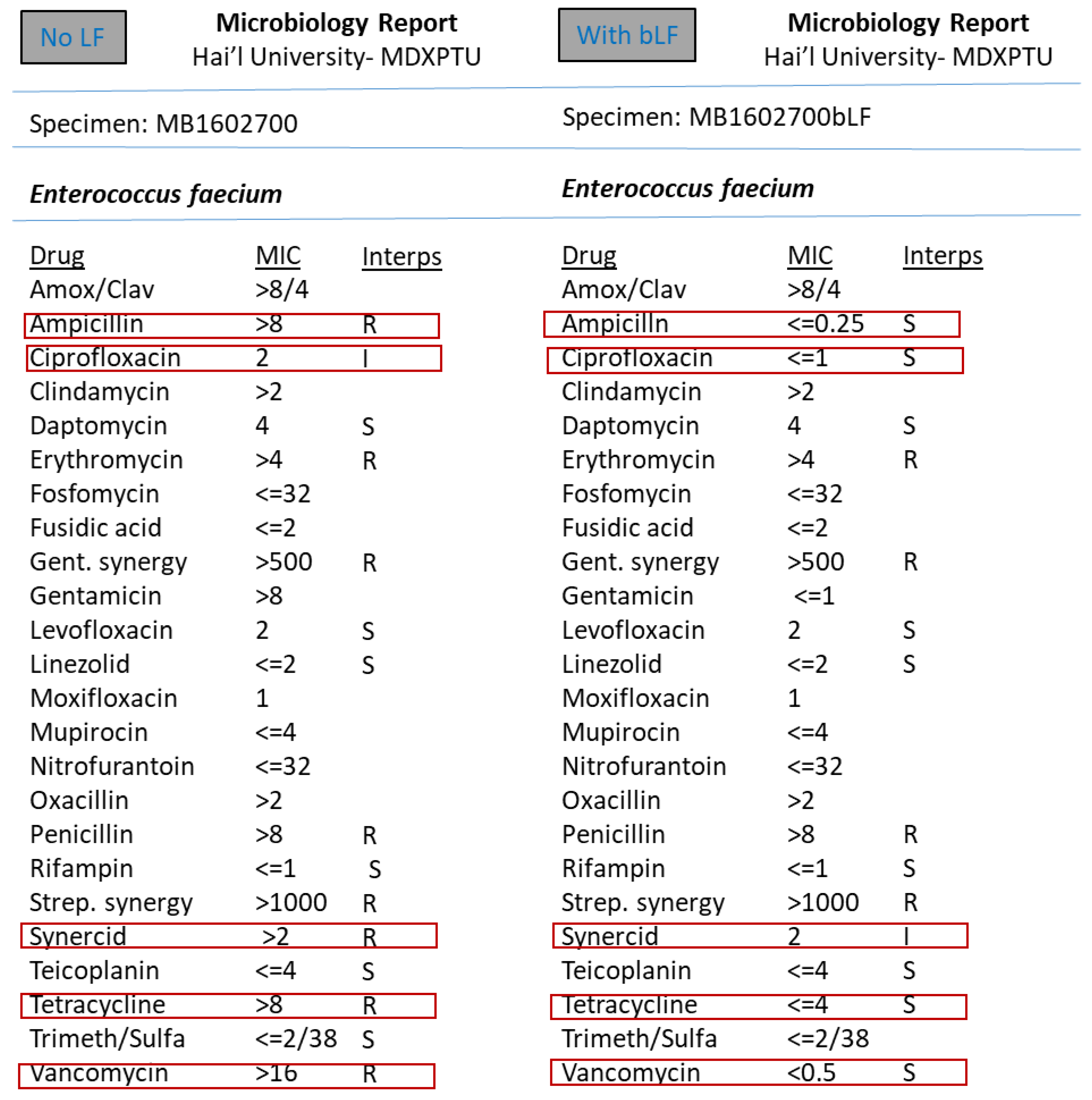
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coombs, M.R.P.; Kronforst, K.; Levy, O. Neonatal Host Defense against Staphylococcal Infections. Clinical and Developmental Immunology. Clin. Dev. Immunol. 2013, 2013, 826303. [Google Scholar] [CrossRef]
- Steijns, J.M.; van Hooijdonk, A.C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84 (Suppl. 1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Oztas, Y.E.; Ozgunes, N. Lactoferrin: A multifunctional protein. Adv. Mol. Med. 2005, 1, 149–154. [Google Scholar] [CrossRef]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef]
- Bennett, R.M.; Kokocinski, T. Lactoferrin turnover in man. Clin. Sci. 1979, 57, 453–460. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Schonne, E. Lactoferrin an iron binding protein in neutrophilic leukocytes. J. Exp. Med. 1969, 130, 643–656. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, S.A.; Gallegos, S.A.; Cruz, Q.R. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.P.; Rahman, M.; Welling, M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides 2011, 32, 1953–1963. [Google Scholar] [CrossRef]
- Lee, N.; Kawai, K.; Nakamura, I.; Tanaka, T.; Kumura, H.; Shimazaki, K. Susceptibilities against bovine lactoferrin with microorganisms isolated from mastitic milk. J. Vet. Med. Sci. 2004, 66, 1267–1269. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin research, technology and applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Mosquito, S.; Zegarra, G.; Villanueva, C.; Ruiz, J.; Ochoa, T.J. Effect of bovine lactoferrin on the minimum inhibitory concentrations of ampicillin and trimethoprim-sulfamethoxazole for clinical Shigella spp. strains. Biochem. Cell Biol. 2012, 90, 412–416. [Google Scholar] [CrossRef]
- de Andrade, F.B.; de Oliveira, J.C.; Yoshie, M.T.; Guimarães, B.M.; Gonçalves, R.B.; Schwarcz, W.D. Antimicrobial activity and synergism of lactoferrin and lysozyme against cariogenic microorganisms. Braz. Dent. J. 2014, 25, 165–169. [Google Scholar] [CrossRef]
- van Veen, S.Q.; Claas, E.C.J. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 2010, 48, 900–907. [Google Scholar] [CrossRef]
- Shahid, M.; Malik, A.; Agrawal, M.; Singhal, S. Phenotypic detection of extended-spectrum and AmpC β-lactamases by a new spot-inoculation method and modified three-dimensional extract test: Comparison with the conventional three-dimensional extract test. J. Antimicrob. Chemother. 2004, 54, 684–687. [Google Scholar] [CrossRef][Green Version]
- Yan, J.J.; Ko, W.C.; Jung, Y.C.; Chuang, C.L.; Wu, J.J. Emergence of Klebsiella pneumoniae isolates producing inducible DHA-1 β-lactamase in a University Hospital in Taiwan. J. Clin. Microbiol. 2002, 40, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Chong, Y.; Shin, H.B.; Kim, Y.A.; Yong, D.; Yum, J.H. Modified Hodge and EDTA—Disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 2001, 7, 88–91. [Google Scholar] [CrossRef]
- Felmingham, D. The need for antimicrobial resistance surveillance. J. Antimicrob. Chemother. 2002, 50 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- Miele, A.; Bandera, M.; Goldstein, B.P. Use of primers selective for vancomycin resistance genes to determine van genotype in enterococci and to study gene organization in VanA isolates. Antimicrob. Agents Chemother. 1995, 39, 1772–1778. [Google Scholar] [CrossRef]
- Menezes, G.A.; Harish, B.N.; Khan, M.A.; Goessens, W.H.; Hays, J.P. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005–2009. Clin. Microbiol. Infect. 2012, 18, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.R.; Grant, J.M.; Plewes, K.; Roscoe, D.; Boyd, D.A. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg. Infect. Dis. 2011, 17, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Mazurier, J.; Spik, G. Comparative study of the iron-binding properties of the human transferrins. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochem. Biophys. Acta 1980, 629, 408. [Google Scholar] [CrossRef]
- Gill, E.E.; Franco, O.L.; Hancock, R.E.W. Antibiotic Adjuvants: Diverse Strategies for Controlling Drug-Resistant Pathogens. Chem. Biol. Drug Des. 2015, 85, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Suppression of Bacterial Biofilm Formation by Iron Limitation. Med. Hypotheses 2004, 63, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.R.; Cole, M.F.; McGhee, J.R. A bactericidal effect for human lactoferrin. Science 1977, 197, 263–265. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Rev. Biochim. 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Bhimani, R.S.; Vendrov, Y.; Furmanski, P. Influence of Lactoferrin Feeding and Injection against Systemic Staphylococcal Infections in Mice. J. Appl. Microbiol. 1999, 86, 135–144. [Google Scholar] [CrossRef]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A Component of Innate Immunity Prevents Bacterial Biofilm Development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Kutila, T.; Pyörälä, S.; Saloniemi, H.; Kaartinen, L. Antibacterial Effect of Bovine Lactoferrin against Udder Pathogens. Acta Vet. Scand. 2003, 44, 35. [Google Scholar] [CrossRef]
- Nonnecke, B.J.; Smith, K.L. Inhibition of mastitic bacteria by bovine milk apo-lactoferrin evaluated by in vitro microassay of bacterial growth. J. Dairy Sci. 1984, 67, 606–613. [Google Scholar] [CrossRef]
- Dionysius, D.A.; Grieve, P.A.; Milne, J.M. Forms of lactoferrin: Their antibacterial effect on enterotoxigenic Escherichia coli. J. Dairy Sci. 1993, 76, 2597–2606. [Google Scholar] [CrossRef]
| Sl. No. | Clinical Isolates Tested | bLF |
|---|---|---|
| 1 | Methicillin resistant Staphylococcus aureus (MRSA)—30 isolates | All 30 tested. All positive for LF action. |
| 2 | Methicillin resistant Coagulase negative Staphylococcus—30 isolates. | All 30 tested. All positive for LF action. |
| 3 | Extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaciae—40 isolates. | All 40 tested. All positive for LF action. |
| 4 | Fluoroquinolone resistant Gram negative pathogens—30 isolates. | All 30 tested. All positive for LF action. |
| 5 | Multidrug resistant Pseudomonas species—05 isolates. | All 05 tested. All positive for LF action. |
| 6 | Carbapenem resistant Gram negative pathogens—05 isolates. | All 05 tested. All positive for LF action. |
| 7 | AmpC β-lactamase producing Gram negative pathogens—05 isolates. | All 05 tested. All positive for LF action. |
| 8 | Vancomycin resistant Enterococci (VRE)—02 isolates | All 02 tested. All positive for LF action. |
| Total isolates tested | n = 147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mogbel, M.S.; Menezes, G.A.; Elabbasy, M.T.; Alkhulaifi, M.M.; Hossain, A.; Khan, M.A. Effect of Synergistic Action of Bovine Lactoferrin with Antibiotics on Drug Resistant Bacterial Pathogens. Medicina 2021, 57, 343. https://doi.org/10.3390/medicina57040343
Al-Mogbel MS, Menezes GA, Elabbasy MT, Alkhulaifi MM, Hossain A, Khan MA. Effect of Synergistic Action of Bovine Lactoferrin with Antibiotics on Drug Resistant Bacterial Pathogens. Medicina. 2021; 57(4):343. https://doi.org/10.3390/medicina57040343
Chicago/Turabian StyleAl-Mogbel, Mohammed S., Godfred A. Menezes, Mohamed T. Elabbasy, Manal M. Alkhulaifi, Ashfaque Hossain, and Mushtaq A. Khan. 2021. "Effect of Synergistic Action of Bovine Lactoferrin with Antibiotics on Drug Resistant Bacterial Pathogens" Medicina 57, no. 4: 343. https://doi.org/10.3390/medicina57040343
APA StyleAl-Mogbel, M. S., Menezes, G. A., Elabbasy, M. T., Alkhulaifi, M. M., Hossain, A., & Khan, M. A. (2021). Effect of Synergistic Action of Bovine Lactoferrin with Antibiotics on Drug Resistant Bacterial Pathogens. Medicina, 57(4), 343. https://doi.org/10.3390/medicina57040343







