Oral and Maxillo-Facial Manifestations of Systemic Diseases: An Overview
Abstract
1. Introduction
2. Infectious Diseases
3. Autoimmune and Disimmune Diseases
4. Granulomatous Disease
5. Drug-Induced Oral Lesions
6. Hematologic Disorders
7. Endocrine Diseases (Miscellanea)
8. Genetic Diseases and Head and Neck Syndrome (Miscellanea)
9. Metastases to the Oro-Facial Tissues
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Karanfilian, K.M.; Valentin, M.N.; Kapila, R.; Bhate, C.; Fatahzadeh, M.; Micali, G.; Schwartz, R.A. Cervicofacial actinomycosis. Int. J. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Urbán, E.; Terhes, G. Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview. Dent. J. 2019, 7, 85. [Google Scholar] [CrossRef]
- Hwang, C.S.; Lee, H.; Hong, M.P.; Kim, J.H.; Kim, K.S. Brain abscess caused by chronic invasive actinomycosis in the nasopharynx: A case report and literature review. Medicine 2018, 97, e0406. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawee, R.Y.; Jawhar, N.M.T.; Saeed, M.M. Challenge dilemma of actinomycosis in the tongue: Review and case report. Int. J. Surg. Case Rep. 2020, 75, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Azaïs, M.; Ghannoum, J. Actinomycosis Presenting as Macroglossia: Case Report and Review of Literature. Head Neck Pathol. 2019, 13, 327–330. [Google Scholar] [CrossRef] [PubMed]
- El-Wajeh, Y.A.M.; Watson, M.G.; Igoumenakis, D.; Stathopoulos, P. Tuberculosis: The great imitator in the head and neck—Our experience of 24 cases in 22 years. Br. J. Oral. Maxillofac. Surg. 2018, 56, 168–172. [Google Scholar] [CrossRef]
- Issa, S.A.; Abdulnabi, H.A.; Jameel, M.E. Orofacial tuberculosis: A diagnostic challenge. IDCases 2020, 21, e00825. [Google Scholar] [CrossRef]
- Burns, B.V.; al-Ayoubi, A.; Ray, J.; Schofield, J.B.; Shotton, J.C. Actinomycosis of the posterior triangle: A case report and review of the literature. J. Laryngol. Otol. 1997, 111, 1082–1085. [Google Scholar] [CrossRef]
- Braz-Silva, P.H.; Schussel, J.L.; López Ortega, K.; Gallottini, M. Oral lesions as an important marker for HIV progression. Dermatol. Online J. 2017, 23, 13. [Google Scholar]
- Pedreira, E.N.; Cardoso, C.L.; Barroso Edo, C.; Santos, J.A.; Fonseca, F.P.; Taveira, L.A. Epidemiological and oral manifestations of HIV-positive patients in a specialized service in Brazil. J. Appl. Oral Sci. 2008, 16, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, A.; Falaki, F.; Delavarian, Z.; Dalirsani, Z.; Sanatkhani, M.; Zabihi Marani, M. Oral manifestations of human immunodeficiency virus-infected patients. Iran. J. Otorhinolaryngol. 2015, 27, 43–54. [Google Scholar]
- Bajpai, S.; Pazare, A.R. Oral manifestations of HIV. Contemp. Clin. Dent. 2010, 1, 1–5. [Google Scholar] [CrossRef]
- Matee, M.I.; Scheutz, F.; Moshy, J. Occurrence of oral lesions in relation to clinical and immunological status among HIV-infected adult Tanzanians. Oral Dis. 2000, 6, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.V.; Prabhu, V.; Chatra, L.; Shenai, P. Oral manifestations of HIV. J. Trop. Dis. 2013, 1, 1–9. [Google Scholar]
- Coogan, M.M.; Greenspan, J.; Challacombe, S.J. Oral lesions in infection with human immunodeficiency virus. Bull. World Health Organ. 2005, 83, 700–706. [Google Scholar] [PubMed]
- Favia, G.; Capodiferro, S.; Scivetti, M.; Lacaita, M.G.; Filosa, A.; Lo Muzio, L. Multiple parotid lymphoepithelial cysts in patients with HIV-infection: Report of two cases. Oral Dis. 2004, 10, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Baccaglini, L.; Atkinson, J.C.; Patton, L.L.; Glick, M.; Ficarra, G.; Peterson, D.E. Management of oral lesions in HIV-positive patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103 (Suppl. Sl), S50.e1–S50.e23. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Betz, S.J. HPV-Related Papillary Lesions of the Oral Mucosa: A Review. Head Neck Pathol. 2019, 13, 80–90. [Google Scholar] [CrossRef]
- Capodiferro, S.; Maiorano, E.; Scully, C.; Favia, G. Does a clinico-pathological correlation exist between tonsillar carcinoma and oral proliferative verrucous leukoplakia? Minerva Stomatol. 2007, 56, 153–154. [Google Scholar]
- Mascitti, M.; Tempesta, A.; Togni, L.; Capodiferro, S.; Troiano, G.; Rubini, C.; Maiorano, E.; Santarelli, A.; Favia, G.; Limongelli, L. Histological features and survival in young patients with HPV-negative oral squamous cell carcinoma. Oral Dis. 2020, 26, 1640–1648. [Google Scholar] [CrossRef]
- Limongelli, L.; Capodiferro, S.; Tempesta, A.; Sportelli, P.; Dell’Olio, F.; Angelelli, G.; Maiorano, E.; Favia, G. Early tongue carcinomas (clinical stage I and II): Echo-guided three-dimensional diode laser mini-invasive surgery with evaluation of histological prognostic parameters. A study of 85 cases with prolonged follow-up. Lasers Med. Sci. 2020, 35, 751–758. [Google Scholar] [CrossRef]
- Girardi, F.M.; Wagner, V.P.; Martins, M.D.; Abentroth, A.L.; Hauth, L.A. Prevalence of p16 expression in oropharyngeal squamous cell carcinoma in southern Brazil. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 26. [Google Scholar] [CrossRef]
- Favia, G.; Maiorano, E.; Capodiferro, S.; Pilolli, G.P.; Lacaita, M.G.; Lajolo, C.; Giuliani, M.; Martinelli, D.; Germinario, C. Oral squamous cell carcinoma: A mono-institutional epidemiological study on 462 cases highlighting differences among young and adult patients. Minerva Stomatol. 2008, 57, 413–421. [Google Scholar] [PubMed]
- Tam, S.; Fu, S.; Xu, L.; Krause, K.J.; Lairson, D.R.; Miao, H.; Sturgis, E.M.; Dahlstrom, K.R. The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis. Oral Oncol. 2018, 82, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Martinelli-Kläy, C.P.; Lombardi, T. Clinical, histopathological and immunohistochemical study of oral squamous papillomas. Acta Odontol. Scand. 2015, 73, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Yang, X.; Shi, L.; Feng, J.; Liu, W.; Zhou, Z. Clinicopathologic features of oral squamous papilloma and papillary squamous cell carcinoma: A study of 197 patients from eastern China. Ann. Diagn. Pathol. 2012, 16, 454–458. [Google Scholar] [CrossRef]
- Tamiolakis, P.; Theofilou, V.I.; Tosios, K.I.; Sklavounou-Andrikopoulou, A. Oral verruciform xanthoma: Report of 13 new cases and review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e429–e435. [Google Scholar] [CrossRef]
- Houston, G.D. The giant cell fibroma. A review of 464 cases. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 582–587. [Google Scholar] [CrossRef]
- Mehrad, M.; Carpenter, D.H.; Chernock, R.D.; Wang, H.; Ma, X.J.; Luo, Y.; Luo, J.; Lewis, J.S., Jr.; El-Mofty, S.K. Papillary squamous cell carcinoma of the head and neck: Clinicopathologic and molecular features with special reference to human papillomavirus. Am. J. Surg. Pathol. 2013, 37, 1349–1356. [Google Scholar] [CrossRef]
- Kui, L.L.; Xiu, H.Z.; Ning, L.Y. Condyloma acuminatum and human papilloma virus infection in the oral mucosa of children. Pediatr. Dent. 2003, 25, 149–153. [Google Scholar]
- Tsao, S.W.; Tsang, C.M.; Pang, P.S.; Zhang, G.; Chen, H.; Lo, K.W. The biology of EBV infection in human epithelial cells. Semin. Cancer Biol. 2012, 22, 137–143. [Google Scholar] [CrossRef]
- Kikuchi, K.; Noguchi, Y.; de Rivera, M.W.; Hoshino, M.; Sakashita, H.; Yamada, T. Detection of Epstein–Barr virus genome and latent infection gene expression in normal epithelia, epithelial dysplasia, and squamous cell carcinoma of the oral cavity. Tumour Biol. 2016, 37, 3389–3404. [Google Scholar] [CrossRef] [PubMed]
- Houldcroft, C.J.; Kellam, P. Host genetics of Epstein–Barr virus infection, latency and disease. Rev. Med. Virol. 2015, 25, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Inoue, H.; Miyazaki, Y.; Ide, F.; Kojima, M.; Kusama, K. Epstein-Barr virus (EBV)-associated epithelial and non-epithelial lesions of the oral cavity. Jpn. Dent. Sci. Rev. 2017, 53, 95–109. [Google Scholar] [CrossRef]
- Olivieri, C.V.; Raybaud, H.; Tonoyan, L.; Abid, S.; Marsault, R.; Chevalier, M.; Doglio, A.; Vincent-Bugnas, S. Epstein-Barr virus-infected plasma cells in periodontitis lesions. Microb. Pathog. 2020, 143, 104128. [Google Scholar] [CrossRef]
- Li, D.T.S.; Lo, A.W.I.; Su, Y.X. Oral Epstein-Barr virus-positive mucocutaneous ulcer: Gingival presentation of a benign lymphoproliferative lesion. Int. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaqa, K.; Sullivan, J.L. Infectious mononucleosis. N. Engl. J. Med. 2010, 362, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Vockerodt, M.; Yap, L.-F.; Shannon-Lowe, C.; Curley, H.; Wei, W.; Vizalikova, K. The Epstein–Barr virus and the pathogenesis of lymphoma. J. Pathol. 2015, 235, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Herbst, H.; Niedobitek, G. Sporadic EBV-associated lymphoepithelial salivary gland carcinoma with EBV-positive low-grade myoepithelial component. Virchows Arch. 2006, 448, 648–654. [Google Scholar] [CrossRef]
- Leiba, M.; Jarjoura, S.; Abboud, W.; Nagler, A.; Yahalom, R.; Duek, A.; Yarom, N. Role of oral examination in newly diagnosed multiple myeloma patients: A safe and simple way to detect light chain amyloidosis. Oral Dis. 2018, 24, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, F.S.; de Paulo, L.F.B.; Servato, J.P.; de Faria, P.R.; Cardoso, S.V.; Loyola, A.M. Involvement of oral tissues by AL amyloidosis: A literature review and report of eight new cases. Clin. Oral Investig. 2016, 20, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Gasparro, R.; Marenzi, G.; Mascolo, M.; Cervasio, M.; Cerciello, G.; De Novellis, D.; Mignogna, M.D. Amyloidoma of the Tongue: Case Report, Surgical Management, and Review of the Literature. J. Oral Maxillofac. Surg. 2020, 78, 1572–1582. [Google Scholar] [CrossRef]
- Lane, T.; Pinney, J.H.; Gilbertson, J.A.; Hutt, D.F.; Rowczenio, D.M.; Mahmood, S.; Sachchithanantham, S.; Fontana, M.; Youngstein, T.; Quarta, C.C.; et al. Changing epidemiology of AA amyloidosis: Clinical observations over 25 years at a single national referral centre. Amyloid 2017, 24, 162. [Google Scholar] [CrossRef]
- Quock, T.P.; Yan, T.; Chang, E.; Guthrie, S.; Broder, M.S. Epidemiology of AL amyloidosis: A real-world study using US claims data. Blood Adv. 2018, 2, 1046. [Google Scholar] [CrossRef]
- Nandapalan, V.; Jones, T.M.; Morar, P.; Clark, A.H.; Jones, A.S. Localized amyloidosis of the parotid gland: A case report and review of the localized amyloidosis of the head and neck. Head Neck 1998, 20, 73–78. [Google Scholar] [CrossRef]
- Pentenero, M.; Davico Bonino, L.; Tomasini, C.; Conrotto, D.; Gandolfo, S. Localized oral amyloidosis of the palate. Amyloid 2006, 13, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Aono, J.; Yamagata, K.; Yoshida, H. Local amyloidosis in the hard palate: A case report. Oral Maxillofac. Surg. 2009, 12, 119. [Google Scholar] [CrossRef]
- Musat, G.; Evsei, A.; Calina, D.; Docea, A.O.; Doukas, S.G.; Vageli, D.P.; Nepka, C.; Spandidos, D.A.; Mitroi, M. Rare amyloidoma of the tongue base: A case report and review of the literature. Mol. Clin. Oncol. 2020, 12, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Maturana-Ramírez, A.; Ortega, A.V.; Labbé, F.C.; de Moraes, Ê.; Aitken-Saavedra, J.P. Macroglossia, the first manifestation of systemic amyloidosis associated with multiple myeloma: Case report. J Stomatol. Oral Maxillofac. Surg. 2018, 119, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Molina, G.; Avila-Casado, C.; Cárdenas-Velázquez, F.; Hernández-Hernández, C.; Calderillo, M.L.; Marroquín, V.; Soto-Abraham, V.; Recillas-Gispert, C.; Sánchez-Guerrero, J. Similarities and differences between primary and secondary Sjögren’s syndrome. J. Rheumatol. 2010, 37, 800–808. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. The geoepidemiology of Sjogren’s syndrome. Autoimmun. Rev. 2010, 9, A305–A310. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. International Sjögren’s Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Moutsopoulos, H.M. Therapy of Sjogren’s syndrome. Springer Semin. Immunopathol. 2001, 23, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Moutsopoulos, N.M.; Moutsopoulos, H.M. The management of Sjogren’s syndrome. Nat. Clin. Pract. Rheumatol. 2006, 2, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ingravallo, G.; Maiorano, E.; Moschetta, M.; Limongelli, L.; Mastropasqua, M.G.; Agazzino, G.F.; De Ruvo, V.; Tarantino, P.; Favia, G.; Capodiferro, S. Primary Breast Extranodal Marginal Zone Lymphoma in Primary Sjögren Syndrome: Case Presentation and Relevant Literature. J. Clin. Med. 2020, 9, 3997. [Google Scholar] [CrossRef] [PubMed]
- Huda Raza, N.U.; Ghafoor, S. Desmosomal protein regulation and clinical implications in oral mucosal tissues. J. Pak. Med. Assoc. 2020, 70, 1425–1431. [Google Scholar] [CrossRef]
- Calabria, E.; Fortuna, G.; Aria, M.; Mignogna, M.D. Autoimmune mucocutaneous blistering diseases in the south of Italy: A 25-year retrospective study on 169 patients. J. Oral Pathol. Med. 2020, 49, 672–680. [Google Scholar] [CrossRef]
- Pettini, F.; Ballini, A.; Capodiferro, S.; Cantore, S.; Cirulli, N.; Garofalo, A.; Coscia, M.F.; De Vito, D.; Foti, C. Management of oral pemphigus vulgaris: A case report and a clinical update. Eur. J. Inflamm. 2015, 53–57. [Google Scholar] [CrossRef]
- Macklis, P.; Adams, K.; Kaffenberger, J.; Kumar, P.; Krispinsky, A.; Kaffenberger, B. The Association Between Oral Health and Skin Disease. J. Clin. Aesthet. Dermatol. 2020, 13, 48–53. [Google Scholar]
- Al Ismaili, A.; Al Busaidi, K.; Nalawade, T.; Saraf, S. Immune-mediated Skin Disorders and their Oral Manifestations in the Omani Population: A Hospital-based Study. Oman Med. J. 2020, 35, e84. [Google Scholar] [CrossRef] [PubMed]
- Kuten-Shorrer, M.; Menon, R.S.; Lerman, M.A. Mucocutaneous Diseases. Dent. Clin. N. Am. 2020, 64, 139–162. [Google Scholar] [CrossRef]
- Odani, K.; Itoh, A.; Yanagita, S.; Kaneko, Y.; Tachibana, M.; Hashimoto, T.; Tsutsumi, Y. Paraneoplastic Pemphigus Involving the Respiratory and Gastrointestinal Mucosae. Case Rep. Pathol. 2020, 2020, 7350759. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, A.; Aydin, F.; Sumer, P.; Keskiner, I.; Koc, S.; Bozkurt, S.; Mumcu, G.; Alpsoy, E.; Uzun, S.; Akman-Karakas, A. Oral health related qualıty of lıfe and dısease severıty ın autoımmune bullous dıseases. Niger. J. Clin. Pract. 2020, 23, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ormond, M.; McParland, H.; Thakrar, P.; Donaldson, A.N.A.; Andiappan, M.; Cook, R.J.; Escudier, M.E.; Higham, J.; Hullah, E.; McMillan, R.; et al. Validation of an Oral Disease Severity Score (ODSS) tool for use in oral mucous membrane pemphigoid. Br. J. Dermatol. 2020, 183, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mumcu, G.; Yay, M.; Karaçaylı, Ü.; Aksoy, A.; Taş, M.N.; Armağan, B.; Sarı, A.; Bozca, B.C.; Tekgöz, E.; Temiz Karadağ, D.; et al. Moderation analysis exploring associations between age and mucocutaneous activity in Behçet’s syndrome: A multicenter study from Turkey. J. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yazici, H.; Tüzün, Y.; Pazarli, H.; Yurdakul, S.; Ozyazgan, Y.; Ozdoğan, H.; Serdaroğlu, S.; Ersanli, M.; Ulkü, B.Y.; Müftüoğlu, A.U. Influence of age of onset and patient’s sex on the prevalence and severity of manifestations of Behçet’s syndrome. Ann. Rheum. Dis. 1984, 43, 783–789. [Google Scholar] [CrossRef]
- Yilmaz, S.; Simsek, I.; Cinar, M.; Erdem, H.; Kose, O.; Yazici, Y.; Pay, S. Patient-driven assessment of disease activity in Behcet’s syndrome: Cross-cultural adaptation, reliability and validity of the Turkish version of the Behcet’s Syndrome Activity Score. Clin. Exp. Rheumatol. 2013, 31, 77–83. [Google Scholar]
- Senusi, A.; Higgins, S.; Fortune, F. The influence of oral health and psycho-social well-being on clinical outcomes in Behcet’s disease. Rheumatol. Int. 2018, 38, 1873–1883. [Google Scholar] [CrossRef]
- Senusi, A.; Seoudi, N.; Bergmeier, L.A.; Fortune, F. Genital ulcer severity score and genital health quality of life in Behçet’s disease. Orphanet J. Rare Dis. 2015, 10, 117. [Google Scholar] [CrossRef]
- Hamuryudan, V.; Hatemi, G.; Sut, N.; Ugurlu, S.; Yurdakul, S.; Yazici, H. Frequent oral ulceration during early disease may predict a severe disease course in males with Behcet’s syndrome. Clin. Exp. Rheumatol. 2012, 30, S32–S34. [Google Scholar] [PubMed]
- Lawton, G.; Bhakta, B.B.; Chamberlain, M.A.; Tennant, A. The Behcet’s disease activity index. Rheumatology (Oxford) 2004, 43, 73–78. [Google Scholar] [CrossRef]
- Mumcu, G.; Inanc, N.; Taze, A.; Ergun, T.; Direskeneli, H. A new mucocutaneous activity index for Behcet’s disease. Clin. Exp. Rheumatol. 2014, 32, S80–S86. [Google Scholar]
- Mumcu, G.; Yazici, Y.; Hatemi, G. Disease Assessment in Behcet’s Syndrome: Behcet’s Syndrome; Springer Nature: Cham, Switzerland, 2020; pp. 261–278. [Google Scholar]
- Soung, J.; Lebwohl, M. Psoriasis: Clinical presentation. Psoriasis and Psoriatic Arthritis: An Integrated Approach; Gordon, K.B., Ruderman, E.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 67–72. [Google Scholar] [CrossRef]
- Younai, S.F.; Phelan, J.A. Oral mucositis with features of psoriasis: Report of a case and review of the literature. Oral Surg. Oral Med. Oral Pathol. Endod. 1997, 84, 61–67. [Google Scholar] [CrossRef]
- Wu, I.B.; Schwartz, R.A. Reiter’s syndrome: The classic triad and more. J. Am. Acad. Dermatol. 2008, 59, 113–121. [Google Scholar] [CrossRef]
- Mattsson, U.; Warfvinge, G.; Jontell, M. Oral psoriasis—A diagnostic dilemma: A report of two cases and a review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, e183–e189. [Google Scholar] [CrossRef]
- Pérez, F.; Aveldañez, A.; Ruvalcaba, M.; Barcelot, M.; Camacho, M.; Memije, M.; Taylor, A. Prevalence of oral lesions in patients with psoriasis. Med. Oral Pathol. Oral Cir. Bucal. 2008, 13, e703–e708. [Google Scholar]
- Zhu, J.-F.; Kaminski, M.J.; Pulitzer, D.R.; Hu, J.; Thomas, H.F. Psoriasis: Pathophysiology and oral manifestations. Oral Dis. 1996, 2, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Weathers, D.R.; Baker, G.; Archard, H.O.; Jefferson Burke, E. Psoriasiform lesions of the oral mucosa (with emphasis on ‘ectopic geographic tongue’). Oral Pathol. 1974, 37, 872–888. [Google Scholar] [CrossRef]
- Ulmansky, M.; Michelle, R.; Azaz, B. Oral psoriasis: Report of six new cases. J. Oral Pathol. Med. 1995, 24, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Reis, V.; Artico, G.; Seo, J.; Bruno, I.; Hirota, S.K.; Lemos, C., Jr.; Martins, M.; Migliari, D. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int. J. Dermatol. 2013, 52, 113–115. [Google Scholar] [CrossRef]
- Picciani, B.; Silva-Junior, G.; Carneiro, S.; Sampaio, A.L.; Goldemberg, D.C.; Oliveira, J.; Porto, L.C.; Dias, E.P. Geographic stomatitis: An oral manifestation of psoriasis? J. Dermatol. Case Rep. 2012, 6, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Oral Psoriasis: An Overlooked Enigma. Dermatology 2016, 232, 319–325. [Google Scholar] [CrossRef]
- Arnold, D.L.; Krishnamurthy, K. Lichen Planus; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, D.; Chisnoiu, R.; Picos, A.M.; Picos, A.; Chisnoiu, A. Treatment trends in oral lichen planus and oral lichenoid lesions (Review). Exp. Ther. Med. 2020, 20, 198. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Clinicopathological and prognostic characteristics of oral squamous cell carcinomas arising in patients with oral lichen planus: A systematic review and a comprehensive meta-analysis. Oral Oncol. 2020, 106, 104688. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Richards, D. Malignant transformation rates in Oral Lichen Planus. Evid. Based Dent. 2018, 19, 122. [Google Scholar] [CrossRef]
- Lourenço, S.V.; de Carvalho, F.R.; Boggio, P.; Sotto, M.N.; Vilela, M.A.; Rivitti, E.A.; Nico, M.M. Lupus erythematosus: Clinical and histopathological study of oral manifestations and immunohistochemical profile of the inflammatory infiltrate. J. Cutan Pathol. 2007, 34, 558–564. [Google Scholar] [CrossRef]
- Schiødt, M. Oral manifestations of lupus erythematosus. Int. J. Oral Surg. 1984, 13, 101–147. [Google Scholar] [CrossRef]
- Nico, M.M.; Vilela, M.A.; Rivitti, E.A.; Lourenço, S.V. Oral lesions in lupus erythematosus: Correlation with cutaneous lesions. Eur. J. Dermatol. 2008, 18, 376–381. [Google Scholar] [PubMed]
- Jessop, S.; Whitelaw, D.A.; Delamere, F.M. Drugs for discoid lupus erythematosus. Cochrane Database Syst. Rev. 2009, 4, CD002954. [Google Scholar]
- Schubert, M.M.; Correa, M.E. Oral graft-versus-host disease. Dent. Clin. N. Am. 2008, 52, 79–109. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, M.C.; Wang, Z.; Horowitz, M.M.; Gale, R.P. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transpl. 2010, 1, 87–105. [Google Scholar]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease:, I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Tamma, R.; Limongelli, L.; Maiorano, E.; Pastore, D.; Cascardi, E.; Tempesta, A.; Carluccio, P.; Mastropasqua, M.G.; Capodiferro, S.; Covelli, C.; et al. Vascular density and inflammatory infiltrate in primary oral squamous cell carcinoma and after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2019, 98, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.E.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative analysis of risk factors for acute graft versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Alawi, F.; Shields, B.E.; Omolehinwa, T.; Rosenbach, M. Oral Granulomatous Disease. Dermatol. Clin. 2020, 38, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Al Johani, K.; Moles, D.R.; Hodgson, T.; Porter, S.R.; Fedele, S. Onset and progression of clinical manifestations of orofacial granulomatosis. Oral Dis. 2009, 15, 214–219. [Google Scholar] [CrossRef]
- Bansal, S.; Garg, A.; Khurana, R.; Bansal, A. Primary orofacial granulomatous involvement of lip and gingiva only: A diagnostic challenge. J. Indian Soc. Periodontol. 2020, 24, 575–578. [Google Scholar] [CrossRef]
- Ketabchi, S.; Massi, D.; Ficarra, G.; Rubino, I.; Franchi, A.; Paglierani, M.; Simoni, A.; Capodiferro, S.; Favia, G.; Maiorano, E.; et al. Expression of protease-activated receptor-1 and -2 in orofacial granulomatosis. Oral Dis. 2007, 13, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Scully, C.; Ficarra, G.; De Frenza, G.; Grassi, R.; Maiorano, E.; Favia, G.; Tetè, S. Orofacial Granulomatosis: Report of Two Cases with Gingival Onset. Eur. J. Inflamm. 2007, 51–56. [Google Scholar] [CrossRef]
- Miest, R.Y.; Bruce, A.J.; Comfere, N.I.; Hadjicharalambous, E.; Endly, D.; Lohse, C.M.; Rogers, R.S., III. A Diagnostic Approach to Recurrent Orofacial Swelling: A Retrospective Study of 104 Patients. Mayo Clin. Proc. 2017, 92, 1053–1060. [Google Scholar] [CrossRef]
- Capodiferro, S.; Maiorano, E.; Tempesta, A.; Limongelli, L.; Favia, G. Ulcerated nodules of the tongue. Neth. J. Med. 2018, 76, 347. [Google Scholar]
- Gulseren, D.; Elçin, G. Images of the month: Demonstrative oral mucosal sarcoidosis in a patient with pulmonary disease. Clin. Med. 2020, 20, e127–e128. [Google Scholar] [CrossRef]
- Brown, F.; Modi, P.; Tanner, L.S. Lofgren Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gavioli, C.F.B.; Nico, M.M.S.; Florezi, G.P.; Lourenço, S.V. The histopathological spectrum of Melkersson-Rosenthal syndrome: Analysis of 47 cases. J. Cutan. Pathol. 2020, 47, 1010–1017. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q. Orofacial Edema and Facial Paralysis: Melkersson-Rosenthal Syndrome. J. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Apoita-Sanz, M.; Blanco-Jauset, P.; Polis-Yanes, C.; Penin-Mosquera, R.M.; Montserrat-Gomá, G.; Pozuelo-Arquimbau, L.; Vidal-Bell, A.; Arranz-Obispo, C.; López-López, J. Granulomatosis with Poliangiitis (Wegener’s Granulomatosis): Orofacial Manifestations. Systematic Review and Case Report. Oral Health Prev. Dent. 2020, 18, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Dhalkari, C.D.; Patil, S.C.; Indurkar, M.S. Strawberry gingivitis—First sign of Wegener’s granulomatosis. J. Oral Maxillofac. Pathol. 2020, 24, 172–175. [Google Scholar] [CrossRef]
- Kertesz, T.; Soowamber, M.; Bubola, J.; Psutka, D.J.; Bradley, G. Gingival Swelling as the Initial Manifestation of Granulomatosis with Polyangiitis. Head Neck Pathol. 2020. [Google Scholar] [CrossRef]
- Patrick, A.; Altman, K. Granulomatosis with polyangiitis: Potentially lethal gingival lesions presenting to the dentist. BMJ Case Rep. 2019, 12, e229607. [Google Scholar] [CrossRef] [PubMed]
- Jajam, M.; Bozzolo, P.; Niklander, S. Oral manifestations of gastrointestinal disorders. J. Clin. Exp. Dent. 2017, 10, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Fleming, P.; Bourke, B. Looking in the mouth for Crohn’s disease. Inflamm. Bowel Dis. Feb. 2010, 16, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Bogenrieder, T.; Rogler, G.; Vogt, T.; Landthaler, M.; Stolz, W. Orofacial granulomatosis as the initial presentation of Crohn’s disease in an adolescent. Dermatology 2003, 206, 273–278. [Google Scholar] [CrossRef]
- Eckel, A.; Lee, D.; Deutsch, G.; Maxin, A.; Oda, D. Oral manifestations as the first presenting sign of Crohn’s disease in a pediatric patient. J. Clin. Exp. Dent. 2017, 97, 934–938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Favia, G.; Limongelli, L.; Tempesta, A.; Maiorano, E.; Capodiferro, S. Oral lesions as first clinical manifestations of Crohn’s disease in paediatric patients: A report on 8 cases. Eur. J. Paediatr. Dent. 2020, 21, 66–69. [Google Scholar] [CrossRef]
- Capodiferro, S.; Maiorano, E.; Limongelli, L.; Tempesta, A.; Favia, G. Cheilitis and gingivitis as first signs of Crohn’s disease in a pediatric patient. Clin. Case Rep. 2019, 7, 387–388. [Google Scholar] [CrossRef]
- Dongari-Bagtzoglou, A. Research, Science and Therapy Committee, American Academy of Periodontology. Drug-associated gingival enlargement. J. Periodontol. 2004, 75, 1424–1431. [Google Scholar]
- Seymour, R.A.; Thomason, J.M.; Ellis, J.S. The pathogenesis of drug-induced gingival overgrowth. J. Clin. Periodontol. 1996, 23, 165–175. [Google Scholar] [CrossRef]
- Bharti, V.; Bansal, C. Drug-induced gingival overgrowth: The nemesis of gingiva unravelled. J. Indian Soc. Periodontol. 2013, 17, 182–187. [Google Scholar] [CrossRef]
- Agrawal, A.A. Gingival enlargements: Differential diagnosis and review of literature. World J. Clin. Cases. 2015, 3, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Tungare, S.; Paranjpe, A.G. Drug Induced Gingival Overgrowth; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Capodiferro, S.; Tempesta, A.; Limongelli, L.; Maiorano, E.; Benedicenti, S.; Favia, G. Nonsurgical Periodontal Treatment by Erbium:YAG Laser Promotes Regression of Gingival Overgrowth in Patient Taking Cyclosporine A: A Case Report. Photobiomodul. Photomed. Laser Surg. 2019, 37, 53–56. [Google Scholar] [CrossRef]
- Srivastava, A.; Nogueras Gonzalez, G.M.; Geng, Y.; Won, A.M.; Cabanillas, M.E.; Naing, A.; Myers, J.N.; Li, Y.; Chambers, M.S. Prevalence of medication related osteonecrosis of the jaw in patients treated with sequential antiresorptive drugs: Systematic review and meta-analysis. Support Care Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Maiorano, E. Medication-Related Osteonecrosis of the Jaws: Considerations on a New Antiresorptive Therapy (Denosumab) and Treatment Outcome after a 13-Year Experience. Int. J. Dent. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Querrer, R.; Ferrare, N.; Melo, N.; Stefani, C.M.; Dos Reis, P.E.D.; Mesquita, C.R.M.; Borges, G.A.; Leite, A.F.; Figueiredo, P.T. Differences between bisphosphonate-related and denosumab-related osteonecrosis of the jaws: A systematic review. Support Care Cancer 2020. [Google Scholar] [CrossRef]
- Antonuzzo, L.; Lunghi, A.; Petreni, P.; Brugia, M.; Laffi, A.; Giommoni, E.; Mela, M.M.; Mazzoni, F.; Balestri, V.; Costanzo, F.D. Osteonecrosis of the Jaw and Angiogenesis inhibitors: A Revival of a Rare but Serous Side Effect. Curr. Med. Chem. 2017, 24, 3068–3076. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Iannone, F.; Lapadula, G.; Maiorano, E. A Case of Osteonecrosis of the Jaw in a Patient with Crohn’s Disease Treated with Infliximab. Am. J. Case Rep. 2017, 18, 1351–1356. [Google Scholar] [CrossRef]
- Favia, G.; Piattelli, A.; Sportelli, P.; Capodiferro, S.; Iezzi, G. Osteonecrosis of the posterior mandible after implant insertion: A clinical and histological case report. Clin. Implant Dent. Relat. Res. 2011, 13, 58–63. [Google Scholar] [CrossRef]
- Lervat, C.; Auperin, A.; Patte, C.; Méchinaud, F.; Leverger, G.; Nelken, B.; Bertrand, Y.; Baruchel, A.; Coze, C.; Munzer, M.; et al. Head and neck presentations of B-NHL and B-AL in children/adolescents: Experience of the LMB89 study. Pediatr. Blood Cancer 2014, 61, 473–478. [Google Scholar] [CrossRef]
- Liu, H.W.; Wen, W.S.; Yang, G. Clinical manifestation and diagnosis of non-Hodgkin’s lymphoma (NHL) in head and neck: Analysis of 138 cases. Shanghai Kou Qiang Yi Xue 2011, 20, 179–182. [Google Scholar]
- Singh, R.; Shaik, S.; Negi, B.S.; Rajguru, J.P.; Patil, P.B.; Parihar, A.S.; Sharma, U. Non-Hodgkin’s lymphoma: A review. J. Family Med. Prim. Care 2020, 9, 1834–1840. [Google Scholar] [CrossRef]
- Kusuke, N.; Custódio, M.; de Sousa, S.C.O.M. Oral lesion as the primary diagnosis of non-Hodgkin’s lymphoma: A 20-year experience from an oral pathology service and review of the literature. Eur. Arch. Otorhinolaryngol. 2019, 276, 2873–2879. [Google Scholar] [CrossRef]
- Nicollas, R.; Rome, A.; Belaich, H.; Roman, S.; Volk, M.; Gentet, J.C.; Michel, G.; Triglia, J.M. Head and neck manifestation and prognosis of Langerhans’ cell histiocytosis in children. Int. J. Pediatr. Otorhi. 2010, 74, 669–673. [Google Scholar] [CrossRef]
- Haupt, R.; Minkov, M.; Astigarraga, I.; Schäfer, E.; Nanduri, V.; Jubran, R.; Egeler, R.M.; Janka, G.; Micic, D.; Rodriguez-Galindo, C.; et al. Langerhans cell histiocytosis (LCH): Guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr. Blood Cancer 2013, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Azouz, E.M.; Saigal, G.; Rodriguez, M.M.; Podda, A. Langerhans’ cell histiocytosis: Pathology, imaging and treatment of skeletal involvement. Pediatr. Radiol. 2005, 35, 103–115. [Google Scholar] [CrossRef]
- Windebank, K.; Nanduri, V. Langerhans cell histiocytosis. Arch. Dis. Child. 2009, 94, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Aimoni, C.; Trevisani, M.; Paola, M. Langerhans’ cell histiocytosis: Report of a case with temporal localization. Int. J. Pediatr. Otorhi. 2000, 55, 51–56. [Google Scholar] [CrossRef]
- Madrigal-Martinez-Pereda, C.; Guerrero-Rodriguez, V.; Guisado-Moya, B.; Meniz-García, C. Langerhans cell histiocytosis: Literature review and descriptive analysis of oral manifestations. Med. Oral Patol. Oral 2009, 14, E222–E228. [Google Scholar]
- Capodiferro, S.; Tempesta, A.; Limongelli, L.; Ingravallo, G.; Maiorano, E.; Sfasciotti, G.L.; Bossù, M.; Polimeni, A.; Favia, G. Primary Oro-Facial Manifestations of Langerhans Cell Histiocytosis in Pediatric Age: A Bi-Institutional Retrospective Study on 45 Cases. Children 2020, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Zgibor, J.C.; Dasanayake, A.P. Diabetes: A growing epidemic of all ages. J. Am. Dent. Assoc. 2003, 134, 11–15. [Google Scholar] [CrossRef]
- Saini, R.; Al-Maweri, S.A.; Saini, D.; Ismail, N.M.; Ismail, A.R. Oral mucosal lesions in non-oral habit diabetic patients and association of diabetes mellitus with oral precancerous lesions. Diabetes Res. Clin. Pract. 2010, 89, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lian, C.; Vaidya, A.; Tsibris, H.C. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020, 6, 993–995. [Google Scholar] [CrossRef]
- Dos Santos, B.; Koth, V.S.; Figueiredo, M.A.; Salum, F.G.; Cherubini, K. Brown tumor of the jaws as a manifestation of tertiary hyperparathyroidism: A literature review and case report. Spec. Care Dentist 2018, 38, 163–171. [Google Scholar] [CrossRef]
- Guimarães, L.M.; Gomes, I.P.; Pereira, T.D.S.F.; de Andrade, B.A.B.; Romañach, M.J.; de Lacerda, J.C.T.; Pontes, H.A.R.; Brennan, P.A.; Rahimi, S.; Carlos, R.; et al. KRAS mutations in brown tumor of the jaws in hyperparathyroidism. J. Oral Pathol. Med. 2020, 49, 796–802. [Google Scholar] [CrossRef]
- Lajolo, C.; Patini, R.; Limongelli, L.; Favia, G.; Tempesta, A.; Contaldo, M.; De Corso, E.; Giuliani, M. Brown tumors of the oral cavity: Presentation of 4 new cases and a systematic literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 575–584.e4. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, L.; Tempesta, A.; Lauritano, D.; Maiorano, E.; Ingravallo, G.; Favia, G.; Capodiferro, S. Peripheral Giant Cell Granuloma of the Jaws as First Sign of Primary Hyperparathyroidism: A Case Series. J. Clin. Med. 2020, 9, 4042. [Google Scholar] [CrossRef]
- Manjunatha, B.S.; Purohit, S.; Harsh, A.; Vangala, N. A complex case of brown tumors as initial manifestation of primary hyperparathyroidism in a young female. J. Oral Maxillofac. Pathol. 2019, 23, 477. [Google Scholar] [CrossRef]
- Thakker, R.V. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol. Cell Endocrinol. 2014, 386, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Alotaiby, F.M.; Fitzpatrick, S.; Upadhyaya, J.; Islam, M.N.; Cohen, D.; Bhattacharyya, I. Demographic, Clinical and Histopathological Features of Oral Neural Neoplasms: A Retrospective Study. Head Neck Pathol. 2019, 13, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Tomasi, A.; Manfredini, M.; Pellacani, G. Oral mucosal stigmata in hereditary-cancer syndromes: From germline mutations to distinctive clinical phenotypes and tailored therapies. Gene 2016, 582, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.L. Inherited syndromes involving pancreatic neuroendocrine tumors. J. Gastrointest. Oncol. 2020, 11, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Anik, A.; Abaci, A. Endocrine cancer syndromes: An update. Minerva Pediatr. 2014, 66, 533–547. [Google Scholar]
- Ben Hammouda, S.; Njima, M.; Ben Abdeljelil, N.; Bellalah, A.; Njim, L.; Zakhama, A. An unusual presentation revealing Peutz-Jeghers syndrome in adult. Ann. Med. Surg. 2020, 58, 87–90. [Google Scholar] [CrossRef]
- Nevozinskaya, Z.; Korsunskaya, I.; Sakaniya, L.; Perlamutrov, Y.; Sobolev, V. Peutz-Jeghers syndrome in dermatology. Acta Dermatovenerol. Alp. Pannonica Adriat. 2019, 28, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.O.; Meliţ, L.E.; Patraulea, F.; Iunius, S.; Mărginean, M.O. Early onset Peutz-Jeghers syndrome, the importance of appropriate diagnosis and follow-up: A case report. Medicine 2019, 98, e16381. [Google Scholar] [CrossRef]
- Onodera, S.; Nakamura, Y.; Azuma, T. Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research. Int. J. Mol. Sci. 2020, 21, 7559. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, N.; Yang, C.; Yang, K.; Bian, Z. Novel PTCH1 mutation in Gorlin-Goltz syndrome potentially altered interactions with lipid bilayer. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Thavaraj, S.; Diaz-Cano, S. An Overview of Autosomal Dominant Tumour Syndromes with Prominent Features in the Oral and Maxillofacial Region. Head Neck Pathol. 2017, 11, 364–376. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Rubin, A.I.; Smith, A.; Castelo-Soccio, L. Retrospective analysis of the histopathologic features of basal cell carcinomas in pediatric patients with basal cell nevus syndrome. J. Cutan. Pathol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mello, R.N.; Khan, Z.; Choudry, U. A multidisciplinary approach to the successful management of Gorlin syndrome. J. Surg. Case Rep. 2017, 2017, rjw224. [Google Scholar] [CrossRef][Green Version]
- Akbari, M.; Chen, H.; Guo, G.; Legan, Z.; Ghali, G. Basal cell nevus syndrome (Gorlin syndrome): Genetic insights, diagnostic challenges, and unmet milestones. Pathophysiology 2018, 25, 77–82. [Google Scholar] [CrossRef]
- Yu, F.; Cai, W.; Jiang, B.; Xu, L.; Liu, S.; Zhao, S. A novel mutation of adenomatous polyposis coli (APC) gene results in the formation of supernumerary teeth. J. Cell Mol. Med. 2018, 22, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, A.B.; Erdem, M.A.; Isler, S.C.; Cifter, M.; Olgac, V.; Kasapoglu, C.; Oral, C.K. Oral and maxillofacial considerations in Gardner’s Syndrome. Int. J. Med. Sci. 2012, 9, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Capodiferro, S.; Lacaita, M.G.; Scivetti, M.; Manco, R.; Testa, N.F. Multiple and giant mandibular osteomas in a Gardner’s syndrome. Case Rep. Minerva Stomatol. 2005, 54, 165–169. [Google Scholar]
- Pereira, D.L.; Carvalho, P.A.; Achatz, M.I.; Rocha, A.; TardinTorrezan, G.; Alves, F.A. Oral and maxillofacial considerations in Gardner’s syndrome: A report of two cases. E Cancer Med. Sci. 2016, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Adisen, M.Z.; Okkesim, A.; Misirlioglu, M. The importance of early diagnosis of gardner’s syndrome in dental examination. Niger. J. Clin. Pract. 2018, 21, 114–116. [Google Scholar] [CrossRef]
- Hirshberg, A.; Berger, R.; Allon, I.; Kaplan, I. Metastatic tumors to the jaws and mouth. Head Neck Pathol. 2014, 8, 463–474. [Google Scholar] [CrossRef]
- McClure, S.A.; Movahed, R.; Salama, A.; Ord, R.A. Maxillofacial metastases: A retrospective review of one institution’s 15-year experience. J. Oral Maxillofac. Surg. 2013, 71, 178–188. [Google Scholar] [CrossRef]
- Shen, M.L.; Kang, J.; Wen, Y.L.; Ying, W.M.; Yi, J.; Hua, C.G.; Tang, X.F.; Wen, Y.M. Metastatic tumors of the oral and maxillofacial region: A retrospective study of 19 cases in Western China and review of Chinese and English literature. J. Oral Maxillofac. Surg. 2009, 67, 718–737. [Google Scholar] [CrossRef]
- Capodiferro, S.; Limongelli, L.; Mastropasqua, M.G.; Favia, G.; Lajolo, C.; Colella, G.; Tempesta, A.; Maiorano, E. Metastatic Tumors of the Oro-Facial Tissues: Clear Cell Renal Cell Carcinoma. A Clinico-Pathological and Immunohistochemical Study of Seven Cases. J. Clin. Med. 2020, 9, 1151. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Piattelli, A.; Maiorano, E. Metastatic Breast Cancer in Medication-Related Osteonecrosis Around Mandibular Implants. Am. J. Case Rep. 2015, 16, 621–626. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastaic tumors t the Jawbones: Analysis of 390 cases. J. Oral Pathol. Med. 1994, 23, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, A.; Leibovich, P.; Horowitz, I.; Buchner, A. Metastatic tumors to postextraction sites. J. Oral Maxillofac. Surg. 1993, 51, 1334–1337. [Google Scholar] [CrossRef]
- Hirshberg, A.; Leibovich, P.; Buchner, A. Metastasis to the oral mucosa: Analysis of 157 cases. J. Oral Pathol. Med. 1993, 22, 358–390. [Google Scholar] [CrossRef] [PubMed]
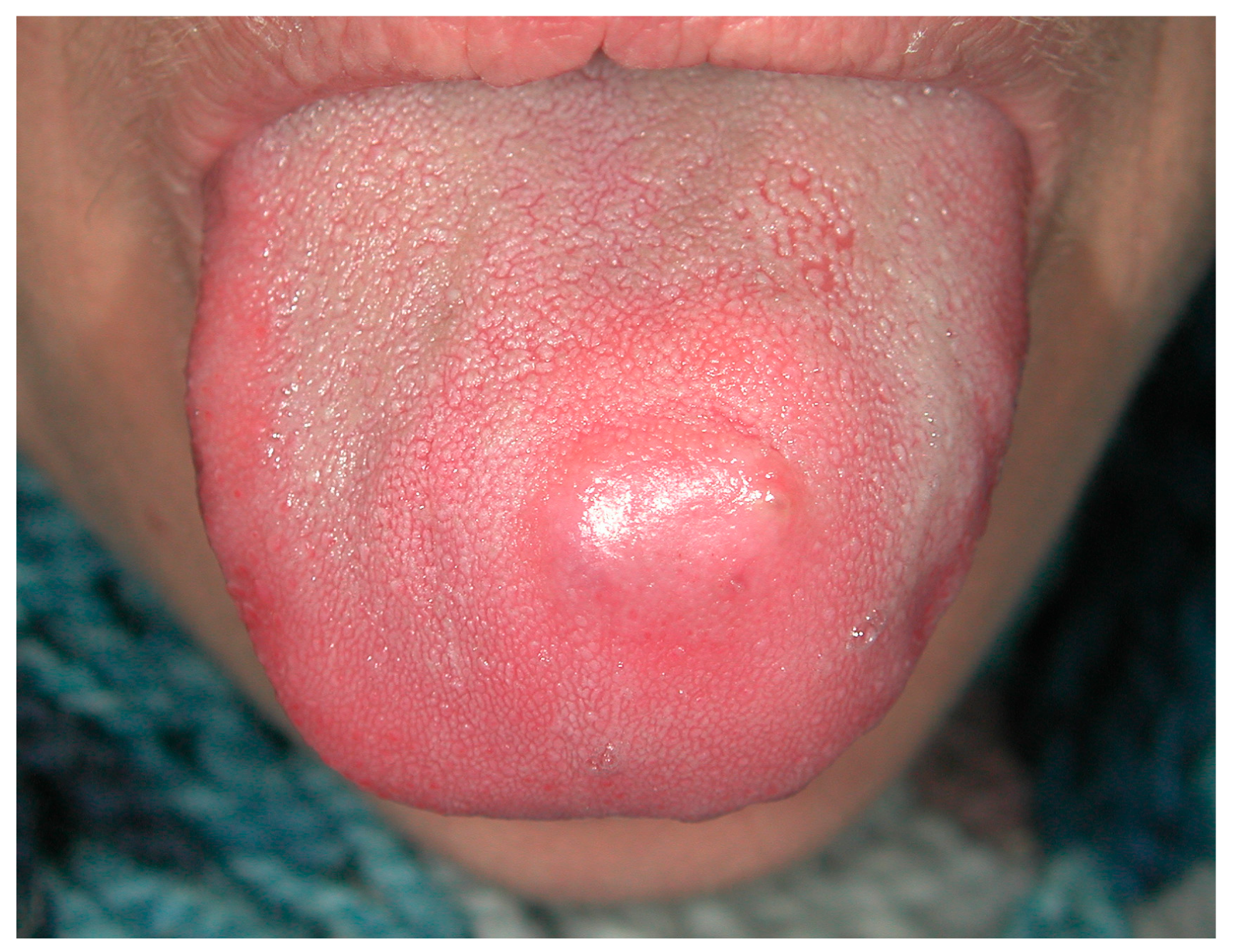
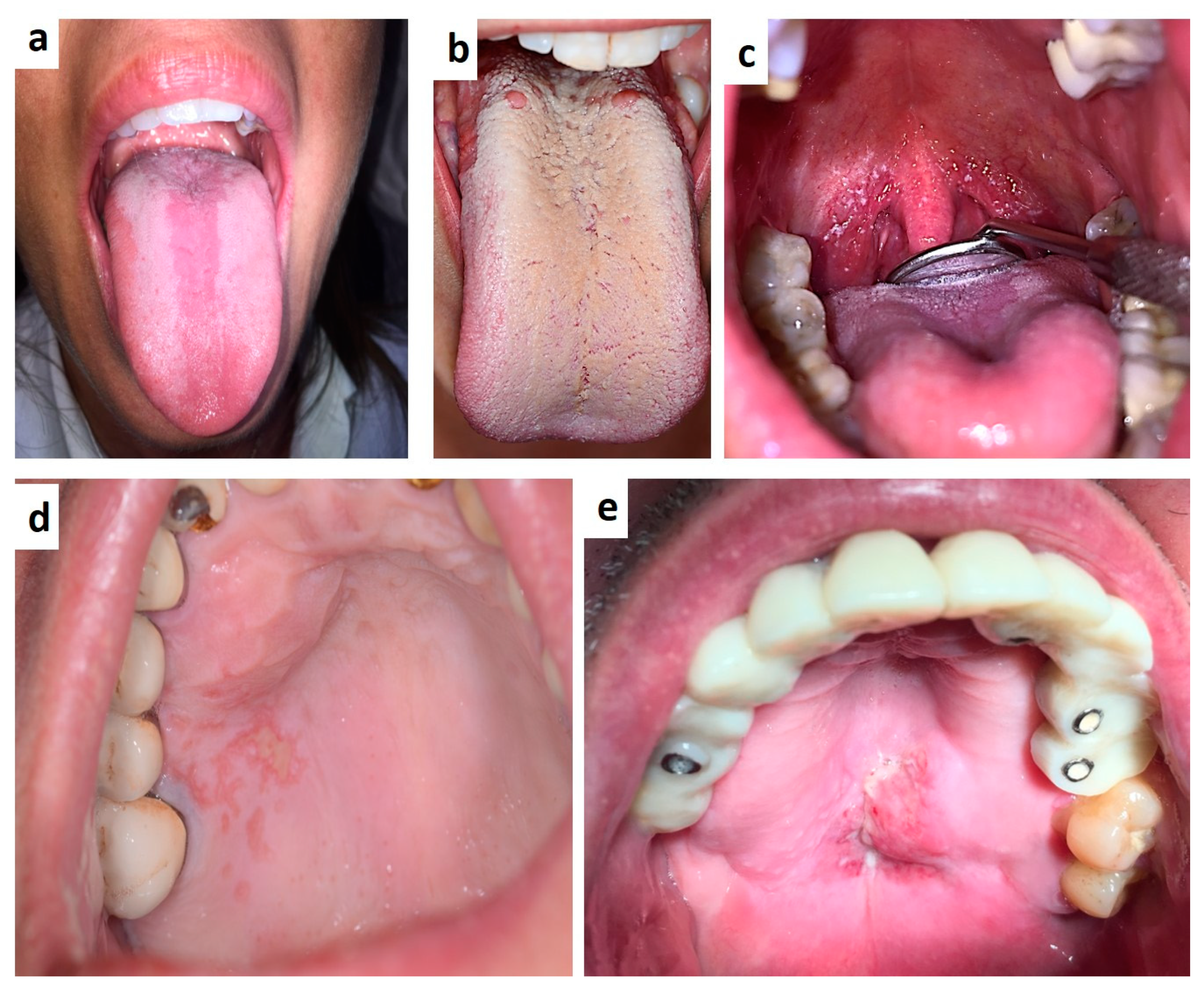
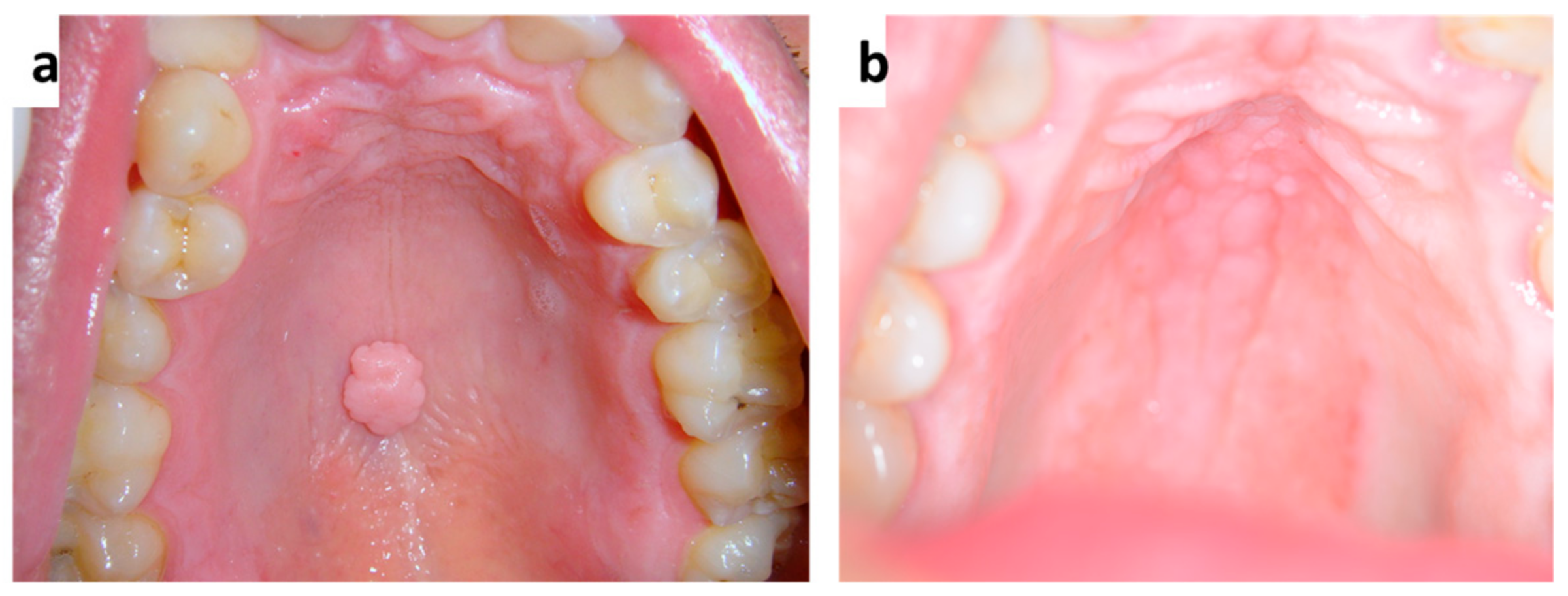
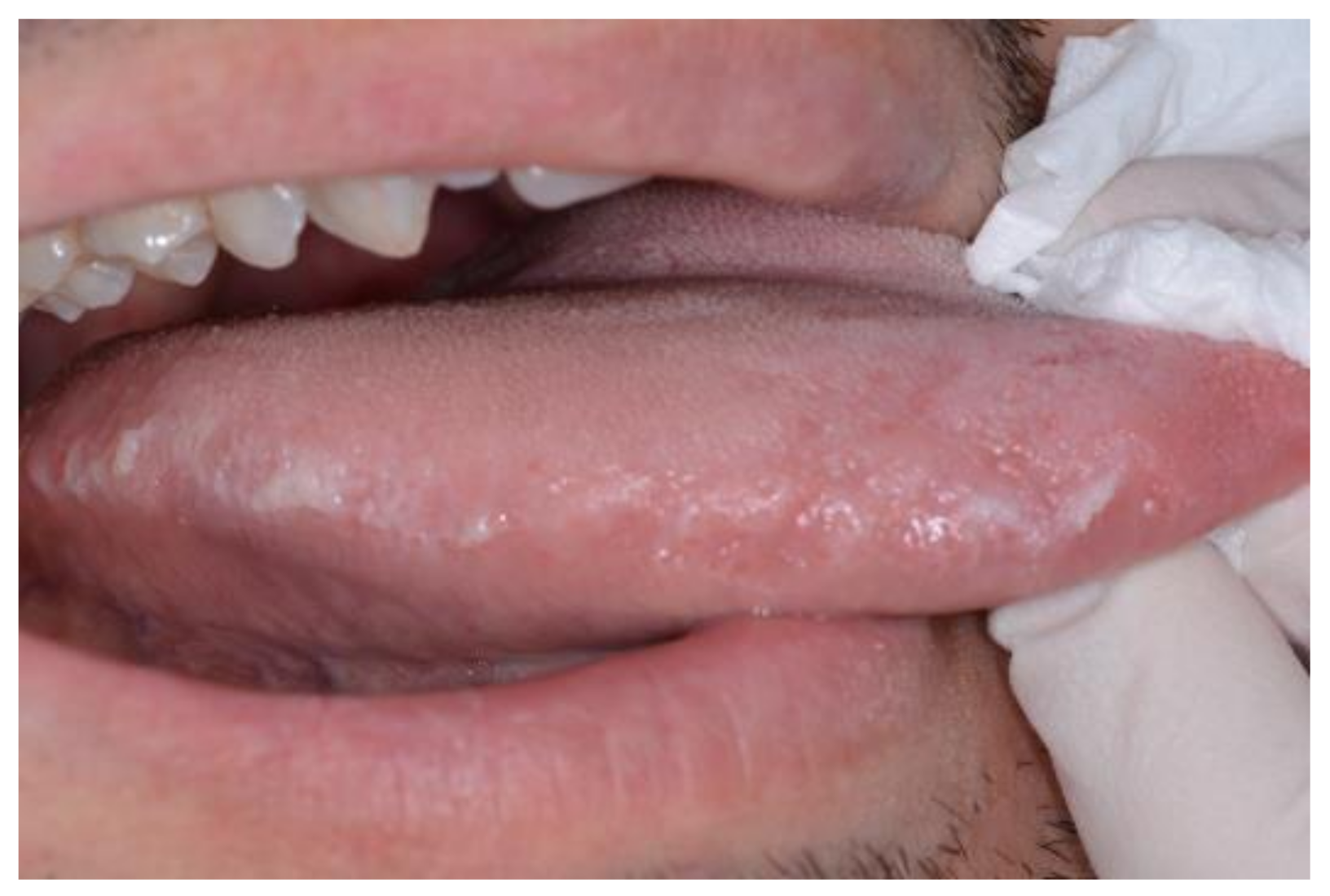
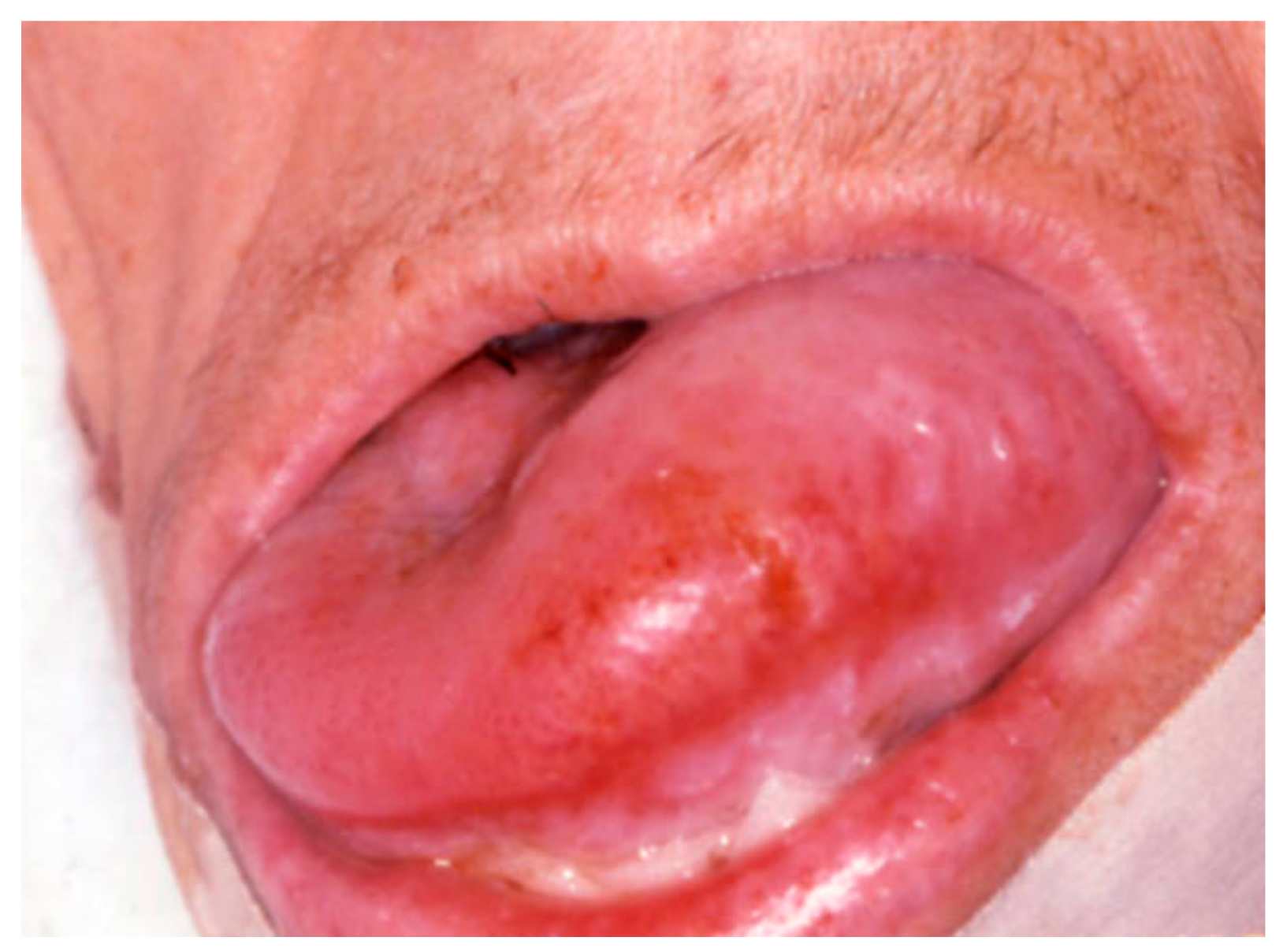
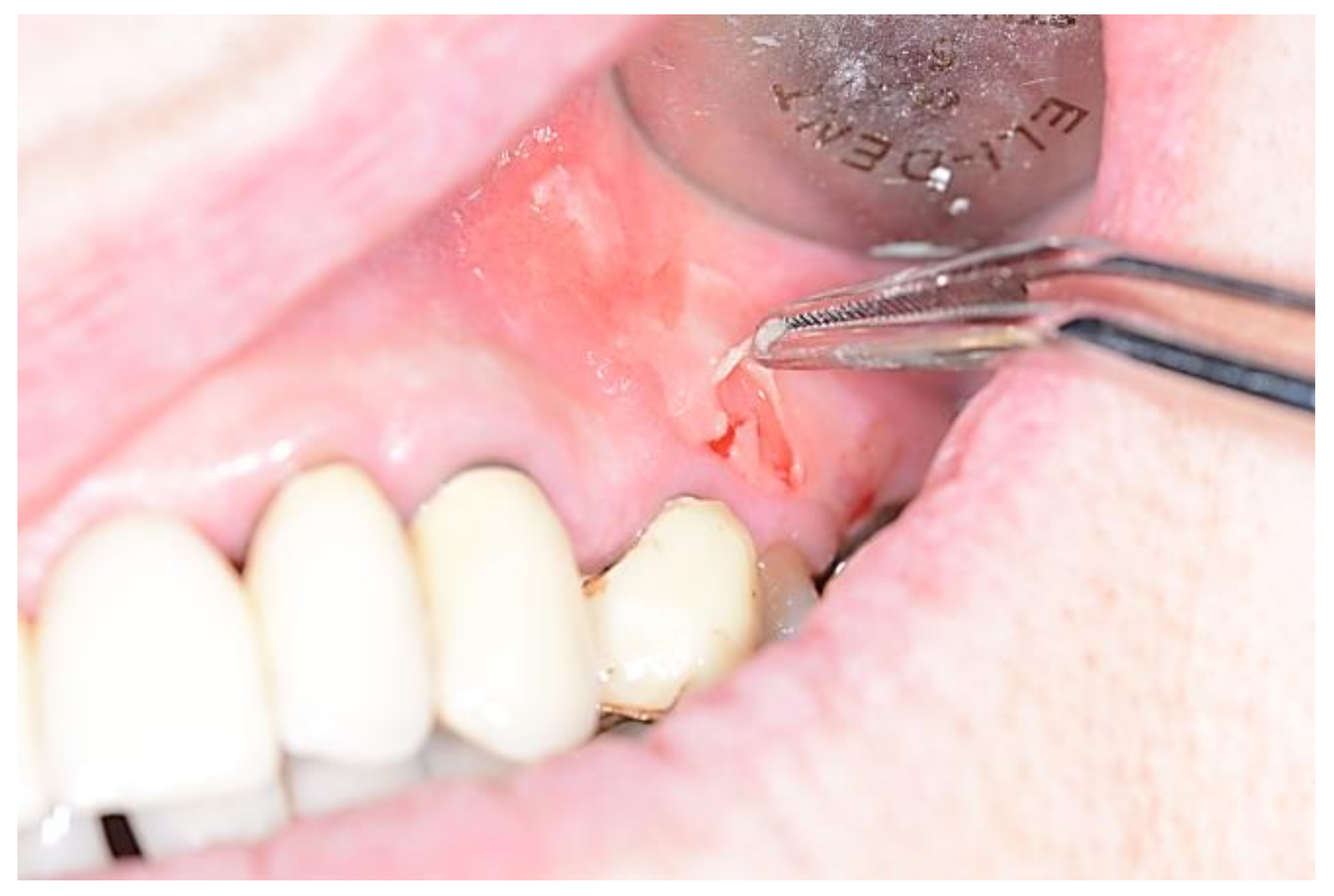
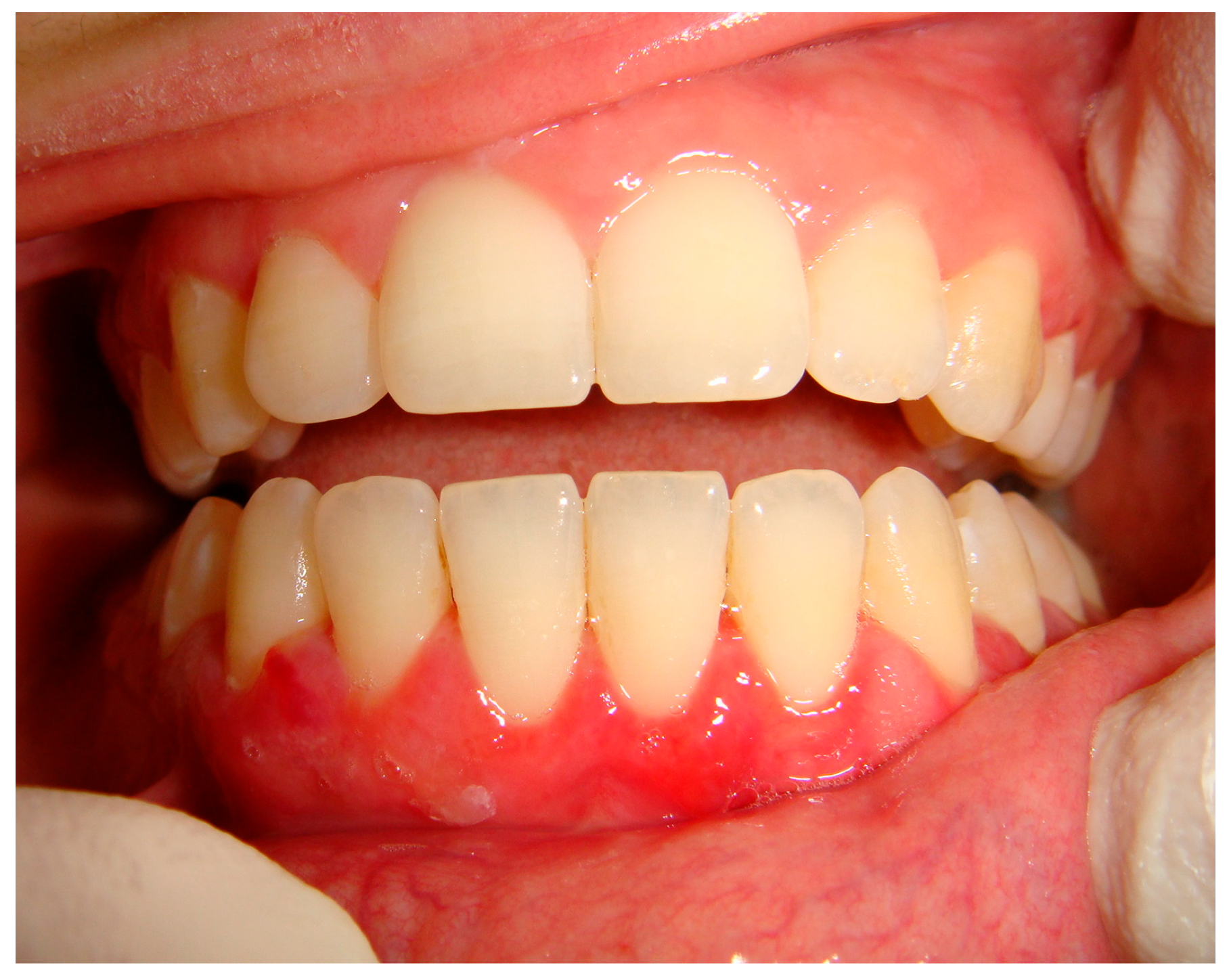
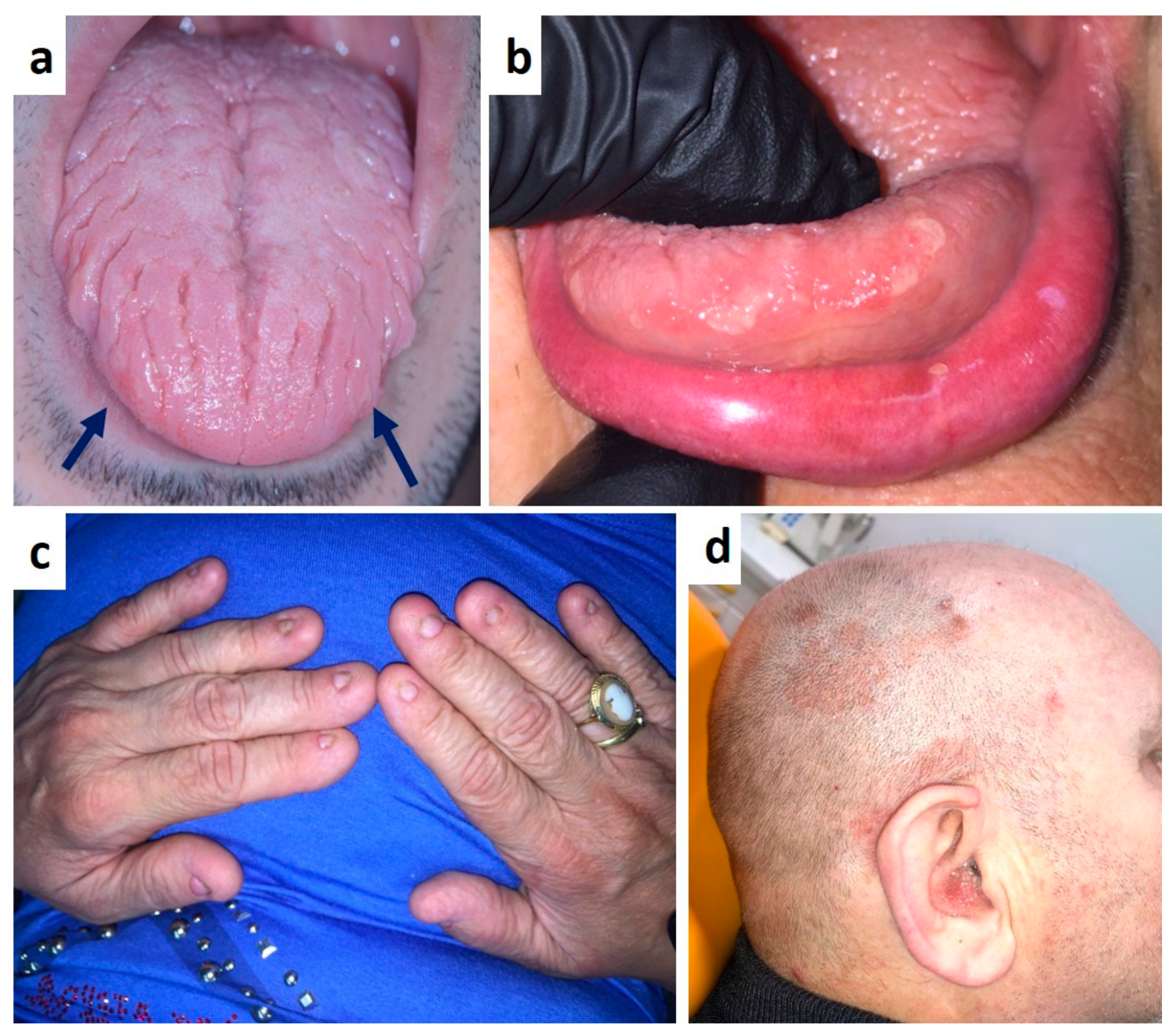
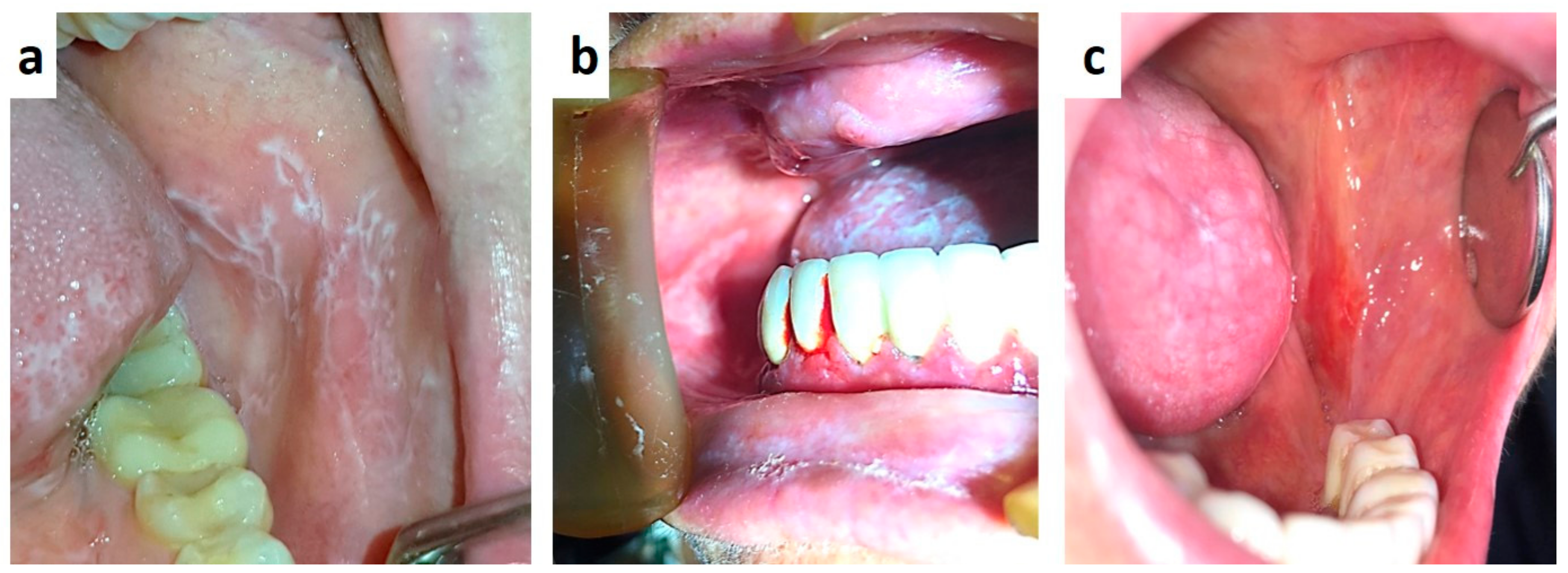
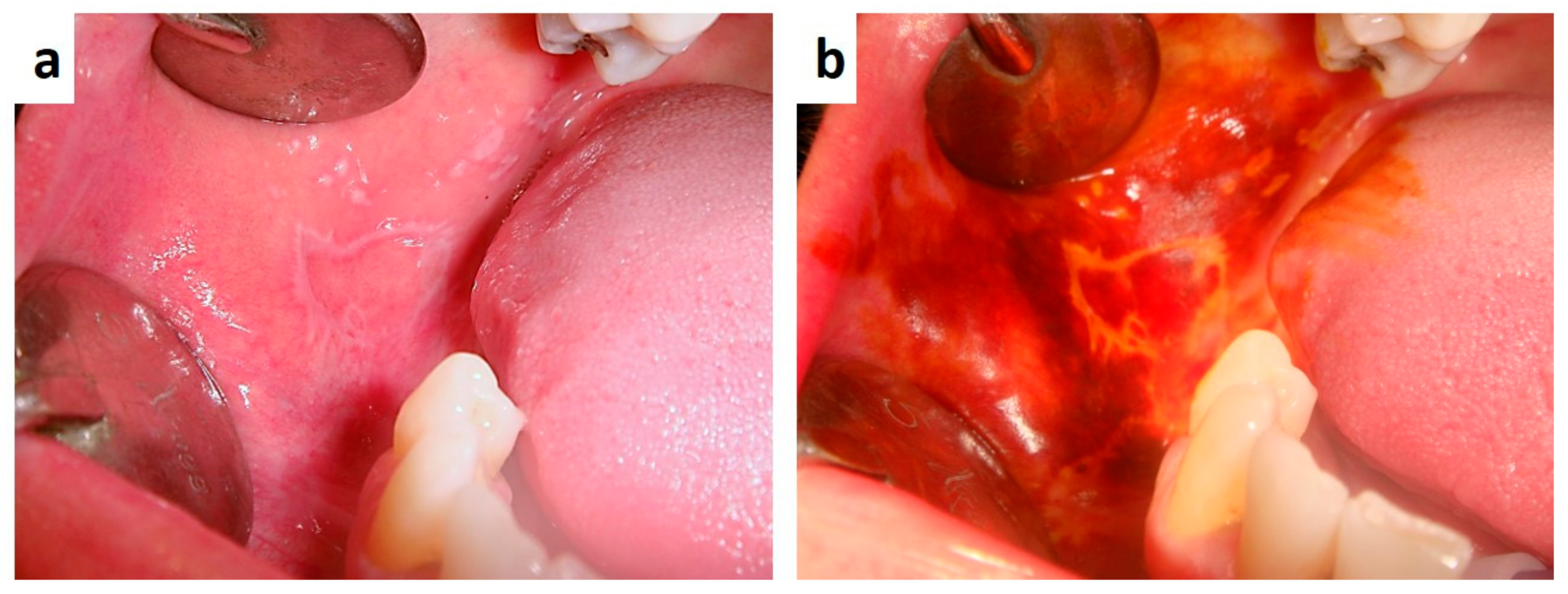
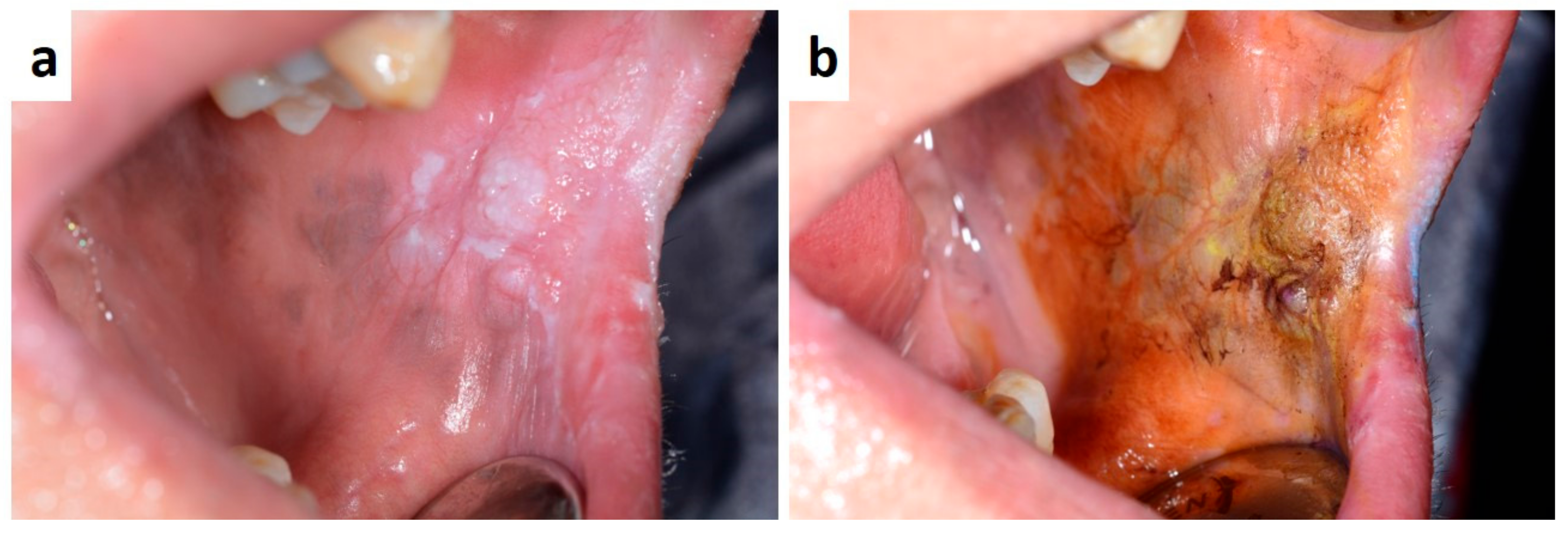
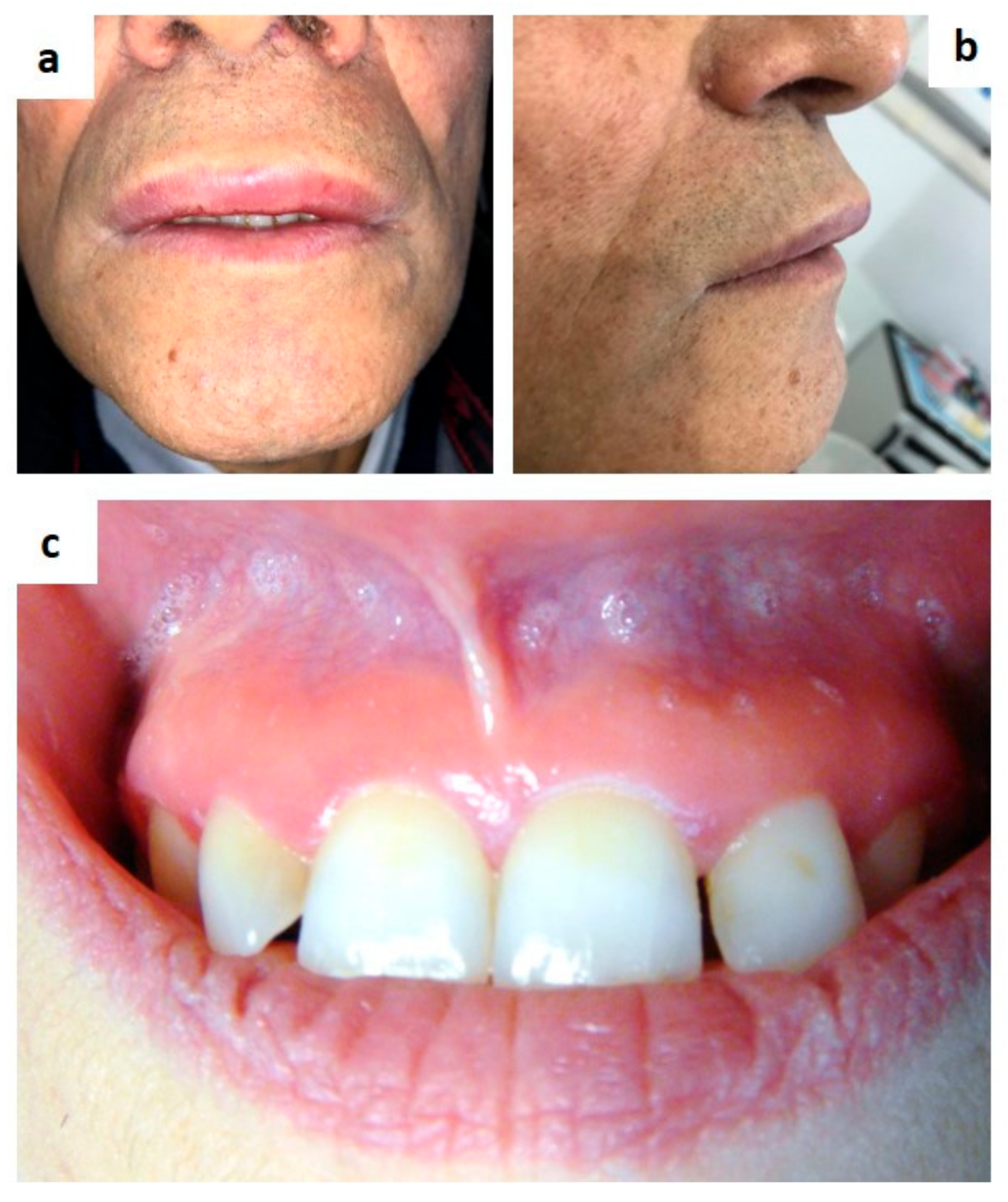
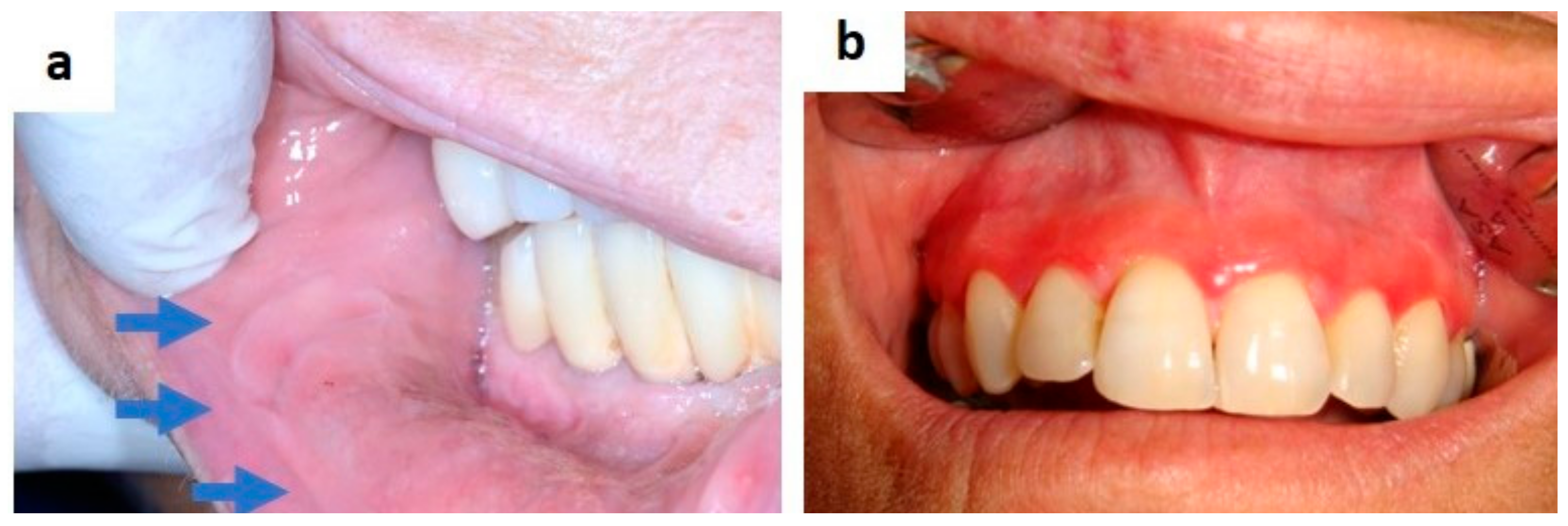
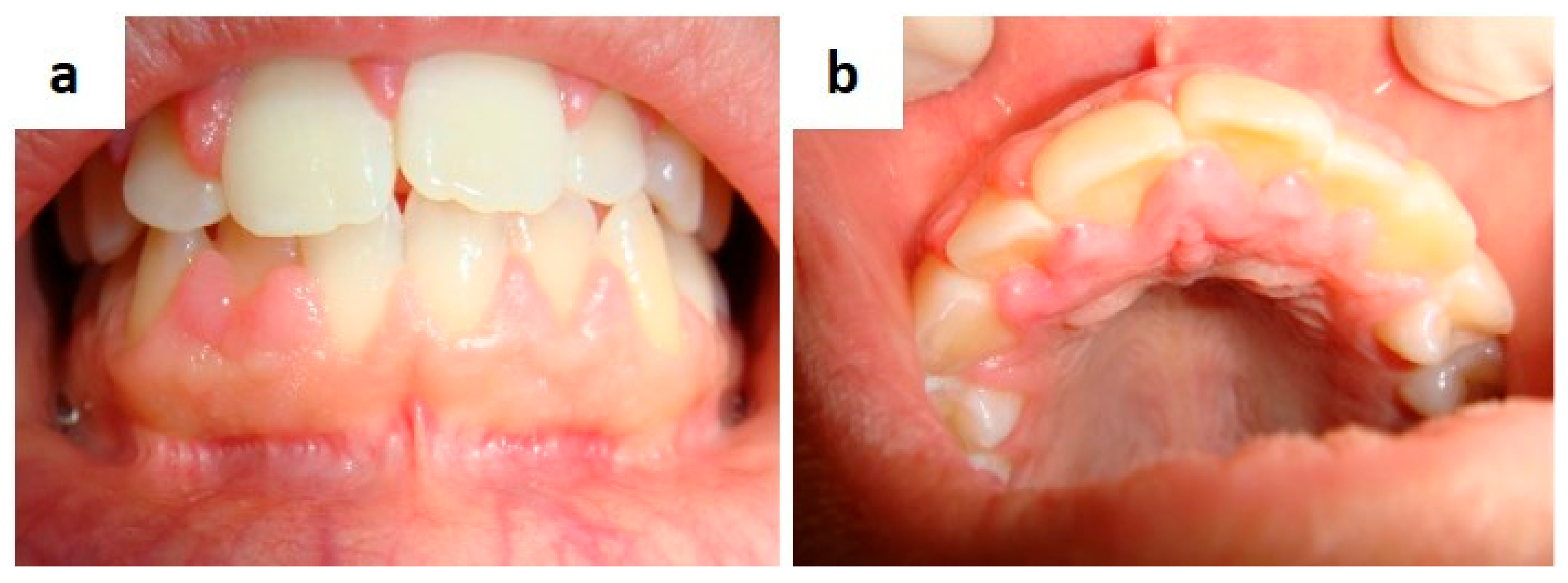
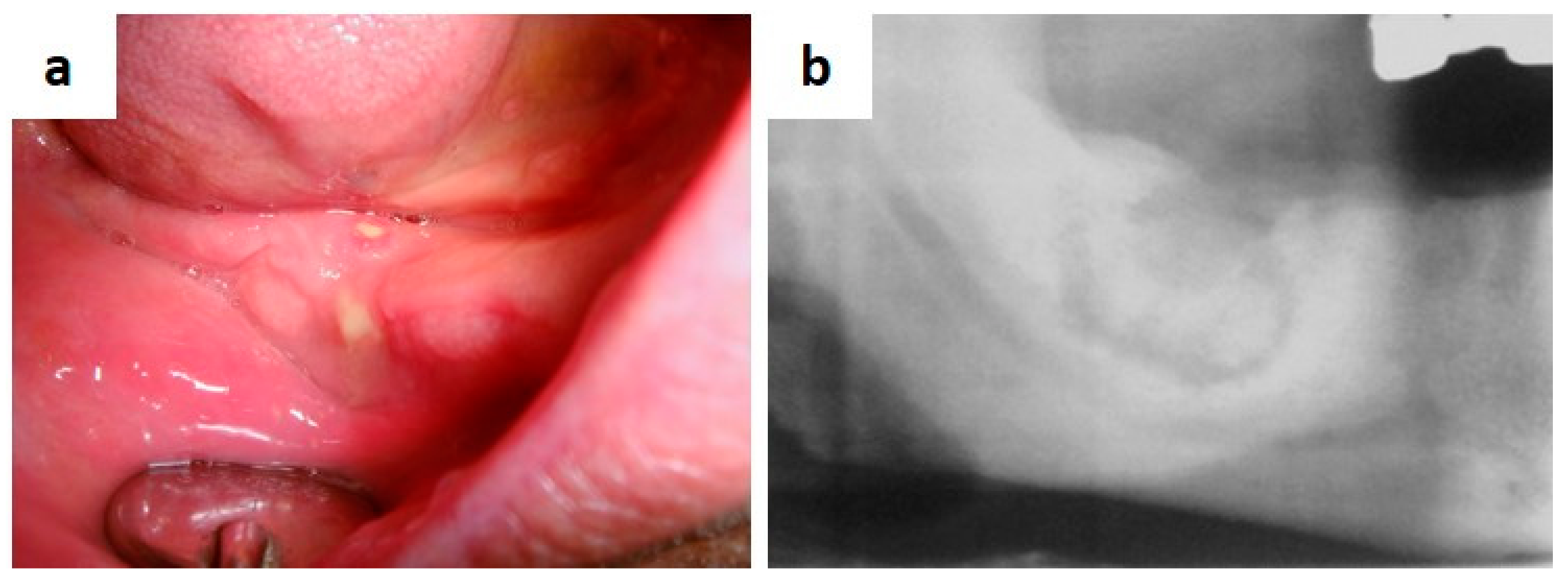

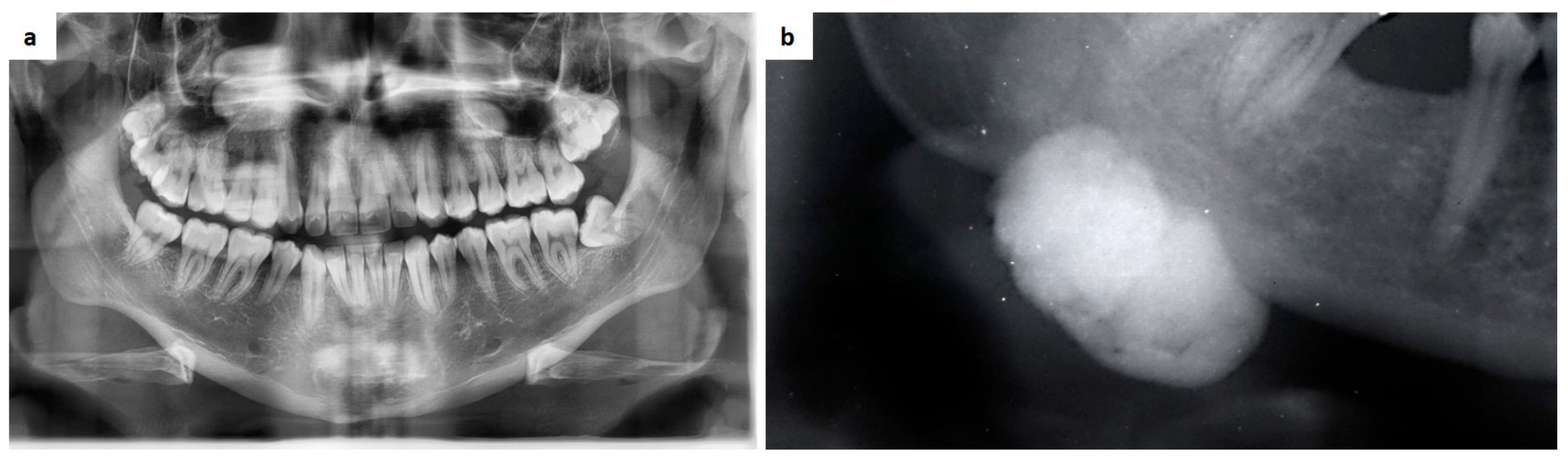
| Pemphigus | Target Antigens * | Ig ** |
| Vulgaris | Dsg 3 in mucosal PV, Dsg 1 and 3 in muco-cutaneous PV | IgG |
| Foliaceus | Dsg 1 | IgG |
| Paraneoplastic | Envoplakin, periplacin, Dsg 1/3, BP180, others | IgG |
| IgA | Dsg 1/3, Dsc 1–3 | IgA |
| Herpetiform | Dsg 1 | IgG |
| Pemphigoid | ||
| Bullous | BP180-NC16A, BP230 | IgG/IgE |
| Mucous membrane | BP180, BP230, laminin-332, α4β6 integrin, laminin-331, COL7 | IgG |
| Gestationis | BP180-NC16A | IgG |
| Linear IgA disease | LAD-1 | IgA |
| Epidermolysis bullosa acquisita | COL7 | IgG/IgA |
| Anti-p200 | Laminin γ1 | IgG |
| Lichen planus pemphigoides | BP180-NC16A, BP230 | IgG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capodiferro, S.; Limongelli, L.; Favia, G. Oral and Maxillo-Facial Manifestations of Systemic Diseases: An Overview. Medicina 2021, 57, 271. https://doi.org/10.3390/medicina57030271
Capodiferro S, Limongelli L, Favia G. Oral and Maxillo-Facial Manifestations of Systemic Diseases: An Overview. Medicina. 2021; 57(3):271. https://doi.org/10.3390/medicina57030271
Chicago/Turabian StyleCapodiferro, Saverio, Luisa Limongelli, and Gianfranco Favia. 2021. "Oral and Maxillo-Facial Manifestations of Systemic Diseases: An Overview" Medicina 57, no. 3: 271. https://doi.org/10.3390/medicina57030271
APA StyleCapodiferro, S., Limongelli, L., & Favia, G. (2021). Oral and Maxillo-Facial Manifestations of Systemic Diseases: An Overview. Medicina, 57(3), 271. https://doi.org/10.3390/medicina57030271








