Prognostic Significance of Cytoplasmic SPNS2 Expression in Patients with Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. OSCC Patients and Ethics Statement
2.2. Preparation and Evaluation of Tissue Microarrays
2.3. Immunochemical Staining
2.4. Immunochemistry Scoring
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of OSCC Patients
3.2. Correlations between SPNS2 Expression and Clinicopathologic Variables
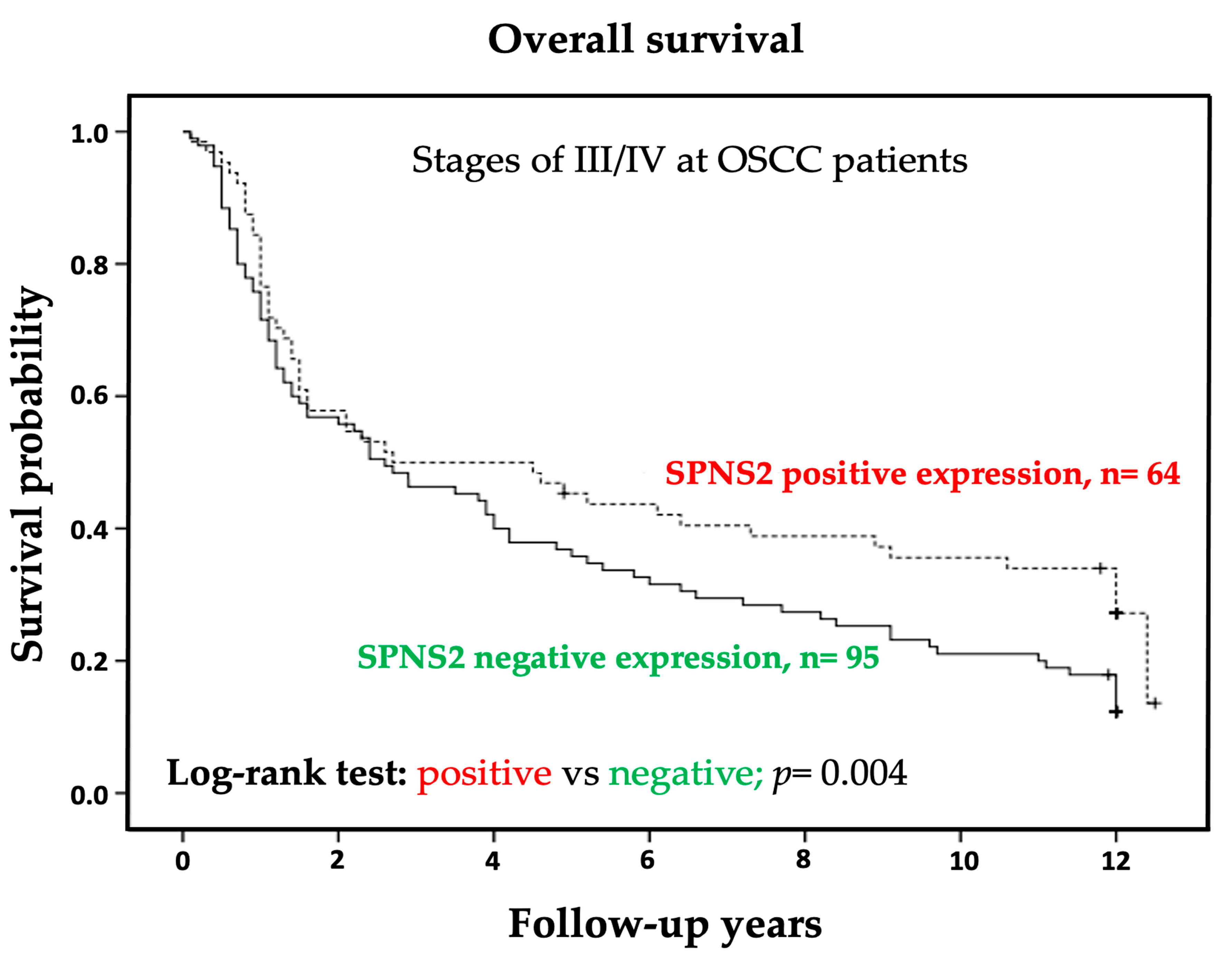
3.3. Negative Cytoplasmic SPNS2 Expression was Associated with Short Overall Survival of Patients with OSCC Stage III/IV
3.4. Cox Proportional Hazard Model Analysis to Identify Prognostic Indicators in OSCC Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Fedele, S.; Lo Russo, L. The World Cancer Report and the burden of oral cancer. Eur. J. Cancer Prev. 2004, 13, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, A.; Chalastras, T.; Ferekidou, E.; Yiotakis, I.; Kyriou, L.; Tzagarakis, M.; Ferekidis, E.; Kandiloros, D. Association between squamous cell carcinoma of the head and neck and serum folate and homocysteine. Anticancer Res. 2006, 26, 2345–2348. [Google Scholar] [PubMed]
- Lo, A.K.; Lo, K.W.; Tsao, S.W.; Wong, H.L.; Hui, J.W.; To, K.F.; Hayward, D.S.; Chui, Y.L.; Lau, Y.L.; Takada, K.; et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia 2006, 8, 173–180. [Google Scholar] [PubMed]
- Hennessey, P.T.; Westra, W.H.; Califano, J.A. Human papillomavirus and head and neck squamous cell carcinoma: Recent evidence and clinical implications. J. Dent. Res. 2009, 88, 300–306. [Google Scholar] [CrossRef]
- Smith, E.M.; Rubenstein, L.M.; Haugen, T.H.; Pawlita, M.; Turek, L.P. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: A case for multifactor disease. J. Oncol. 2012, 2012, 571862. [Google Scholar] [CrossRef]
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr virus infection and oral squamous cell carcinoma risk: A meta-analysis. PLoS ONE 2017, 12, e0186860. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, J.; Wang, J.; Huang, R. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tob. Induc. Dis. 2019, 17, 29. [Google Scholar] [CrossRef]
- Hill, S.J.; D’Andrea, A.D. Predictive Potential of Head and Neck Squamous Cell Carcinoma Organoids. Cancer Discov. 2019, 9, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Maruya, S.; Issa, J.P.; Weber, R.S.; Rosenthal, D.I.; Haviland, J.C.; Lotan, R.; El-Naggar, A.K. Differential methylation status of tumor-associated genes in head and neck squamous carcinoma: Incidence and potential implications. Clin. Cancer Res. 2004, 10, 3825–3830. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Li, D.; Liu, S.; Amizuka, N.; Li, M. Identification of Differentially Expressed Genes Induced by Aberrant Methylation in Oral Squamous Cell Carcinomas Using Integrated Bioinformatic Analysis. Int. J. Mol. Sci. 2018, 19, 1698. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Noguti, J.; De Moura, C.F.; De Jesus, G.P.; Da Silva, V.H.; Hossaka, T.A.; Oshima, C.T.; Ribeiro, D.A. Metastasis from oral cancer: An overview. Cancer Genom. Proteom. 2012, 9, 329–335. [Google Scholar]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.W.; Warnakulasuriya, S.; Gupta, P.C.; Dimba, E.; Chindia, M.; Otoh, E.C.; Sankaranarayanan, R.; Califano, J.; Kowalski, L. Global oral health inequalities in incidence and outcomes for oral cancer: Causes and solutions. Adv. Dent. Res. 2011, 23, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Oliveira, A.K.; Costa, R.A.P.; De Rossi, T.; Paes Leme, A.F. Prognostic biomarkers in oral squamous cell carcinoma: A systematic review. Oral. Oncol. 2017, 72, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, X.; Fu, Y.; Liu, Y.; Zheng, L.; Liu, F.; Li, T.; Yin, X.; Qiao, X.; Xu, X. Identification of AUNIP as a candidate diagnostic and prognostic biomarker for oral squamous cell carcinoma. EBioMedicine 2019, 47, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Chen, M.L.; Chen, C.L.; Yeh, C.M.; Sung, W.W. Overexpression of EIF5A2 Predicts Poor Prognosis in Patients with Oral Squamous Cell Carcinoma. Diagnostics 2020, 10, 436. [Google Scholar] [CrossRef]
- Hisano, Y.; Nishi, T.; Kawahara, A. The functional roles of S1P in immunity. J. Biochem. 2012, 152, 305–311. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2019, 195, 85–99. [Google Scholar] [CrossRef]
- Huang, Y.L.; Huang, W.P.; Lee, H. Roles of sphingosine 1-phosphate on tumorigenesis. World J. Biol. Chem. 2011, 2, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef]
- Spiegel, S.; Cuvillier, O.; Edsall, L.; Kohama, T.; Menzeleev, R.; Olivera, A.; Thomas, D.; Tu, Z.; Van Brocklyn, J.; Wang, F. Roles of sphingosine-1-phosphate in cell growth, differentiation, and death. Biochemistry 1998, 63, 69–73. [Google Scholar]
- Chalfant, C.E.; Spiegel, S. Sphingosine 1-phosphate and ceramide 1-phosphate: Expanding roles in cell signaling. J. Cell Sci. 2005, 118, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Du, W.; Kaneko, E.; Okamoto, Y.; Yoshioka, K.; Takuwa, Y. Tumor-suppressive sphingosine-1-phosphate receptor-2 counteracting tumor-promoting sphingosine-1-phosphate receptor-1 and sphingosine kinase 1—Jekyll Hidden behind Hyde. Am. J. Cancer Res. 2011, 1, 460–481. [Google Scholar]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Lian, S.; Khan, A.; Llop, J.R.; Samuelson, A.V.; Chen, W.; Klionsky, D.J.; Kishi, S. Autolysosome biogenesis and developmental senescence are regulated by both Spns1 and v-ATPase. Autophagy 2017, 13, 386–403. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Ishii, T.; Endo, K.; Kawakami, E.; Nagao, K.; Miyashita, T.; Akiyama, K.; Watabe, K.; Komatsu, M.; Yamamoto, D.; et al. L-leucine and SPNS1 coordinately ameliorate dysfunction of autophagy in mouse and human Niemann-Pick type C disease. Sci. Rep. 2017, 7, 15944. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr.; Beatty, J.T.; Goffeau, A.; Harley, K.T.; Heijne, W.H.; Huang, S.C.; Jack, D.L.; Jahn, P.S.; Lew, K.; Liu, J.; et al. The major facilitator superfamily. J. Mol. Microbiol. Biotechnol. 1999, 1, 257–279. [Google Scholar]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ingham, N.; Kelly, J.; Jadeja, S.; Goulding, D.; Pass, J.; Mahajan, V.B.; Tsang, S.H.; Nijnik, A.; Jackson, I.J.; et al. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS Genet. 2014, 10, e1004688. [Google Scholar] [CrossRef] [PubMed]
- Donoviel, M.S.; Hait, N.C.; Ramachandran, S.; Maceyka, M.; Takabe, K.; Milstien, S.; Oravecz, T.; Spiegel, S. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J 2015, 29, 5018–5028. [Google Scholar] [CrossRef] [PubMed]
- Hisano, Y.; Kobayashi, N.; Yamaguchi, A.; Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE 2012, 7, e38941. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Dasgupta, S.; Jiang, X.; Zhao, X.; Zhu, G.; He, Q.; Dinkins, M.; Bieberich, E.; Wang, G. Critical role of Spns2, a sphingosine-1-phosphate transporter, in lung cancer cell survival and migration. PLoS ONE 2014, 9, e110119. [Google Scholar] [CrossRef]
- Van der Weyden, L.; Arends, M.J.; Campbell, A.D.; Bald, T.; Wardle-Jones, H.; Griggs, N.; Velasco-Herrera, M.D.; Tuting, T.; Sansom, O.J.; Karp, N.A.; et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 2017, 541, 233–236. [Google Scholar] [CrossRef]
- Gu, X.; Jiang, Y.; Xue, W.; Song, C.; Wang, Y.; Liu, Y.; Cui, B. SPNS2 promotes the malignancy of colorectal cancer cells via regulating Akt and ERK pathway. Clin. Exp. Pharmacol. Physiol. 2019, 46, 861–871. [Google Scholar] [CrossRef]
- Huang, W.; Qian, T.; Cheng, Z.; Zeng, T.; Si, C.; Liu, C.; Deng, C.; Ye, X.; Liu, Y.; Cui, L.; et al. Prognostic significance of Spinster homolog gene family in acute myeloid leukemia. J. Cancer 2020, 11, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Lin, C.W.; Lu, J.W.; Yeh, K.T.; Lin, S.H.; Yang, S.F. Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients. Diagnostics 2020, 10, 122. [Google Scholar] [CrossRef]
- Lu, J.W.; Lin, S.H.; Yeh, C.M.; Yeh, K.T.; Huang, L.R.; Chen, C.Y.; Lin, Y.M. Cytoplasmic CK1epsilon Protein Expression Is Correlated With Distant Metastasis and Survival in Patients With Melanoma. In Vivo 2020, 34, 2905–2911. [Google Scholar] [CrossRef]
- Ho, Y.J.; Chang, J.; Yeh, K.T.; Gong, Z.; Lin, Y.M.; Lu, J.W. Prognostic and Clinical Implications of WNK Lysine Deficient Protein Kinase 1 Expression in Patients With Hepatocellular Carcinoma. In Vivo 2020, 34, 2631–2640. [Google Scholar] [CrossRef]
- Scully, C.; Field, J.K.; Tanzawa, H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral. Oncol. 2000, 36, 256–263. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Lo Russo, L.; Cirillo, N.; Lo Muzio, L. Prognostic significance of CD68(+) and CD163(+) tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral. Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236. [Google Scholar] [CrossRef] [PubMed]
- Beaurivage, C.; Champagne, A.; Tobelaim, W.S.; Pomerleau, V.; Menendez, A.; Saucier, C. SOCS1 in cancer: An oncogene and a tumor suppressor. Cytokine 2016, 82, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.A.; Lu, J.W.; Lin, T.Y.; Tsai, C.H.; Chou, W.C.; Lin, C.C.; Kuo, Y.Y.; Liu, C.Y.; Tseng, M.H.; Chiang, Y.C.; et al. Clinico-biological significance of suppressor of cytokine signaling 1 expression in acute myeloid leukemia. Blood Cancer J. 2017, 7, e588. [Google Scholar] [CrossRef]
- Zhang, X.H.; Yang, L.; Liu, X.J.; Zhan, Y.; Pan, Y.X.; Wang, X.Z.; Luo, J.M. Association between methylation of tumor suppressor gene SOCS1 and acute myeloid leukemia. Oncol. Rep. 2018, 40, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Tomiuk, S.; Hofmann, K.; Nix, M.; Zumbansen, M.; Stoffel, W. Cloned mammalian neutral sphingomyelinase: Functions in sphingolipid signaling? Proc. Natl. Acad. Sci. USA 1998, 95, 3638–3643. [Google Scholar] [CrossRef]
- Han, M.; Chang, H.; Zhang, P.; Chen, T.; Zhao, Y.; Zhang, Y.; Liu, P.; Xu, T.; Xu, P. C13C4.5/Spinster, an evolutionarily conserved protein that regulates fertility in C. elegans through a lysosome-mediated lipid metabolism process. Protein Cell 2013, 4, 364–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakurai, A.; Nakano, Y.; Koganezawa, M.; Yamamoto, D. Phenotypic interactions of spinster with the genes encoding proteins for cell death control in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2010, 73, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y. Stories of spinster with various faces: From courtship rejection to tumor metastasis rejection. J. Neurogenet. 2019, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Fu, L.; Chen, H.; Guan, J.; Chen, Y.; Fang, J. Association study of genetic variation in the autophagy lysosome pathway genes and risk of eight kinds of cancers. Int. J. Cancer 2018, 143, 80–87. [Google Scholar] [CrossRef] [PubMed]
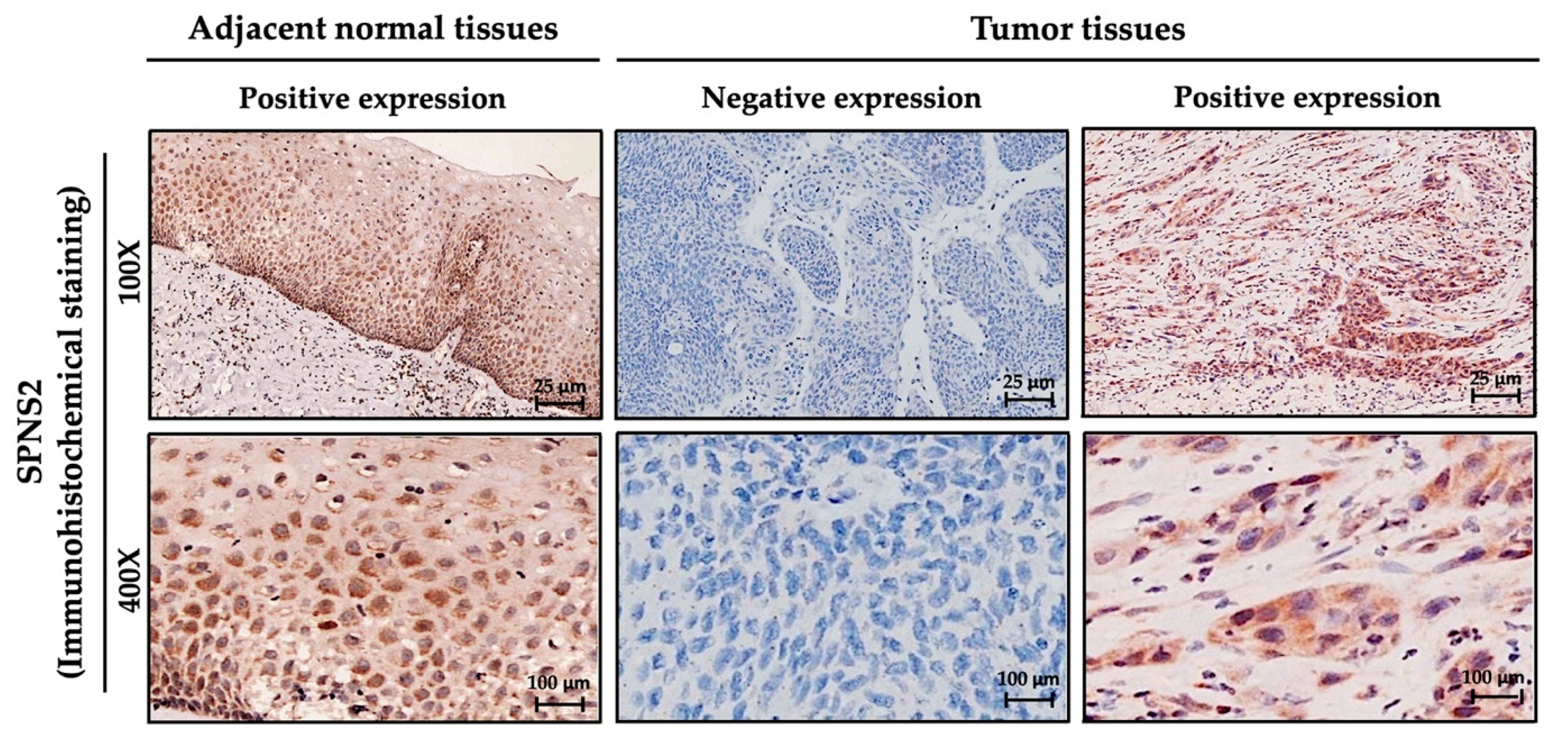
| Factors | (n = 264) | Percentage |
|---|---|---|
| Cytoplasmic staining of SPNS2 | ||
| Negative | 141 | 53.40% |
| Positive | 123 | 46.60% |
| Gender | ||
| Male | 250 | 94.70% |
| Female | 14 | 5.30% |
| Age (Year) | ||
| Range | 31–88 | |
| Mean | 55.3 | |
| Medium | 54 | |
| T (Tumor size) | ||
| I | 65 | 24.60% |
| II | 81 | 30.70% |
| III | 21 | 8.00% |
| IV | 97 | 36.70% |
| N (Lymph node) | ||
| N0 | 169 | 64.00% |
| N1 | 95 | 36.00% |
| M (Metastasis) | ||
| M0 | 262 | 99.20% |
| M1 | 2 | 0.80% |
| AJCC cancer stage | ||
| I | 50 | 18.90% |
| II | 55 | 20.80% |
| III | 31 | 11.70% |
| IV | 128 | 48.50% |
| Histological grade | ||
| WD | 42 | 15.90% |
| MD | 214 | 81.10% |
| PD | 8 | 3.00% |
| Clinical therapy | ||
| Radiotherapy | 162 | 71.40% |
| Chemotherapy | 65 | 28.60% |
| Cytoplasmic Staining of SPNS2 | ||||
|---|---|---|---|---|
| Variables | Negative | Positive | (n = 264) | p-Value a |
| Age | 55.7 ± 10.9 | 55.3 ± 11.2 | 0.803 | |
| Gender | ||||
| Male | 136 (96.5%) | 114 (92.7%) | 250 | 0.173 |
| Female | 5 (3.5%) | 9 (7.3%) | 14 | |
| Histological grade | ||||
| WD | 23 (16.3%) | 19 (15.4%) | 42 | 0.848 |
| MD/PD | 118 (83.7%) | 104 (86.4%) | 222 | |
| T status | ||||
| T1/T2 | 69 (48.9%) | 77 (62.6%) | 146 | 0.026 * |
| T3/T4 | 72 (51.1%) | 46 (37.4%) | 118 | |
| Lymph node metastasis | ||||
| No | 86 (61.0%) | 83 (67.5%) | 169 | 0.273 |
| Yes | 55 (39.0%) | 40 (32.5%) | 95 | |
| Distant metastasis | ||||
| M0 | 139 (98.6%) | 121 (100%) | 262 | 0.501 |
| M1 | 2 (1.4%) | 0 (0%) | 2 | |
| Stage | ||||
| I, II | 46 (32.6%) | 59 (48.0%) | 105 | 0.011 * |
| III, IV | 95 (67.4%) | 64 (52.0%) | 159 | |
| Smoking | ||||
| No | 34 (33.3%) | 34 (41.0%) | 68 | 0.284 |
| Yes | 68 (66.7%) | 49 (59.0%) | 117 | |
| Betel nut chewing | ||||
| No | 30 (50.0%) | 29 (50.9%) | 59 | 0.924 |
| Yes | 30 (50.0%) | 28 (49.1%) | 58 | |
| Survival | ||||
| ≤3 year | 57 (40.4%) | 51 (41.5%) | 108 | 0.865 |
| >3 year | 84 (59.6%) | 72 (58.5%) | 156 | |
| ≤5 year | 71 (50.4%) | 60 (48.8%) | 131 | 0.799 |
| >5 year | 70 (49.6%) | 63 (51.2%) | 133 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables (n = 159) | Hazard Ratio a | 95% CI | p-Value | Hazard Ratio a | 95% CI | p-Value |
| Expression of SPNS2 | ||||||
| Positive | 1 | 1 | ||||
| Negative | 1.44 | 1.001–2.063 | 0.049 * | 1.45 | 1.001–2.083 | 0.049 * |
| T status | ||||||
| T1, T2 | 1 | 1 | ||||
| T3, T4 | 1.1 | 0.761–1.577 | 0.624 | 1.68 | 1.063–2.660 | 0.026 * |
| Lymph node metastasis | ||||||
| No | 1 | 1 | ||||
| Yes | 1.53 | 1.095–2.137 | 0.013 * | 1.73 | 1.153–2.594 | 0.008 * |
| Distant metastasis | ||||||
| M0 | 1 | 1.04 | ||||
| M1 | 1.32 | 0.486–3.557 | 0.590 | 1 | 0.231–4.001 | 0.957 |
| Histological grade | ||||||
| WD | 1 | 1 | ||||
| MD, PD | 1.99 | 1.163–3.402 | 0.012 * | 1.77 | 1.017–3.066 | 0.043 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.-W.; Tseng, Y.-S.; Lo, Y.-S.; Lin, Y.-M.; Yeh, C.-M.; Lin, S.-H. Prognostic Significance of Cytoplasmic SPNS2 Expression in Patients with Oral Squamous Cell Carcinoma. Medicina 2021, 57, 164. https://doi.org/10.3390/medicina57020164
Lu J-W, Tseng Y-S, Lo Y-S, Lin Y-M, Yeh C-M, Lin S-H. Prognostic Significance of Cytoplasmic SPNS2 Expression in Patients with Oral Squamous Cell Carcinoma. Medicina. 2021; 57(2):164. https://doi.org/10.3390/medicina57020164
Chicago/Turabian StyleLu, Jeng-Wei, Yen-Shuo Tseng, Yu-Sheng Lo, Yueh-Min Lin, Chung-Min Yeh, and Shu-Hui Lin. 2021. "Prognostic Significance of Cytoplasmic SPNS2 Expression in Patients with Oral Squamous Cell Carcinoma" Medicina 57, no. 2: 164. https://doi.org/10.3390/medicina57020164
APA StyleLu, J.-W., Tseng, Y.-S., Lo, Y.-S., Lin, Y.-M., Yeh, C.-M., & Lin, S.-H. (2021). Prognostic Significance of Cytoplasmic SPNS2 Expression in Patients with Oral Squamous Cell Carcinoma. Medicina, 57(2), 164. https://doi.org/10.3390/medicina57020164






