Nocturnal Hypoglycaemia in Patients with Diabetes Mellitus: Database Analysis of a Cohort Using Telemedicine Support for Self-Monitoring of Blood Glucose over a 10-Year-Long Period
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Basic Characteristics of the Cohort and Frequency of Nocturnal Hypoglycaemia
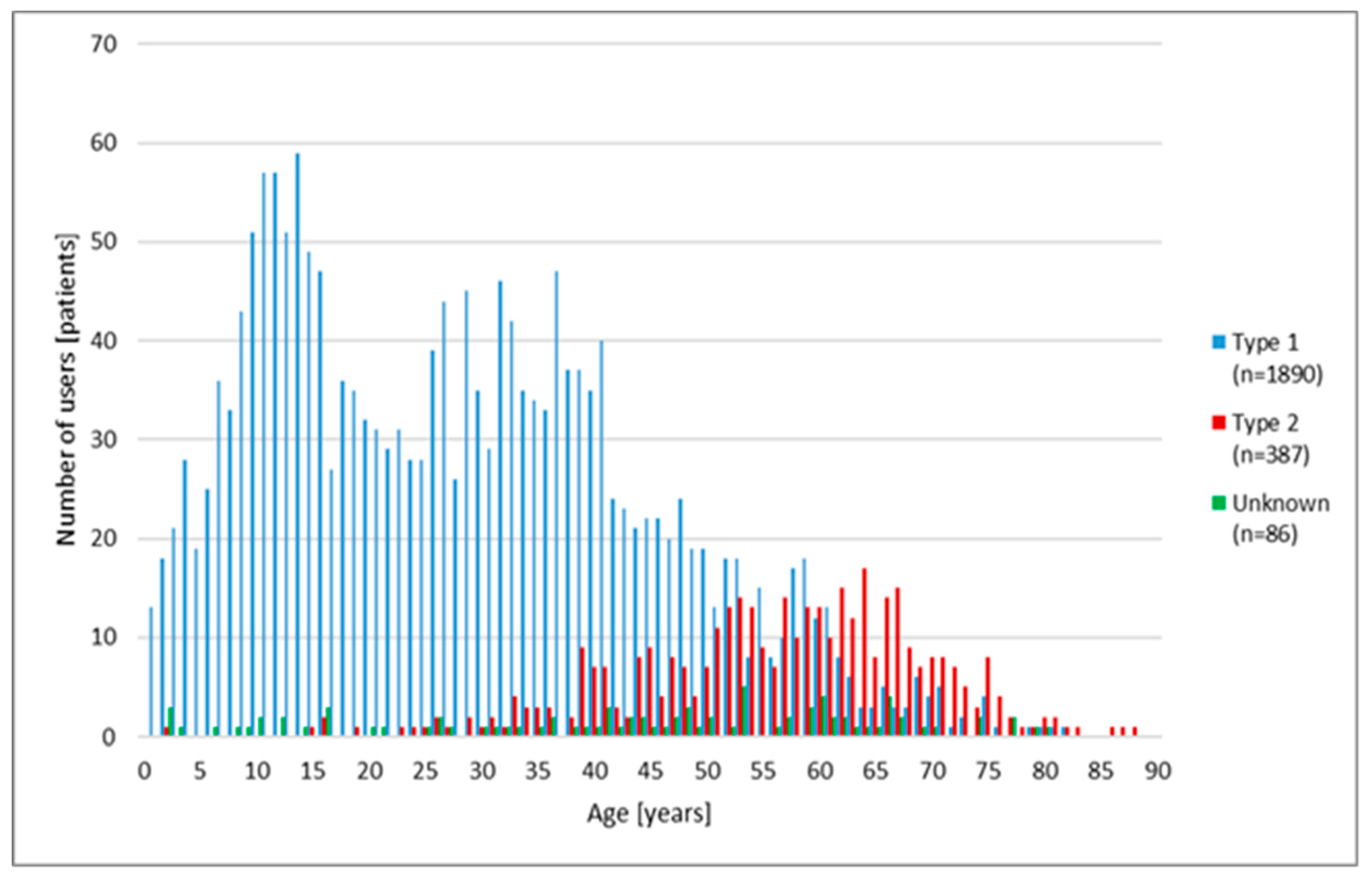
3.2. Nocturnal Hypoglycaemia in Different Age Groups
3.3. Nocturnal Hypoglycaemia according to the Type of Diabetes
3.4. Nocturnal Hypoglycaemia in Patients with Different Treatment Strategies
3.5. Retest (Control Measurement) after the First Nocturnal Hypoglycaemia
4. Discussion
4.1. Frequency of Nocturnal Hypoglycaemia
4.2. Age Groups
4.3. Type of Diabetes
4.4. Antihyperglycaemic Treatment
4.5. Retest after Nocturnal Hypoglycaemia
4.6. Limitations of the Study
4.7. Prevention of Nocturnal Hypoglycaemia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cryer, P.E. The barrier of hypoglycaemia in diabetes. Diabetes 2008, 57, 3169–3176. [Google Scholar] [CrossRef]
- Frier, B.M. Hypoglycaemia and diabetes—The lifestyle perspective. Diabetol. Hung. 2004, 12, 87–92. [Google Scholar]
- Heller, S.R. Hypoglycaemia: Its pathophysiology in insulin treated diabetes and hypoglycaemia unawareness. Br. J. Diabetes Vasc. Dis. 2008, 16, 135–141. [Google Scholar] [CrossRef]
- Anderbro, T.; Amsberg, S.; Adamson, U.; Bolinder, J.; Lins, P.-E.; Wredling, R.; Moberg, E.; Lisspers, J.; Johanssonet, U.-B. Fear of hypoglycaemia in adults with Type 1 diabetes. Diabet. Med. 2010, 27, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Schopman, J.E.; Simon, A.C.; Hoefnagel, S.J.; Hoekstra, J.B.L.; Scholten, R.J.P.M.; Holleman, F. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: A systematic review and meta-analysis. Diabetes/Metab. Res. Rev. 2014, 30, 11–22. [Google Scholar] [CrossRef]
- Weitgasser, R.; Lopes, S. Self-reported frequency and impact of hypoglycaemic events in insulin-treated diabetic patients in Austria. Wien. Klin. Wochenschr. 2014, 127, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Edridge, C.L.; Dunkley, A.J.; Bodicoat, D.H.; Rose, T.C.; Gray, L.J.; Davies, M.J.; Khunti, K. Prevalence and incidence of hypoglycaemia in 532,542 people with type 2 diabetes on oral therapies and insulin: A systematic review and meta-analysis of population-based studies. PLoS ONE 2015, 10, e0126427. [Google Scholar] [CrossRef]
- Khunti, K.; Alsifri, S.; Aronson, R.; Cigrovski Berković, M.; Enters-Weijnen, C.; Forsén, T.; Galstyan, G.; Geelhoed-Duijvestijn, P.; Goldfracht, M.; Gydesen, H.; et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: The global HAT study. Diabetes Obes. Metab. 2016, 18, 907–915. [Google Scholar] [CrossRef]
- Kern, W.; Holstein, A.; Moenninghoff, C.; Kienhöfer, J.; Riedl, M.; Kulzer, B. Self-reported hypoglycaemic events in 2430 patients with insulin-treated diabetes in the German sub-population of the HAT study. Exp. Clin. Endocrinol. Diabetes 2017, 125, 592–597. [Google Scholar] [CrossRef]
- Heller, S.R.; Peyrot, M.; Oates, S.K.; Taylor, A.D. Hypoglycemia in patient with type 2 diabetes treated with insulin: It can happen. BMJ Open Diabetes Res. Care 2020, 8, e001194. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Blonde, L.; Cembrowski, G.; Chacra, A.R.; Charpentier, G.; Colagiuri, S.; Dailey, G.; Gabbay, R.A.; Heinemann, L.; Kerr, D.; et al. Coalition for Clinical Research-Self-Monitoring of Blood Glucose Scientific Board: Consensus report: The current role of self-monitoring of blood glucose in non-insulin-treated type 2 diabetes. J. Diabetes Sci. Technol. 2011, 5, 1529–1548. [Google Scholar] [CrossRef] [PubMed]
- Czupryniak, L.; Barkai, L.; Bolgarska, S.; Bronisz, A.; Broz, J.; Cypryk, K.; Honka, M.; Janez, A.; Krnic, M.; Lalic, N.; et al. Self-monitoring of blood glucose in diabetes: From evidence to clinical reality in Central and Eastern Europe–Recommendations from the International Central-Eastern European Expert Group. Diabetes Technol. Ther. 2014, 16, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.M.; O’Gorman, C.S.; Palmert, M.R. The impact of telemedicine interventions involving routine transmission of blood glucose data with clinician feedback on metabolic control in youth with type 1 diabetes: A systematic review and meta-analysis. Int. J. Pediatr. Endocrinol. 2010, 2010, 536957. [Google Scholar] [CrossRef]
- Tildesley, H.D.; Po, M.D.; Ross, S.A. Internet blood glucose monitoring systems provide lasting glycemic benefit in type 1 and 2 diabetes: A systematic review. Med. Clin. N. Am. 2015, 99, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, C.; Yang, S.J.; Sun, L.; Li, F.; Choi, I.Y.; Cho, J.-H.; Wang, G.; Yoon, K.H. Randomized, open-label, parallel group study to evaluate the effect of internet-based glucose management system on subjects with diabetes in China. Telemed. J. e-Health 2016, 22, 666–674. [Google Scholar] [CrossRef]
- Kulzer, B.; Seitz, L.; Kern, W. Real-world patient-reported rates of non-severe hypoglycaemic events in Germany. Exp. Clin. Endocrinol. Diabetes 2014, 122, 167–172. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, U.; Alsifri, S.; Aronson, R.; Berković, M.C.; Galstyan, G.; Gydesen, H.; Lekdorf, J.B.; Ludvik, B.; Moberg, E.; Ramachandran, A.; et al. Comparison of the HAT study, the largest global hypoglycaemia study to date, with similar large real-world studies. Diabetes Obes. Metabol. 2019, 21, 844–853. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. In Vitro Diagnostic Test Systems: Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus; ISO 15197; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Klatman, E.L.; Jenkins, A.J.; Ahmedani, M.Y.; Ogle, G.D. Blood glucose meters and test strips: Global market and challenges to access in low-resource settings. Lancet Diabetes Endocrinol. 2018, 7, 150–160, Erratum in 2019, 7, 150–160. [Google Scholar] [CrossRef]
- Budnitz, D.S.; Lovegrove, M.C.; Shehab, N.; Richards, C.L. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011, 365, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.I.; Shehab, N.; Lovegrove, M.C.; Kegler, S.R.; Weidenbach, K.N.; Ryan, G.J.; Budnitz, D.S. National estimates of insulin-related hypoglycaemia and errors leading to emergency department visits and hospitalizations. JAMA Intern. Med. 2014, 174, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Benoit, S.R.; Kahn, H.S.; Geller, A.I.; Budnitz, D.S.; Mann, C.; Dai, M.; Gregg, E.W.; Geiss, L.S. Diabetes-related emergency medical service activations in 23 states, United States 2015. Prehospital Emerg. Care 2018, 22, 705–712. [Google Scholar] [CrossRef]
- Goto, A.; Arah, O.A.; Goto, M.; Terauchi, Y.; Noda, M. Severe hypoglycaemia and cardiovascular disease: Systematic review and meta-analysis with bias analysis. BMJ 2013, 347, f4533. [Google Scholar] [CrossRef]
- Fitzpatrick, C.; Chatterjee, S.; Seidu, S.; Bodicoat, D.H.; Ng, G.A.; Davies, M.J.; Khunti, K. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes. Metab. 2018, 20, 2169–2178. [Google Scholar] [CrossRef]
- Briscoe, V.J.; Davis, S.N. Hypoglycaemia in type 1 and type 2 diabetes: Physiology, pathophysiology, and management. Clin. Diabetes 2006, 24, 115–121. [Google Scholar] [CrossRef]
- Schultes, B. Nocturnal hypoglycaemia—Responses, consequences, and prevention. Diabetol. Hung. 2012, 20, 84–91. [Google Scholar]
- Tanenberg, R.J.; Newton, C.A.; Drake, A.J. Confirmation of hypoglycaemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr. Pract. 2010, 16, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.V.; Woodward, A.; Casson, I.F.; Weston, P.J. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes—The ‘dead in bed’ syndrome revisited. Diabetologia 2009, 52, 42–45. [Google Scholar] [CrossRef]
- Andersen, A.; Jørgensen, P.G.; Knop, F.K.; Vilsbøll, T. Hypoglycaemia and cardiac arrhythmias in diabetes. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820911803. [Google Scholar] [CrossRef] [PubMed]
- Shalimova, A.; Graff, B.; Gąsecki, D.; Wolf, J.; Sabisz, A.; Szurowska, E.; Jodzio, K.; Narkiewicz, K. Cognitive dysfunction in type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.J.; Sheu, W.H. Association between hypoglycaemia and dementia in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 116, 279–287. [Google Scholar] [CrossRef]
- Barendse, S.; Singh, H.; Frier, B.M.; Speight, J. The impact of hypoglycaemia on quality of life and related patient-reported outcomes in Type 2 diabetes: A narrative review. Diabet. Med. 2012, 29, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, M.; Lipka, I.; Pawęska, J.; Niewada, M.; Rdzanek, E.; Zaletel, J.; Ramírez de Arellano, A.; Doležal, T.; Mitreva, B.C.; Nagy, B.; et al. Cost of severe hypoglycaemia in nine European countries. J. Med. Econ. 2016, 19, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef]
- International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: A joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017, 40, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Fontaine, P.; Eschwege, E.; Lièvre, M.; Gouet, D.; Huet, D.; Madani, S.; Lavigne, S.; Charbonnel, B. Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: Results from the DIALOG study. Diabetes Metab. 2015, 41, 116–125. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Gu, L.; Babineaux, S.; Colclough, H.; Curtis, B. Glycemic control in Chinese patients with type 2 diabetes mellitus receiving oral antihyperglycemic medication-only or insulin-only treatment: A cross-sectional survey. Diabetes Ther. 2015, 6, 197–211. [Google Scholar] [CrossRef]
- Chiang, J.L.; Maahs, D.M.; Garvey, K.C.; Hood, K.K.; Laffel, L.M.; Weinzimer, S.A.; Wolfsdorf, J.I.; Schatz, D. Type 1 diabetes in children and adolescents: A position statement by the American Diabetes Association. Diabetes Care 2018, 41, 2026–2044. [Google Scholar] [CrossRef]
- Rosenstock, J.; Dailey, G.; Massi-Benedetti, M.; Fritsche, A.; Lin, Z.; Salzmanet, A. Reduced hypoglycaemia risk with insulin glargine: A meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005, 28, 950–955. [Google Scholar] [CrossRef]
- Bartley, P.C.; Bogoev, M.; Larsen, J.; Philotheou, A. Long-term efficacy and safety of insulin detemir compared to neutral protamine Hagedorn insulin in patients with type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: A 2-year, randomized, controlled trial. Diabet. Med. 2008, 25, 442–449. [Google Scholar] [CrossRef]
- Lane, W.; Bailey, T.S.; Gerety, G.; Gumprecht, J.; Philis-Tsimikas, A.; Hansen, C.T.; Nielsen, T.S.S.; Warren, M. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: The SWITCH 1 randomized clinical trial. JAMA 2017, 318, 33–44. [Google Scholar] [CrossRef]
- Home, P.D.; Bergenstal, R.M.; Bolli, G.B.; Ziemen, M.; Rojeski, M.; Espinasse, M.; Riddle, M.C. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: A randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 2015, 38, 2217–2225. [Google Scholar] [CrossRef]
- Farngren, J.; Ahrén, B. Incretin-based medications (GLP-1 receptor agonists, DPP-4 inhibitors) as a means to avoid hypoglycaemic episodes. Metabolism 2019, 99, 25–31. [Google Scholar] [CrossRef]
- Nyström, T.; Bodegard, J.; Nathanson, D.; Thuresson, M.; Norhammar, A.; Eriksson, J.W. Novel oral glucose-lowering drugs are associated with lower risk of all-cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Baker, J.; Cahn, A.; Eckel, R.H.; El Sayed, N.A.; Fischl, A.H.; Gaede, P.; Leslie, D.; Pieralice, S.; Tuccinardi, D.; et al. Hypoglycaemia and its management in primary care setting. Diabetes/Metab. Res. Rev. 2020, 36, e3332. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Bianchi, C.; Del Prato, S. Use of incretin-based medications: What do current international recommendations suggest with respect to GLP-1 receptor agonists and DPP-4 inhibitors? Metabolism 2020, 107, 154242. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Johnson-Rabbett, B.; Seaquist, E.R. Hypoglycemia in diabetes: The dark side of diabetes treatment. A patient-centered review. J. Diabetes 2019, 11, 711–718. [Google Scholar] [CrossRef]
- Adolfsson, P.; Rentoul, D.; Klinkenbijl, B.; Parkin, C.G. Hypoglycaemia remains the key obstacle to optimal glycaemic control–continuous glucose monitoring is the solution. Eur. Endocrinol. 2018, 14, 50–56. [Google Scholar] [CrossRef]
| Number of Nocturnal Hypoglycaemic Values (n) | Females n (%) | Males n (%) | Total n (%) |
|---|---|---|---|
| 1 | 228 (26.1) | 402 (27.0) | 630 (26.7) |
| 2–9 | 418 (47.9) | 688 (46.2) | 1106 (46.8) |
| 10–49 | 193 (22.1) | 340 (22.8) | 533 (22.6) |
| 50–99 | 24 (2.7) | 45 (3.0) | 69 (2.9) |
| 100–199 | 9 (1.0) | 14 (0.9) | 23 (1.0) |
| ≥200 | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Total | 873 (100.0) | 1490 (100.0) | 2363 (100.0) |
| Age-Groups (Years) | Females n (%) | Males n (%) | Total n (%) |
|---|---|---|---|
| <10.0 | 115 (13.2) | 129 (8.7) | 244 (10.3) |
| 10.0–19.9 | 196 (22.5) | 285 (19.1) | 481 (20.4) |
| 20.0–29.9 | 153 (17.5) | 194 (13.0) | 347 (14.7) |
| 30.0–39.9 | 151 (17.3) | 261 (17.5) | 412 (17.4) |
| 40.0–49.9 | 92 (10.5) | 234 (15.7) | 326 (13.8) |
| 50.0–59.9 | 78 (8.9) | 191 (12.8) | 269 (11.4) |
| 60.0–69.9 | 53 (6.1) | 147 (9.9) | 200 (8.5) |
| 70.0–79.9 | 30 (3.4) | 41 (2.8) | 71 (3.0) |
| ≥80.0 | 5 (0.6) | 8 (0.5) | 13 (0.6) |
| Total | 873 (100.0) | 1490 (100.0) | 2363 (100.0) |
| Age at Diabetes Manifestation (Years) | Females n (%) | Males n (%) | Total n (%) |
|---|---|---|---|
| <5 | 94 (11.7) | 117 (9.0) | 211 (10.0) |
| 5–9 | 173 (21.6) | 182 (14.0) | 355 (16.9) |
| 10–19 | 213 (26.6) | 349 (26.8) | 562 (26.7) |
| 20–29 | 126 (15.7) | 221 (16.9) | 347 (16.5) |
| 30–39 | 100 (12.5) | 186 (14.3) | 286 (13.6) |
| 40–49 | 52 (6.5) | 153 (11.7) | 205 (9.7) |
| 50–59 | 34 (4.2) | 62 (14.0) | 96 (4.6) |
| ≥60 | 10 (1.2) | 34 (2.6) | 44 (2.1) |
| Total * | 802 (100.0) | 1304 (100.0) | 2106 (100.0) |
| Antidiabetic Treatment | Patients n (%) | Blood Glucose Values, n (%) | Blood Glucose, Interquartile Ranges (mmol/L) | ||||
|---|---|---|---|---|---|---|---|
| Min | 25% | 50% | 75% | Max | |||
| Diet only | 36 (1.5) | 641 (2.6) | 0.6 | 2.3 | 2.6 | 2.8 | 2.9 |
| Oral drugs | 37 (1.6) | 60 (0.3) | 0.6 | 1.1 | 1.6 | 2.6 | 2.9 |
| Oral drugs + insulin | 209 (8.8) | 1405 (5.7) | 0.6 | 2.3 | 2.6 | 2.8 | 2.9 |
| Insulin | 1854 (78.5) | 20,727 (84.1) | 0.6 | 2.4 | 2.7 | 2.8 | 2.9 |
| Injectables, non-insulin | 6 (0.3) | 12 (0.1) | 0.6 | 2.5 | 2.7 | 2.9 | 2.9 |
| Not known | 221 (9.3) | 1778 (7.2) | 0.6 | 2.3 | 2.6 | 2.8 | 2.9 |
| Total | 2363 (100.0) | 24,623 (100.0) | 0.6 | 2.4 | 2.7 | 2.8 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermendy, G.; Kecskes, A.; Nagy, A. Nocturnal Hypoglycaemia in Patients with Diabetes Mellitus: Database Analysis of a Cohort Using Telemedicine Support for Self-Monitoring of Blood Glucose over a 10-Year-Long Period. Medicina 2021, 57, 167. https://doi.org/10.3390/medicina57020167
Jermendy G, Kecskes A, Nagy A. Nocturnal Hypoglycaemia in Patients with Diabetes Mellitus: Database Analysis of a Cohort Using Telemedicine Support for Self-Monitoring of Blood Glucose over a 10-Year-Long Period. Medicina. 2021; 57(2):167. https://doi.org/10.3390/medicina57020167
Chicago/Turabian StyleJermendy, Gyorgy, Agnes Kecskes, and Attila Nagy. 2021. "Nocturnal Hypoglycaemia in Patients with Diabetes Mellitus: Database Analysis of a Cohort Using Telemedicine Support for Self-Monitoring of Blood Glucose over a 10-Year-Long Period" Medicina 57, no. 2: 167. https://doi.org/10.3390/medicina57020167
APA StyleJermendy, G., Kecskes, A., & Nagy, A. (2021). Nocturnal Hypoglycaemia in Patients with Diabetes Mellitus: Database Analysis of a Cohort Using Telemedicine Support for Self-Monitoring of Blood Glucose over a 10-Year-Long Period. Medicina, 57(2), 167. https://doi.org/10.3390/medicina57020167






