Endovascular Interventional Radiology of the Urogenital Tract
Abstract
1. Introduction
2. Technical Aspects of Arterial Embolizations
- When occlusion of terminal arteries is required (such as in case of intrarenal aneurysms), coil embolization is the first choice of treatment. Coils are mechanical devices with different shapes (tridimensional, helical, complex), diameters, and lengths. Often, nylon filaments are added to their surface. Coils can be pushable or detachable, furthermore they can be distinguished in microcoils that need to be used with microcatheters and standard coils, which advance in standard 4-5 F catheters. Pushable coils, once released from the catheter, cannot be retrieved, while the latter, if the final position is not the correct one, may be replaced in the catheter, removed, and changed with another coil and detached by using special devices [8]. Another possible option of treatment are glues. N-butyl-cyanoacrylate (NBCA) was the first liquid embolic agent applied in clinical practice. It is a monomer acrylic glue which quickly polymerizes when it comes in contact with ionic media, such as blood, and causes a permanent occlusion, and also generates an acute inflammatory process in the wall, which progresses to chronic in about four weeks [4]. NBCA is radiolucent and therefore it is usually mixed with radiopaque materials such as Lipiodol. This is an oily contrast medium that, when mixed with NBCA, makes it radiopaque and increases its polymerization time. The NBCA:Lipiodol mixing ratio determines the effect on polymerization time and is adjusted depending on the clinical situation [9]. To avoid adherence to the thin catheters that are required for the superselective embolization, NBCA has to be injected through a catheter washed with a 5% dextrose solution and the catheter has to be withdrawn promptly after injection. Moreover, NBCA polymerizes with an exothermic reaction, causing pain to the patient. Glubran 2 is an acrylic glue bearing a CE mark authorized for surgical and endovascular use in neuroradiology and interventional radiology. The comonomer of Glubran 2 comprises a monomer of NBCA and a monomer of metacryloxysulpholane (MS) (owned by GEM Srl). MS allows the monomer of NBCA to polymerize with a lower exothermic reaction (45 °C) and a slightly longer polymerization time [10]. Compared to the monomer NBCA, the Glubran 2 causes less pain to the patient and is associated with a lower risk of adherence of the catheter to the tissue, hence showing a greater ease of use. Differently, acrylic glues, once deposited into the nidus, determine its permanent occlusion and prevent its replenishment through feeding branches.
- When occlusion of a main artery is necessary, the possible solutions are coils or plugs. Usually standard catheters and coils are sufficient and rarely are microcatheters required.
3. Kidney
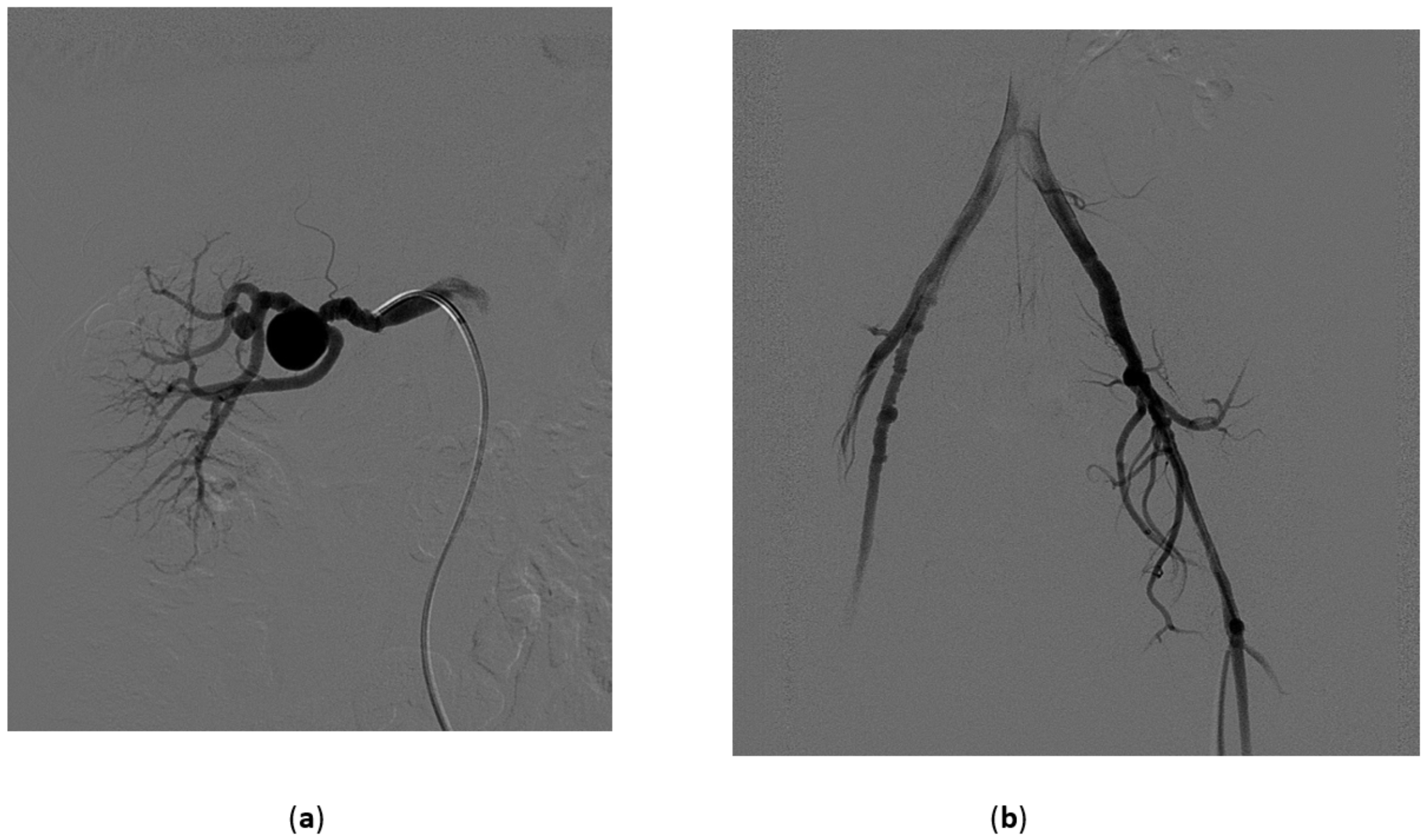
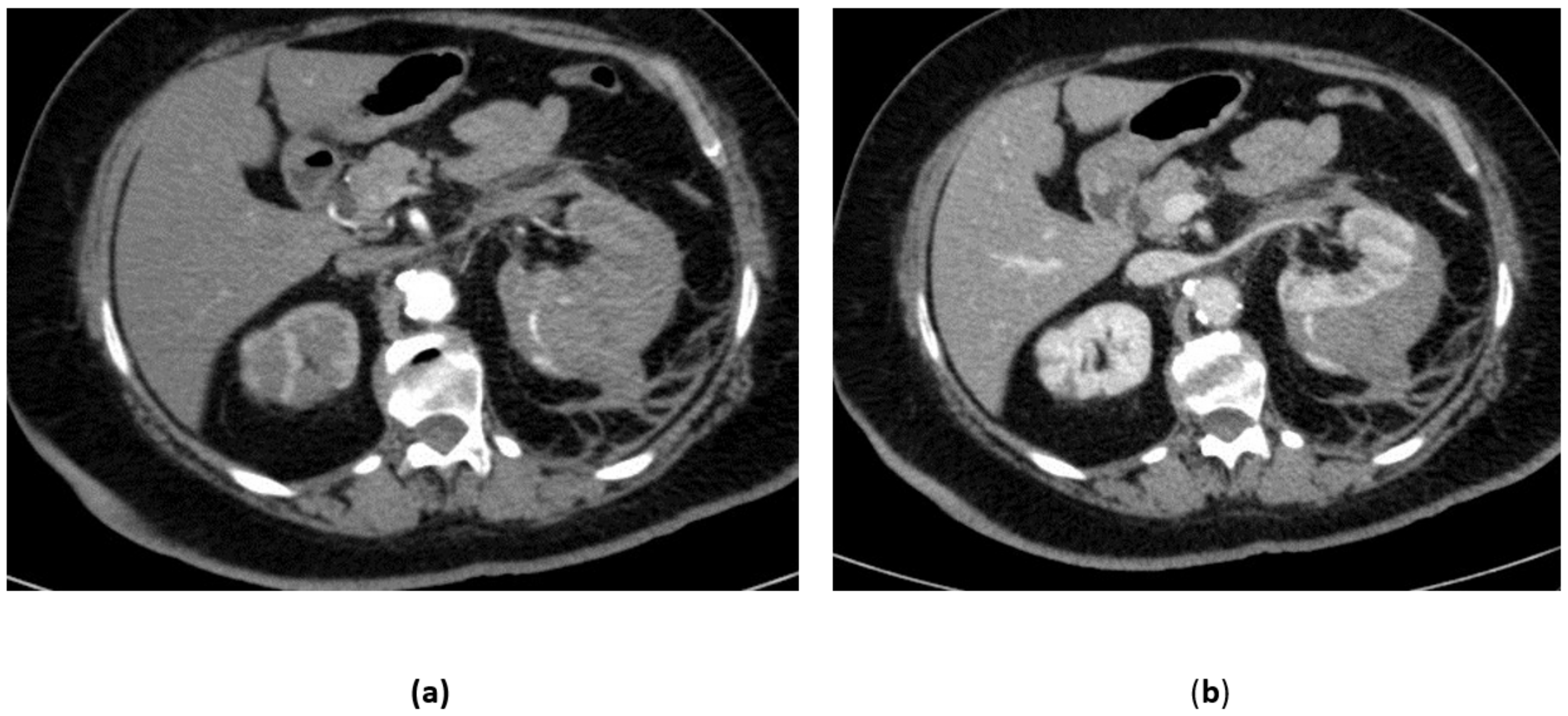
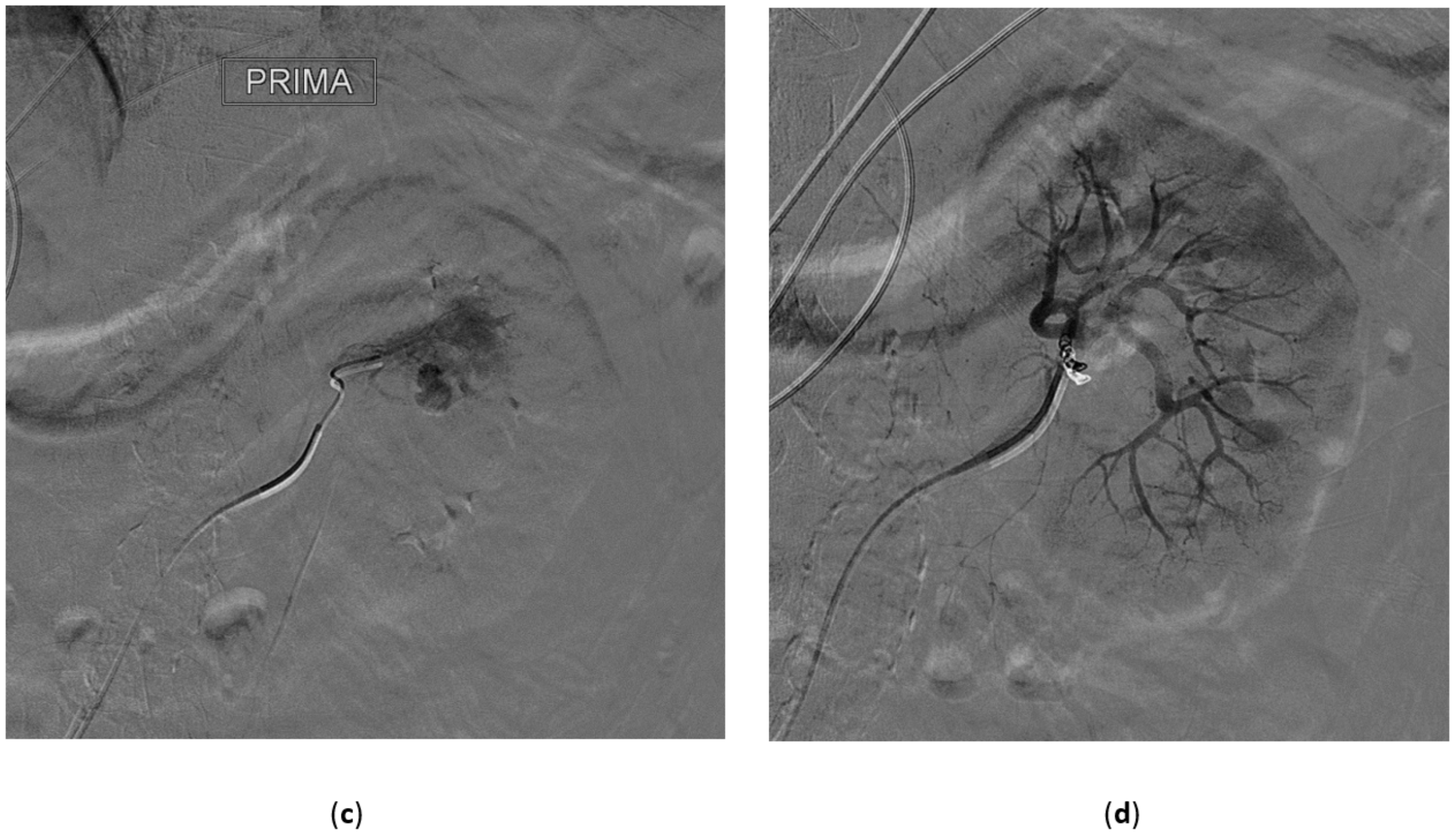
3.1. Renal Artery Aneurysms
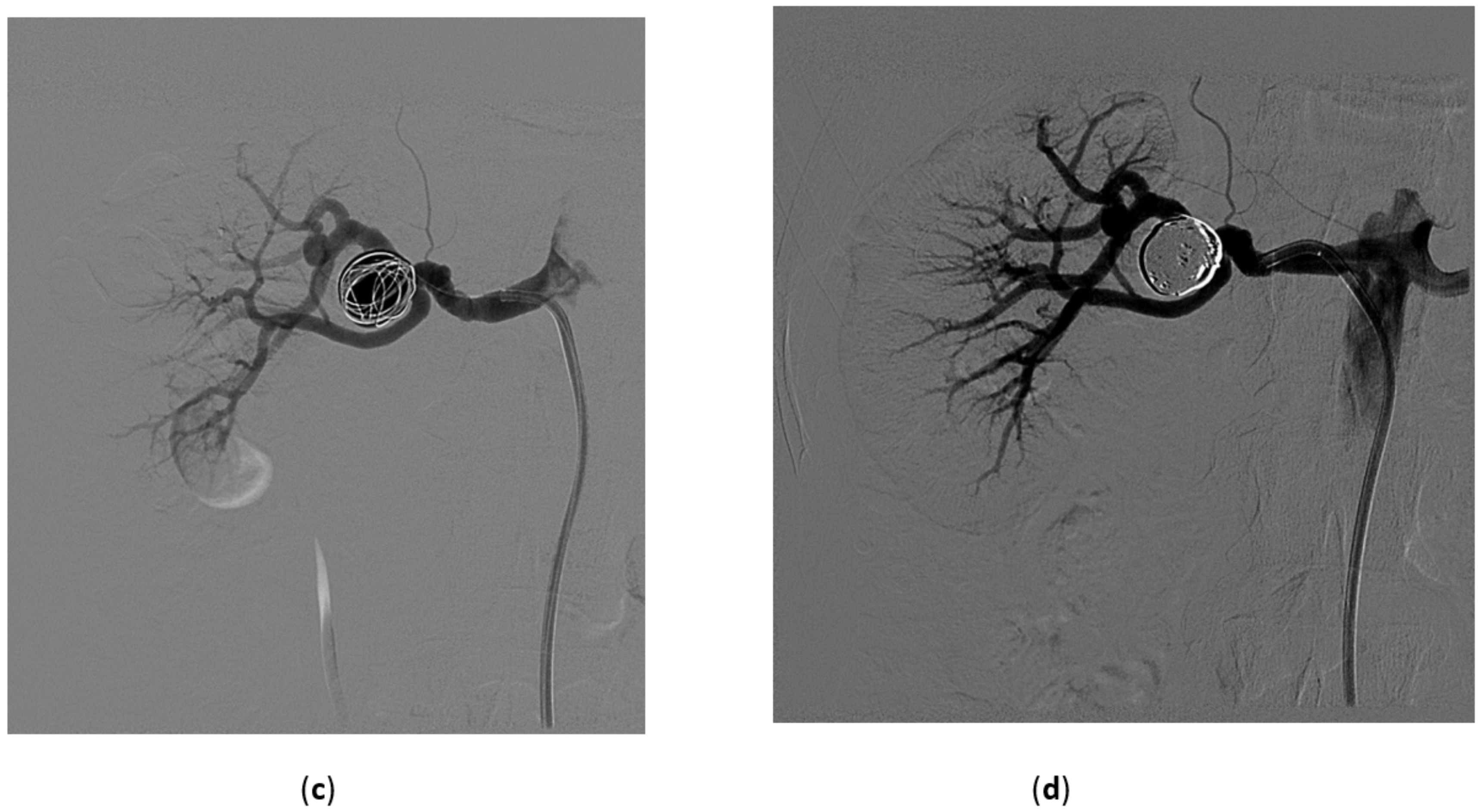
3.2. Renal Artery Pseudoaneurysm (RAP)
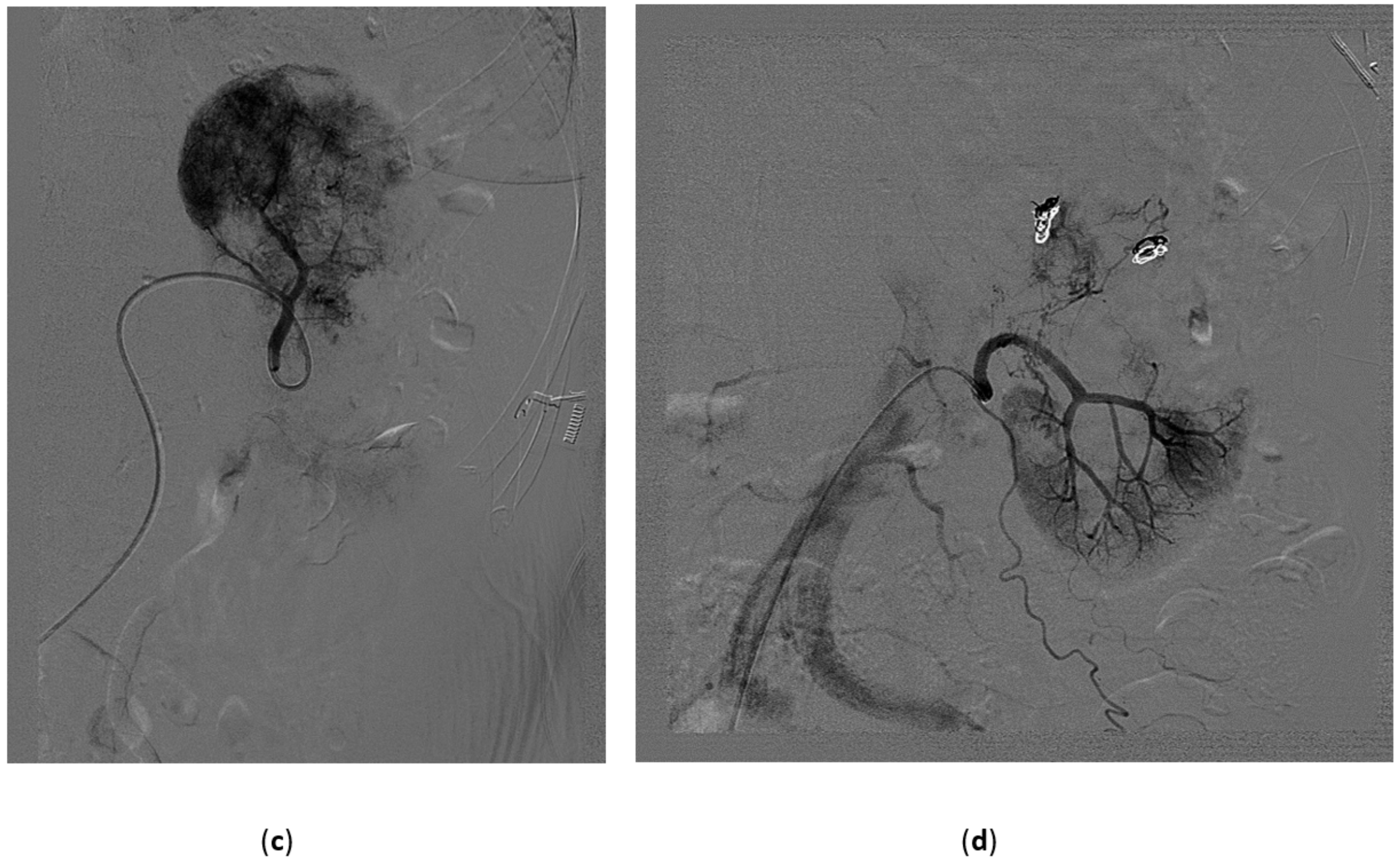
3.3. Renal Arterovenous Fistula (AVF)
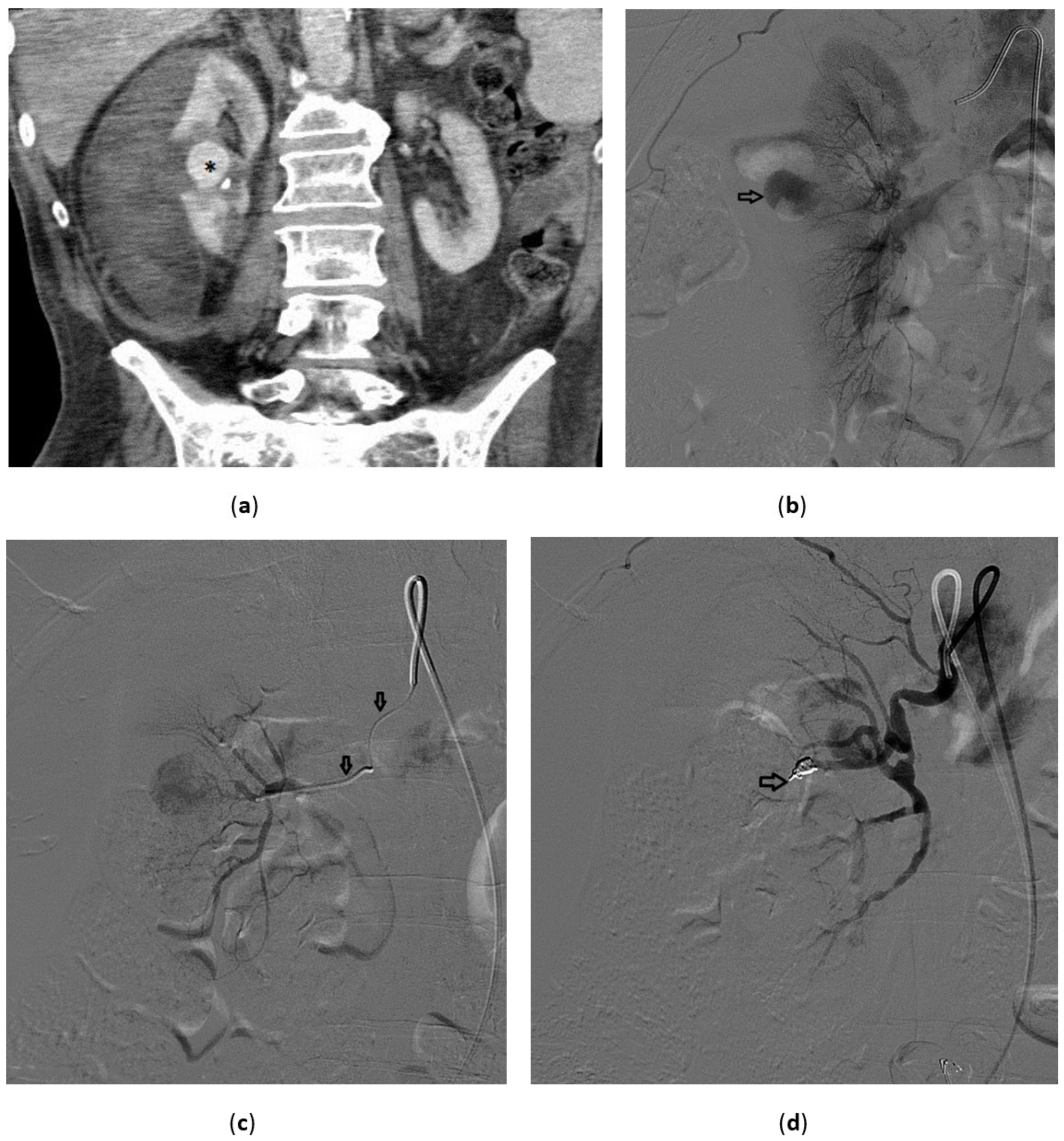
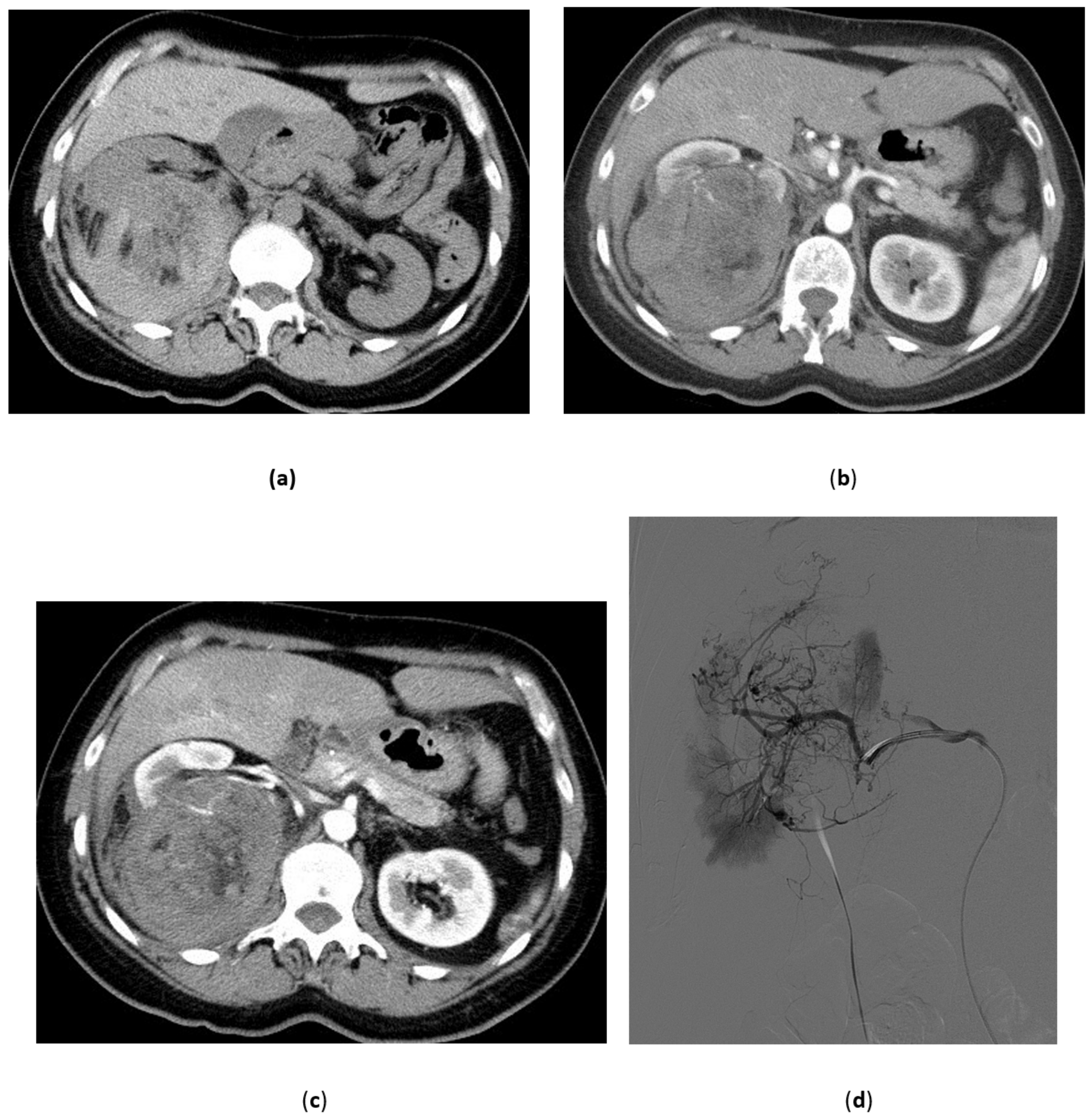
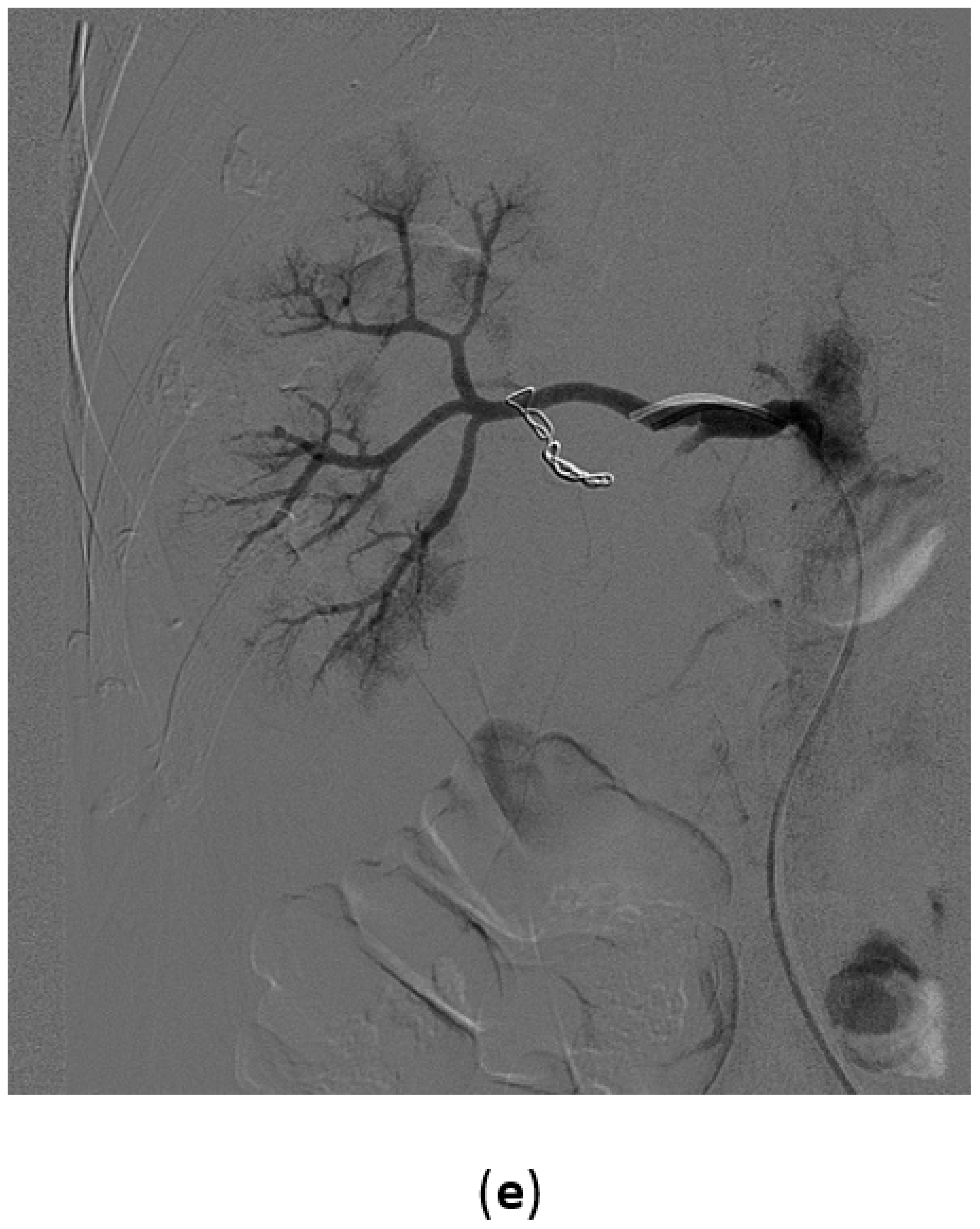
3.4. Renal Trauma
- Grade I: renal contusion, possibly with microscopic gross hematuria, or nonexpanding subcapsular hematoma without a parenchymal laceration;
- Grade II: nonexpanding perirenal hematoma confined to renal retroperitoneum or a renal cortex laceration (<1 cm) without urinary extravasation;
- Grade III: renal cortex laceration (>1 cm) and no urinary extravasation or collecting system rupture;
- Grade IV: renal cortical laceration extending into the collecting system, or a segmental renal artery or vein injury (with parenchymal infarct), or main renal artery or vein injury with contained hematoma;
- Grade V: shattered kidney avulsion of the renal pedicle, or thrombosis of the main renal artery.
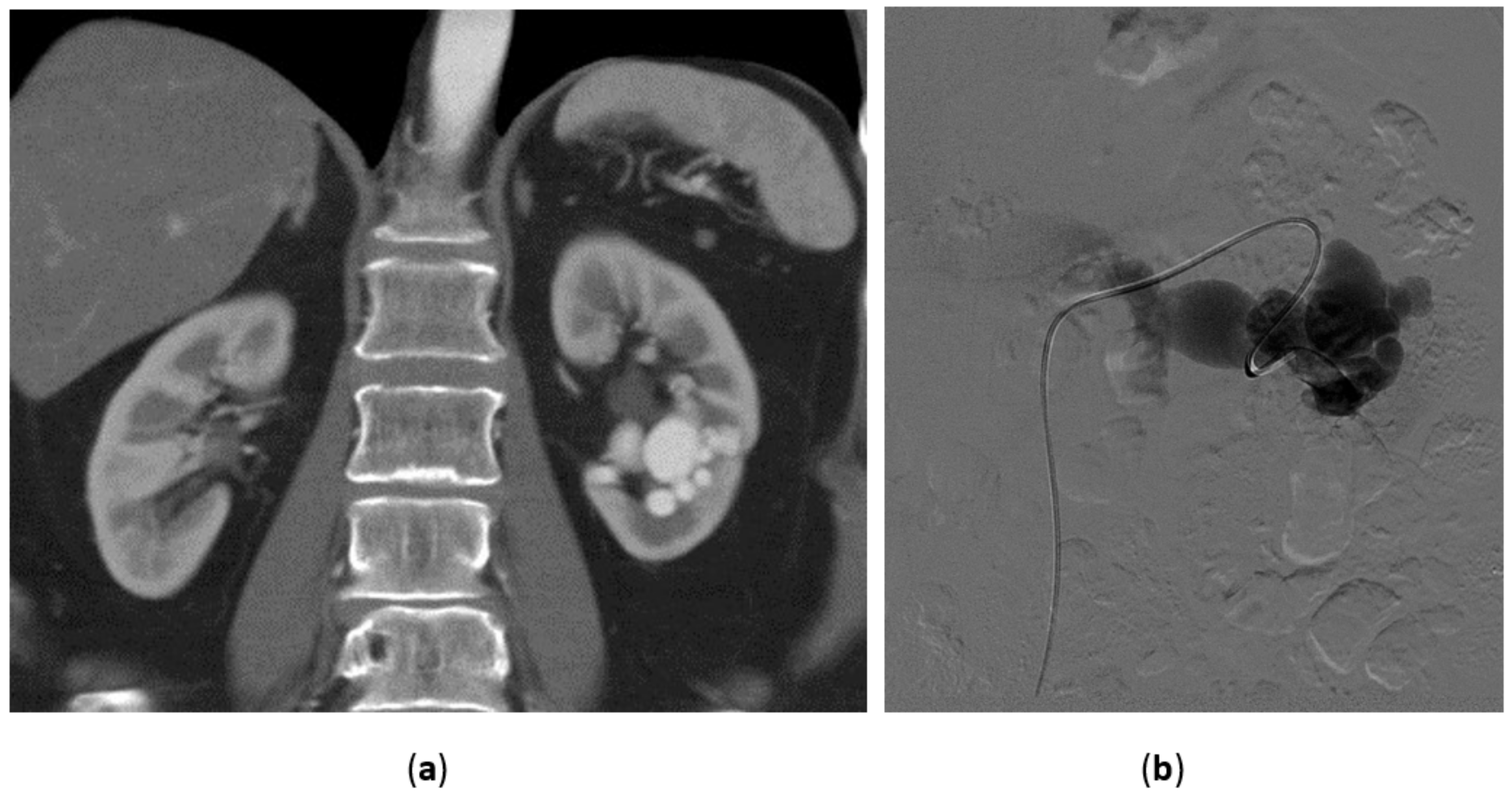
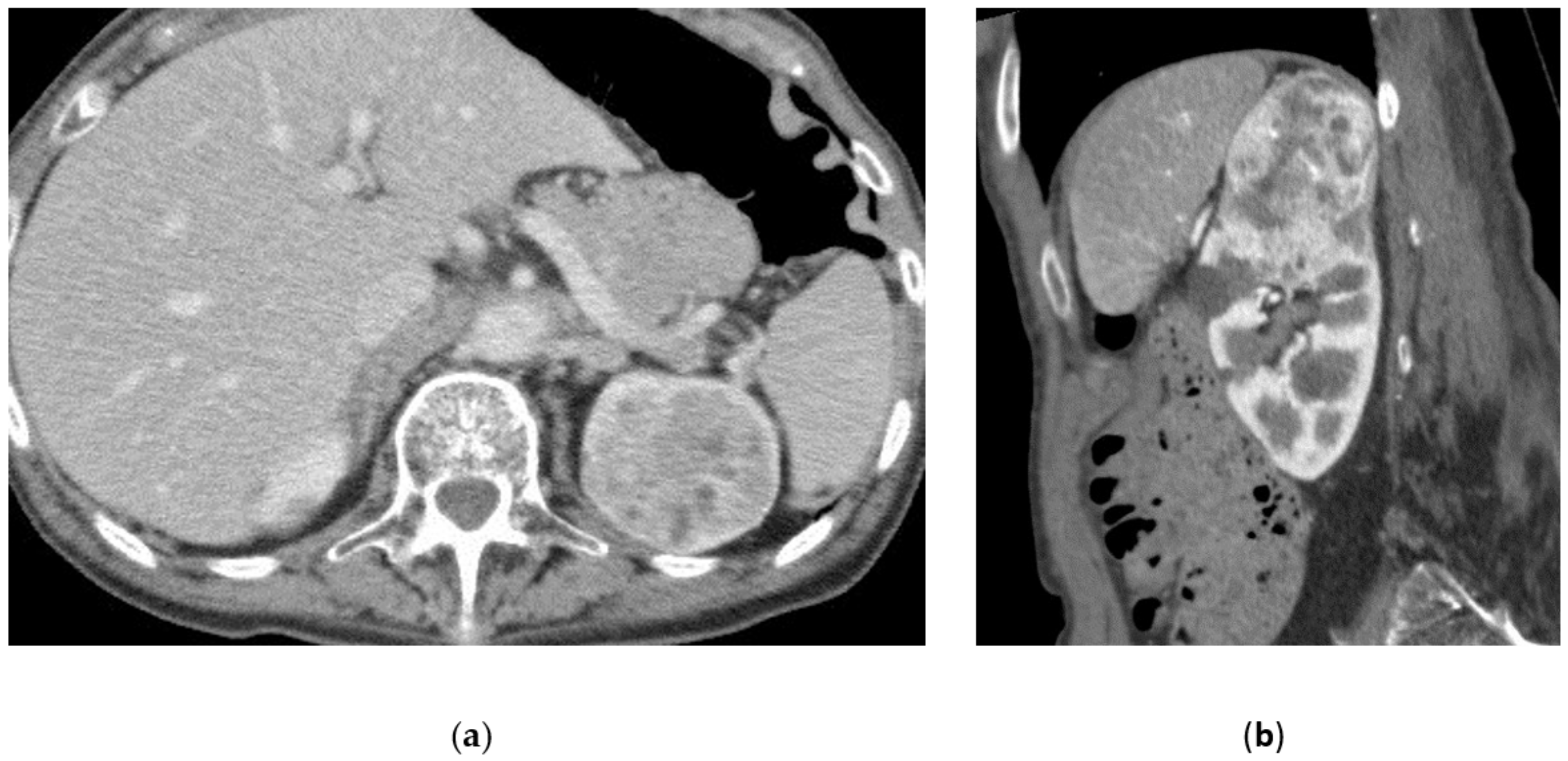
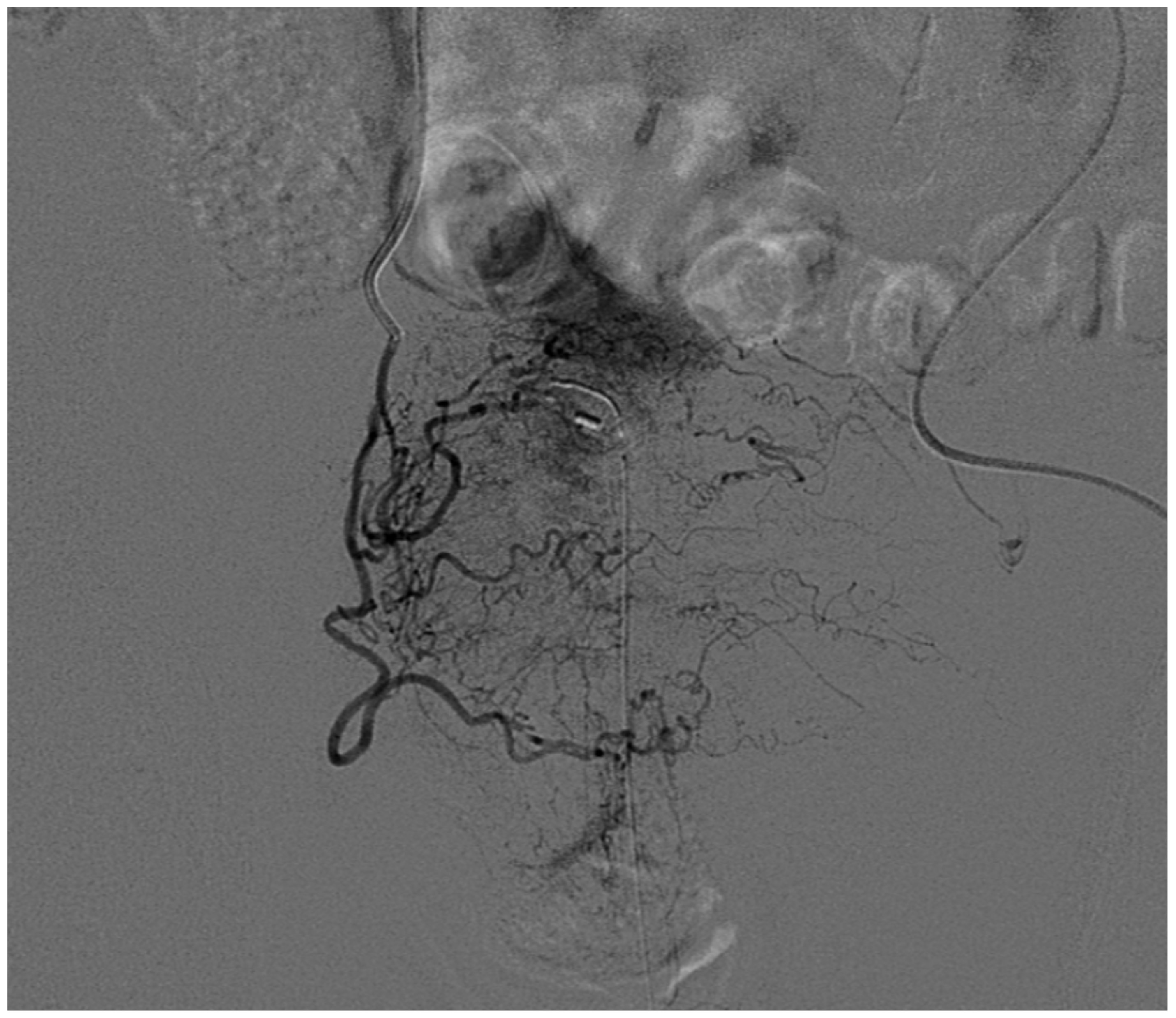
3.5. Renal Angiomyolipoma
3.6. Malignant Renal Tumors
4. Bladder
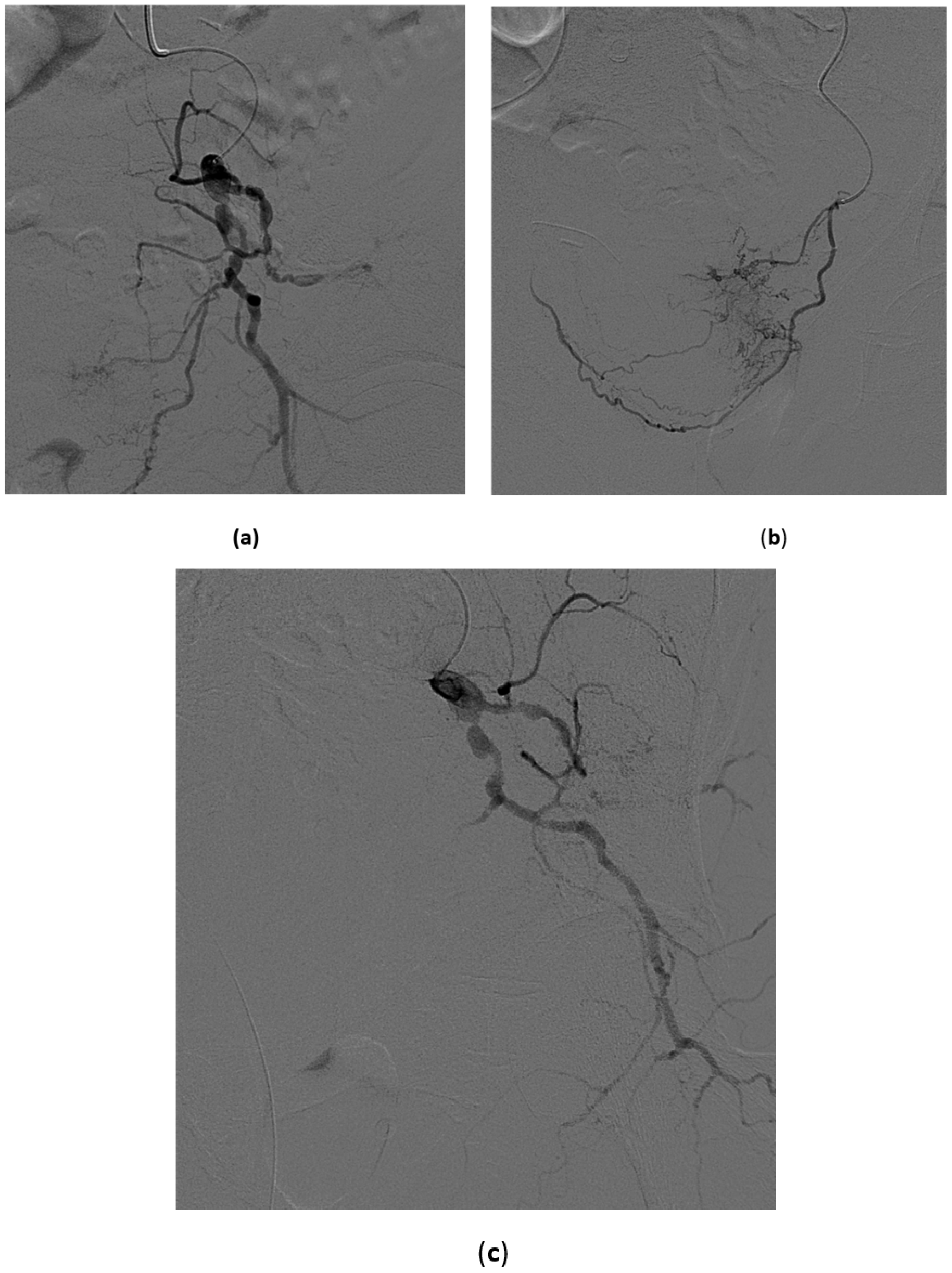
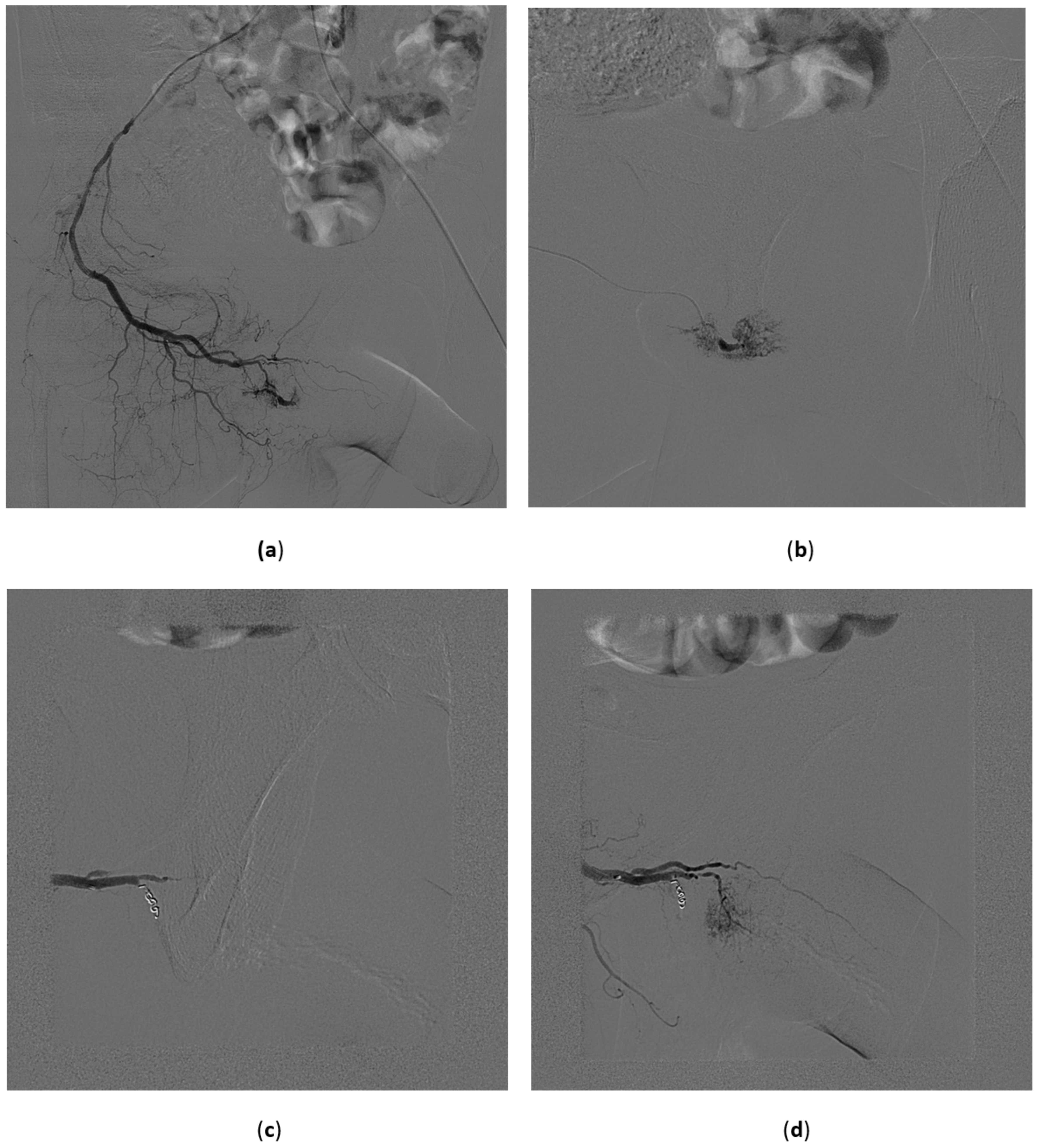
- Superselective catheterization of vesical arteries is the preferred approach as it allows a more targeted delivery of the embolizing particles, thus reducing to a minimum the risk of side-effects from non-target embolization. A coaxial microcatheter is used for selective catheterization. Polyvinyl alcohol particles or tris-acryl gelatin microspheres particles are then released, mixed with contrast medium to make them radiopaque, and detectable with fluoroscopy during delivery. As the most distal branches are embolized, larger particles are injected (Figure 8). In cases in which contrast extravasation is identified, embolization of the feeding artery with n-butyl-2-cyanoacrylate glue mixed with lipiodol has been described [59].
- The coil blockade technique is the alternative approach when the vesical arteries cannot be selectively catheterized. It consists of using 0.018-inch microcoils or a vascular microplug of the appropriate length and diameter to occlude a distal branch at its ostium. Then, embolizing particles are released. In this way the distal branch is protected from unwanted embolization and the particles tend to flow selectively into the vesical vessels, also because of the lower peripheral resistance of bleeding vessels.
- When the catheterization of the main distal branches of the anterior division of the hypogastric artery is not possible, the catheter tip is left in the anterior division and proximal embolization is performed using 0.035-inch coils of adequate length and diameter or mechanically disrupted absorbable gelatin sponge powder sheet.
Results of the Procedure
5. Penis
5.1. High-Flow Priapism
5.2. Clinical, Laboratory, and Imaging Findings
5.3. Treatment of High-Flow Priapism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abt, D.; Bywater, M.; Engeler, D.S.; Schmid, H.-P. Therapeutic options for intractable hematuria in advanced bladder cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2013, 20, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Smit, S.G.; Heyns, C.F. Management of radiation cystitis. Nat. Rev. Urol. 2010, 7, 206–214. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kadioglu, A.; Bivalacqua, T.J.; Ghanem, H.; Nehra, A.; Shamloul, R. Priapism: Pathogenesis, Epidemiology, and Management. J. Sex. Med. 2010, 7, 476–500. [Google Scholar] [CrossRef]
- Londoño, M.A.; Vallejo, A.D.; Argueta, L.F.A.; Uribe, J.R.; Jaramillo, A.R. Transcatheter Embolization. Rev. Colomb. Radiol. 2017, 28, 4773–4781. [Google Scholar]
- Kozar, R.A.; Crandall, M.; Shanmuganathan, K.; Zarzaur, B.L.; Coburn, M.; Cribari, C.; Kaups, K.; Schuster, K.; Tominaga, G.T. AAST Patient Assessment Committee Organ injury scaling 2018 update: Spleen, liver, and kidney. J. Trauma Acute Care Surg. 2018, 85, 1119–1122. [Google Scholar] [CrossRef]
- Carmignani, G.; Belgrano, E.; Puppo, P.; Cichero, A.; Giuliani, L. Transcatheter embilization of the hypogastric arteries in cases of bladder hemorrhage from advanced pelvic cancers: Followup in 9 cases. J. Urol. 1980, 124, 196–200. [Google Scholar] [CrossRef]
- Pisco, J.M.; Martins, J.M.; Correia, M.G. Internal iliac artery: Embolization to control hemorrhage from pelvic neoplasms. Radiology 1989, 172, 337–339. [Google Scholar] [CrossRef]
- Pozzi-Mucelli, F.; Pellegrin, A.; Pozzi-Mucelli, R. Renal Angiography and Vascular Interventional Radiology. In Radiological Imaging of the Kidney; Springer: Berlin/Heidelberg, Germany, 2014; pp. 189–221. [Google Scholar]
- Takeuchi, Y.; Morishita, H.; Sato, Y.; Hamaguchi, S.; Sakamoto, N.; Tokue, H.; Yonemitsu, T.; Murakami, K.; Fujiwara, H.; Sofue, K.; et al. Guidelines for the use of NBCA in vascular embolization devised by the Committee of Practice Guidelines of the Japanese Society of Interventional Radiology (CGJSIR), 2012 edition. Jpn. J. Radiol. 2014, 32, 500–517. [Google Scholar] [CrossRef]
- Leonardi, M.; Barbara, C.; Simonetti, L.; Giardino, R.; Aldini, N.N.; Fini, M.; Martini, L.; Masetti, L.; Joechler, M.; Roncaroli, F. Glubran 2: A new acrylic glue for neuroradiological endovascular use. Experimental study on animals. Interv. Neuroradiol. J. Peritherapeutic Neuroradiol. Surg. Proced. Relat. Neurosci. 2002, 8, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Belli, A.-M.; Markose, G.; Morgan, R. The Role of Interventional Radiology in the Management of Abdominal Visceral Artery Aneurysms. Cardiovasc. Intervent. Radiol. 2012, 35, 234–243. [Google Scholar] [CrossRef] [PubMed]
- White, R.I.; Lynch-Nyhan, A.; Terry, P.; Buescher, P.C.; Farmlett, E.J.; Charnas, L.; Shuman, K.; Kim, W.; Kinnison, M.; Mitchell, S.E. Pulmonary arteriovenous malformations: Techniques and long-term outcome of embolotherapy. Radiology 1988, 169, 663–669. [Google Scholar] [CrossRef]
- Clark, T.W.I.; Sankin, A.; Becske, T.; Nelson, P.K.; Fox, M. Stent-Assisted Gugliemi Detachable Coil Repair of Wide-Necked Renal Artery Aneurysm Using 3-D Angiography. Vasc. Endovascular Surg. 2008, 41, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Somarouthu, B.; Rabinov, J.; Waichi, W.; Kalva, S.P. Stent-Assisted Coil Embolization of an Intraparenchymal Renal Artery Aneurysm in a Patient With Neurofibromatosis. Vasc. Endovascular Surg. 2011, 45, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Rhodes, E.L.; Gewertz, B.L.; Chang, C.Y.; Walter, J.F.; Fry, W.J. Renal artery aneurysms. Significance of macroaneurysms exclusive of dissections and fibrodysplastic mural dilations. Arch. Surg. Chic. Ill 1960 1975, 110, 1327–1333. [Google Scholar] [CrossRef]
- Abeshouse, B.S. Aneurysm of the renal artery; report of two cases and review of the literature. Urol. Cutan. Rev. 1951, 55, 451–463. [Google Scholar]
- Brownstein, A.J.; Erben, Y.; Rajaee, S.; Li, Y.; Rizzo, J.A.; Mojibian, H.; Ziganshin, B.A.; Elefteriades, J.A. Natural history and management of renal artery aneurysms in a single tertiary referral center. J. Vasc. Surg. 2018, 68, 137–144. [Google Scholar] [CrossRef]
- Henke, P.K.; Cardneau, J.D.; Welling, T.H.; Upchurch, G.R.; Wakefield, T.W.; Jacobs, L.A.; Proctor, S.B.; Greenfield, L.J.; Stanley, J.C. Renal Artery Aneurysms: A 35-Year Clinical Experience With 252 Aneurysms in 168 Patients. Ann. Surg. 2001, 234, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, K.; Dougherty, M. Renovascular disease: Aneurysms and arteriovenous fistulae. In Rutherford’s Vascular Surgery; WB Saunders: Philadelphia, PA, USA, 2010; p. 2329. [Google Scholar]
- Liguori, G.; Trombetta, C.; Bucci, S.; Pozzi-Mucelli, F.; Bernobich, E.; Belgrano, E. Percutaneous Management of Renal Artery Aneurysm With a Stent-Graft. J. Urol. 2002, 167, 2518–2519. [Google Scholar] [CrossRef]
- Chimpiri, A.R.; Natarajan, B. Renal Vascular Lesions: Diagnosis and Endovascular Management. Semin. Interv. Radiol. 2009, 26, 253–261. [Google Scholar] [CrossRef]
- Ngo, T.C.; Lee, J.J.; Gonzalgo, M.L. Renal pseudoaneurysm: An overview. Nat. Rev. Urol. 2010, 7, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.; Ward, J.N.; Lavengood, R.W. Intrarenal aneurysm following partial nephrectomy. Urology 1973, 2, 286–288. [Google Scholar] [CrossRef]
- Jain, S.; Nyirenda, T.; Yates, J.; Munver, R. Incidence of Renal Artery Pseudoaneurysm Following Open and Minimally Invasive Partial Nephrectomy: A Systematic Review and Comparative Analysis. J. Urol. 2013, 189, 1643–1648. [Google Scholar] [CrossRef]
- Cohenpour, M.; Strauss, S.; Gottlieb, P.; Peer, A.; Rimon, U.; Stav, K.; Gayer, G. Pseudoaneurysm of the renal artery following partial nephrectomy: Imaging findings and coil embolization. Clin. Radiol. 2007, 62, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Heye, S.; Maleux, G.; Van Poppel, H.; Oyen, R.; Wilms, G. Hemorrhagic complications after nephron-sparing surgery: Angiographic diagnosis and management by transcatheter embolization. AJR Am. J. Roentgenol. 2005, 184, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Chavali, J.S.S.; Bertolo, R.; Kara, O.; Garisto, J.; Mouracade, P.; Nelson, R.J.; Dagenais, J.; Kaouk, J.H. Renal Arterial Pseudoaneurysm After Partial Nephrectomy: Literature Review and Single-Center Analysis of Predictive Factors and Renal Functional Outcomes. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 45–50. [Google Scholar] [CrossRef]
- Wallace, S.; Gianturco, C.; Anderson, J.; Goldstein, H.; Davis, L.; Bree, R. Therapeutic vascular occlusion utilizing steel coil technique: Clinical applications. Am. J. Roentgenol. 1976, 127, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; D’Onofrio, M.; Minniti, S.; Ferrara, R.M.; Procacci, C. Therapeutic Embolization of Idiopathic Renal Arteriovenous Fistula Using the “Stop-Flow” Technique. J. Endovasc. Ther. 2001, 8, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Bryk, D.J.; Zhao, L.C. Guideline of guidelines: A review of urological trauma guidelines. BJU Int. 2016, 117, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, M.; Kondo, Y.; Endo, Y.; Nishimura, T.; Mizunuma, K.; Arai, M.; Yokota, H.; Nakazawa, K.; Murata, S.; Kumita, S. The role of transcatheter arterial embolization (TAE) for deep renal injury. Jpn. J. Urol. 2013, 104, 688–696. [Google Scholar] [CrossRef]
- Hadjipavlou, M.; Grouse, E.; Gray, R.; Sri, D.; Huang, D.; Brown, C.; Sharma, D. Managing penetrating renal trauma: Experience from two major trauma centres in the UK. BJU Int. 2018, 121, 928–934. [Google Scholar] [CrossRef]
- Wang, H.L.; Xu, C.Y.; Wang, H.H.; Xu, W. Emergency Transcatheter Arterial Embolization for Acute Renal Hemorrhage. Medicine 2015, 94, e1667. [Google Scholar] [CrossRef] [PubMed]
- Tinkoff, G.; Esposito, T.J.; Reed, J.; Kilgo, P.; Fildes, J.; Pasquale, M.; Meredith, J.W. American Association for the Surgery of Trauma Organ Injury Scale I: Spleen, Liver, and Kidney, Validation Based on the National Trauma Data Bank. J. Am. Coll. Surg. 2008, 207, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Bookstein, J.J.; Ernst, C.B. Vasodilatory and Vasoconstrictive Pharmacoangiographic Manipulation of Renal Collateral Flow. Radiology 1973, 108, 55–59. [Google Scholar] [CrossRef]
- Keihani, S.; Xu, Y.; Presson, A.P.; Hotaling, J.M.; Nirula, R.; Piotrowski, J.; Dodgion, C.M.; Black, C.M.; Mukherjee, K.; Morris, B.J.; et al. Contemporary management of high-grade renal trauma: Results from the American Association for the Surgery of Trauma Genitourinary Trauma study. J. Trauma Acute Care Surg. 2018, 84, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Chevallier, O.; Gehin, S.; Midulla, M.; Berthod, P.-E.; Galland, C.; Briche, P.; Duperron, C.; Majbri, N.; Mousson, C.; et al. Endovascular management of arterial injuries after blunt or iatrogenic renal trauma. Quant. Imaging Med. Surg. 2017, 7, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, R.S.; Darcy, M.D. Arterial Embolization for the Treatment of Renal Masses and Traumatic Renal Injuries. Tech. Vasc. Interv. Radiol. 2016, 19, 203–210. [Google Scholar] [CrossRef]
- Stewart, A.F.; Brewer, M.E.; Daley, B.J.; Klein, F.A.; Kim, E.D. Intermediate-Term Follow-Up of Patients Treated With Percutaneous Embolization for Grade 5 Blunt Renal Trauma. J. Trauma Inj. Infect. Crit. Care 2010, 69, 468–470. [Google Scholar] [CrossRef]
- Muller, A.; Rouvière, O. Renal artery embolization—indications, technical approaches and outcomes. Nat. Rev. Nephrol. 2015, 11, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.P.; Sanda, M.G. Contemporary diagnosis and management of renal angiomyolipoma. J. Urol. 2002, 168, 1315–1325. [Google Scholar] [CrossRef]
- Yamakado, K.; Tanaka, N.; Nakagawa, T.; Kobayashi, S.; Yanagawa, M.; Takeda, K. Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture. Radiology 2002, 225, 78–82. [Google Scholar] [CrossRef]
- Ewalt, D.H.; Diamond, N.; Rees, C.; Sparagana, S.P.; Delgado, M.; Batchelor, L.; Roach, E.S. Long-term outcome of transcatheter embolization of renal angiomyolipomas due to tuberous sclerosis complex. J. Urol. 2005, 174, 1764–1766. [Google Scholar] [CrossRef]
- Rimon, U.; Duvdevani, M.; Garniek, A.; Golan, G.; Bensaid, P.; Ramon, J.; Morag, B. Large renal angiomyolipomas: Digital subtraction angiographic grading and presentation with bleeding. Clin. Radiol. 2006, 61, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kothary, N.; Soulen, M.C.; Clark, T.W.I.; Wein, A.J.; Shlansky-Goldberg, R.D.; Stavropoulos, S.W.; Crino, P.B. Renal Angiomyolipoma: Long-term Results after Arterial Embolization. J. Vasc. Interv. Radiol. 2005, 16, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Faddegon, S.; So, A. Treatment of angiomyolipoma at a tertiary care centre: The decision between surgery and angioembolization. Can. Urol. Assoc. J. 2011, 5, E138–E141. [Google Scholar] [CrossRef]
- Jou, Y.-C.; Chen, W.-P.; Huang, C.-L. Urgent Angioembolization With Early Elective Nephron-sparing Surgery for Spontaneously Ruptured Renal Angiomyolipoma. J. Chin. Med. Assoc. 2009, 72, 450–452. [Google Scholar] [CrossRef][Green Version]
- Bishay, V.L.; Crino, P.B.; Wein, A.J.; Malkowicz, S.B.; Trerotola, S.O.; Soulen, M.C.; Stavropoulos, S.W. Embolization of Giant Renal Angiomyolipomas: Technique and Results. J. Vasc. Interv. Radiol. 2010, 21, 67–72. [Google Scholar] [CrossRef]
- Huang, Q.; Zhai, R.-Y. Embolization of symptomatic renal angiomyolipoma with a mixture of lipiodol and PVA, a mid-term result. Chin. J. Cancer Res. Chung-Kuo Yen Cheng Yen Chiu 2014, 26, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Thulasidasan, N.; Sriskandakumar, S.; Ilyas, S.; Sabharwal, T. Renal Angiomyolipoma: Mid- to Long-Term Results Following Embolization with Onyx. Cardiovasc. Intervent. Radiol. 2016, 39, 1759–1764. [Google Scholar] [CrossRef]
- Villalta, J.D.; Sorensen, M.D.; Durack, J.C.; Kerlan, R.K.; Stoller, M.L. Selective Arterial Embolization of Angiomyolipomas: A Comparison of Smaller and Larger Embolic Agents. J. Urol. 2011, 186, 921–927. [Google Scholar] [CrossRef]
- Murray, T.E.; Doyle, F.; Lee, M. Transarterial Embolization of Angiomyolipoma: A Systematic Review. J. Urol. 2015, 194, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, R.M.; Stavropoulos, S.W. The Role of Interventional Radiology Techniques in the Management of Renal Angiomyolipomas. Curr. Urol. Rep. 2017, 18, 36. [Google Scholar] [CrossRef]
- Bakal, C.W.; Cynamon, J.; Lakritz, P.S.; Sprayregen, S. Value of Preoperative Renal Artery Embolization in Reducing Blood Transfusion Requirements during Nephrectomy for Renal Cell Carcinoma. J. Vasc. Interv. Radiol. 1993, 4, 727–731. [Google Scholar] [CrossRef]
- Maxwell, N.J.; Saleem Amer, N.; Rogers, E.; Kiely, D.; Sweeney, P.; Brady, A.P. Renal artery embolisation in the palliative treatment of renal carcinoma. Br. J. Radiol. 2007, 80, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Guy, L.; Alfidja, A.T.; Chabrot, P.; Ravel, A.; Boiteux, J.-P.; Boyer, L. Palliative transarterial embolization of renal tumors in 20 patients. Int. Urol. Nephrol. 2007, 39, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Choong, S.K.; Walkden, M.; Kirby, R. The management of intractable haematuria. BJU Int. 2000, 86, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, M.; Şanal, B.; Aras, B.; Bozkaya, H.; Çınar, C.; Güneyli, S.; Gök, M.; Adam, G.; Düzgün, F.; Oran, I. The short- and long-term effectiveness of transcatheter arterial embolization in patients with intractable hematuria. Diagn. Interv. Imaging 2016, 97, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Delgal, A.; Cercueil, J.-P.; Koutlidis, N.; Michel, F.; Kermarrec, I.; Mourey, E.; Cormier, L.; Krausé, D.; Loffroy, R. Outcome of transcatheter arterial embolization for bladder and prostate hemorrhage. J. Urol. 2010, 183, 1947–1953. [Google Scholar] [CrossRef]
- Prasad, V.; Sacks, B.A.; Kraus, S.; Clouse, M.E. Embolotherapy for Lower Urinary Tract Hemorrhage. J. Vasc. Interv. Radiol. 2009, 20, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Amodeo, A.; Mucelli, F.P.; Patel, H.; Marco, D.; Belgrano, E.; Trombetta, C. Intractable haematuria: Long-term results after selective embolization of the internal iliac arteries. BJU Int. 2010, 106, 500–503. [Google Scholar] [CrossRef]
- Mohan, S.; Kumar, S.; Dubey, D.; Phadke, R.V.; Baijal, S.S.; Kathuria, M. Superselective vesical artery embolization in the management of intractable hematuria secondary to hemorrhagic cystitis. World J. Urol. 2019, 37, 2175–2182. [Google Scholar] [CrossRef]
- Loffroy, R.; Guiu, B.; Cercueil, J.-P.; Krausé, D. Endovascular therapeutic embolisation: An overview of occluding agents and their effects on embolised tissues. Curr. Vasc. Pharmacol. 2009, 7, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Nabi, G.; Sheikh, N.; Greene, D.; Marsh, R. Therapeutic transcatheter arterial embolization in the management of intractable haemorrhage from pelvic urological malignancies: Preliminary experience and long-term follow-up. BJU Int. 2003, 92, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Burchell, R.C. Physiology of internal iliac artery ligation. J. Obstet. Gynaecol. Br. Commonw. 1968, 75, 642–651. [Google Scholar] [CrossRef]
- Hald, T.; Mygind, T. Control of life-threatening vesical hemorrhage by unilateral hypogastric artery muscle embolization. J. Urol. 1974, 112, 60–63. [Google Scholar] [CrossRef]
- Taha, D.-E.; Shokeir, A.A.; Aboumarzouk, O.A. Selective embolisation for intractable bladder haemorrhages: A systematic review of the literature. Arab J. Urol. 2018, 16, 197–205. [Google Scholar] [CrossRef]
- Loffroy, R.; Pottecher, P.; Cherblanc, V.; Favelier, S.; Estivalet, L.; Koutlidis, N.; Moulin, M.; Cercueil, J.P.; Cormier, L.; Krausé, D. Current role of transcatheter arterial embolization for bladder and prostate hemorrhage. Diagn. Interv. Imaging 2014, 95, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- El-Assmy, A.; Mohsen, T. Internal iliac artery embolization for the control of severe bladder hemorrhage secondary to carcinoma: Long-term follow-up. ScientificWorldJournal 2007, 7, 1567–1574. [Google Scholar] [CrossRef]
- Appleton, D.S.; Sibley, G.N.; Doyle, P.T. Internal iliac artery embolisation for the control of severe bladder and prostate haemorrhage. Br. J. Urol. 1988, 61, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Sieber, P.R. Bladder necrosis secondary to pelvic artery embolization: Case report and literature review. J. Urol. 1994, 151, 422. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kusano, S.; Matsubayashi, T.; Uchida, T. Selective embolization of the vesical artery in the management of massive bladder hemorrhage. Radiology 1980, 136, 345–348. [Google Scholar] [CrossRef]
- McIvor, J.; Williams, G.; Southcott, R.D. Control of severe vesical haemorrhage by therapeutic embolisation. Clin. Radiol. 1982, 33, 561–567. [Google Scholar] [CrossRef]
- Bertolotto, M.; Quaia, E.; Mucelli, F.P.; Ciampalini, S.; Forgács, B.; Gattuccio, I. Color Doppler Imaging of Posttraumatic Priapism before and after Selective Embolization. RadioGraphics 2003, 23, 495–503. [Google Scholar] [CrossRef]
- Roghmann, F.; Becker, A.; Sammon, J.D.; Ouerghi, M.; Sun, M.; Sukumar, S.; Djahangirian, O.; Zorn, K.C.; Ghani, K.R.; Gandaglia, G.; et al. Incidence of Priapism in Emergency Departments in the United States. J. Urol. 2013, 190, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.B.; Schirmer, H.K.; Scott, W.W. A New Concept in the Management of Priapism. J. Urol. 1960, 83, 60–61. [Google Scholar] [CrossRef]
- Dubocq, F.M.; Tefilli, M.V.; Grignon, D.J.; Pontes, J.E.; Dhabuwala, C.B. High flow malignant priapism with isolated metastasis to the corpora cavernosa. Urology 1998, 51, 324–326. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Harraz, A.M.; Shindel, A.W.; Lue, T.F. Evaluation and management of priapism: 2009 update. Nat. Rev. Urol. 2009, 6, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Halls, J.E.; Patel, D.V.; Walkden, M.; Patel, U. Priapism: Pathophysiology and the role of the radiologist. Br. J. Radiol. 2012, 85, S79–S85. [Google Scholar] [CrossRef]
- Keck, B.; Lotter, G.; Wieland, W.F.; Wullich, B.; Uder, M.; Engehausen, D.G.; Fritsche, H.M. Sonographic diagnosis of a posttraumatic arteriocavernosal fistula resulting in high-flow priapism. J. Clin. Ultrasound 2012, 40, 60–62. [Google Scholar] [CrossRef]
- Savoca, G.; Pietropaolo, F.; Scieri, F.; Bertolotto, M.; Mucelli, F.P.; Belgrano, E. Sexual function after highly selective embolization of cavernous artery in patients with high flow priapism: Long-term followup. J. Urol. 2004, 172, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Volgger, H.; Pfefferkorn, S.; Hobisch, A. Posttraumatic High-Flow Priapism in Children: Noninvasive Treatment by Color Doppler Ultrasound-Guided Perineal Compression. Urology 2007, 70, 590.e3–590.e5. [Google Scholar] [CrossRef] [PubMed]
- Wear, J.B.; Crummy, A.B.; Munson, B.O. A New Approach to the Treatment of Priapism. J. Urol. 1977, 117, 252–254. [Google Scholar] [CrossRef]
- Kim, K.R.; Shin, J.H.; Song, H.-Y.; Ko, G.-Y.; Yoon, H.-K.; Sung, K.-B.; Ahn, T.-Y.; Kim, C.W.; Kim, Y.H.; Ko, H.-K.; et al. Treatment of High-flow Priapism with Superselective Transcatheter Embolization in 27 Patients: A Multicenter Study. J. Vasc. Interv. Radiol. 2007, 18, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Montague, D.K.; Jarow, J.; Broderick, G.A.; Dmochowski, R.R.; Heaton, J.P.W.; Lue, T.F.; Nehra, A.; Sharlip, I.D. Members of the Erectile Dysfunction Guideline Update Panel; Americal Urological Association American Urological Association guideline on the management of priapism. J. Urol. 2003, 170, 1318–1324. [Google Scholar] [CrossRef]
- De Magistris, G.; Pane, F.; Giurazza, F.; Corvino, F.; Coppola, M.; Borzelli, A.; Silvestre, M.; Amodio, F.; Cangiano, G.; Cavaglià, E.; et al. Embolization of high-flow priapism: Technical aspects and clinical outcome from a single-center experience. Radiol. Med. 2020, 125, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ettorre, G.C.; Francioso, G.; Genchi, V.; Prattichizzo, A. Post-traumatic priapism with high flow treated with embolization with N-butyl-cyanoacrylate. Apropos of a case. Radiol. Med. 2000, 99, 403–405. [Google Scholar] [PubMed]
- Burnett, A.L.; Sharlip, I.D. Standard Operating Procedures for Priapism. J. Sex. Med. 2013, 10, 180–194. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzi Mucelli, F.; Pozzi Mucelli, R.A.; Marrocchio, C.; Tollot, S.; Cova, M.A. Endovascular Interventional Radiology of the Urogenital Tract. Medicina 2021, 57, 278. https://doi.org/10.3390/medicina57030278
Pozzi Mucelli F, Pozzi Mucelli RA, Marrocchio C, Tollot S, Cova MA. Endovascular Interventional Radiology of the Urogenital Tract. Medicina. 2021; 57(3):278. https://doi.org/10.3390/medicina57030278
Chicago/Turabian StylePozzi Mucelli, Fabio, Roberta A. Pozzi Mucelli, Cristina Marrocchio, Saverio Tollot, and Maria A. Cova. 2021. "Endovascular Interventional Radiology of the Urogenital Tract" Medicina 57, no. 3: 278. https://doi.org/10.3390/medicina57030278
APA StylePozzi Mucelli, F., Pozzi Mucelli, R. A., Marrocchio, C., Tollot, S., & Cova, M. A. (2021). Endovascular Interventional Radiology of the Urogenital Tract. Medicina, 57(3), 278. https://doi.org/10.3390/medicina57030278






