Pulp Vitality Testing with a Developed Universal Pulse Oximeter Probe Holder
Abstract
1. Introduction
2. Materials and Methods
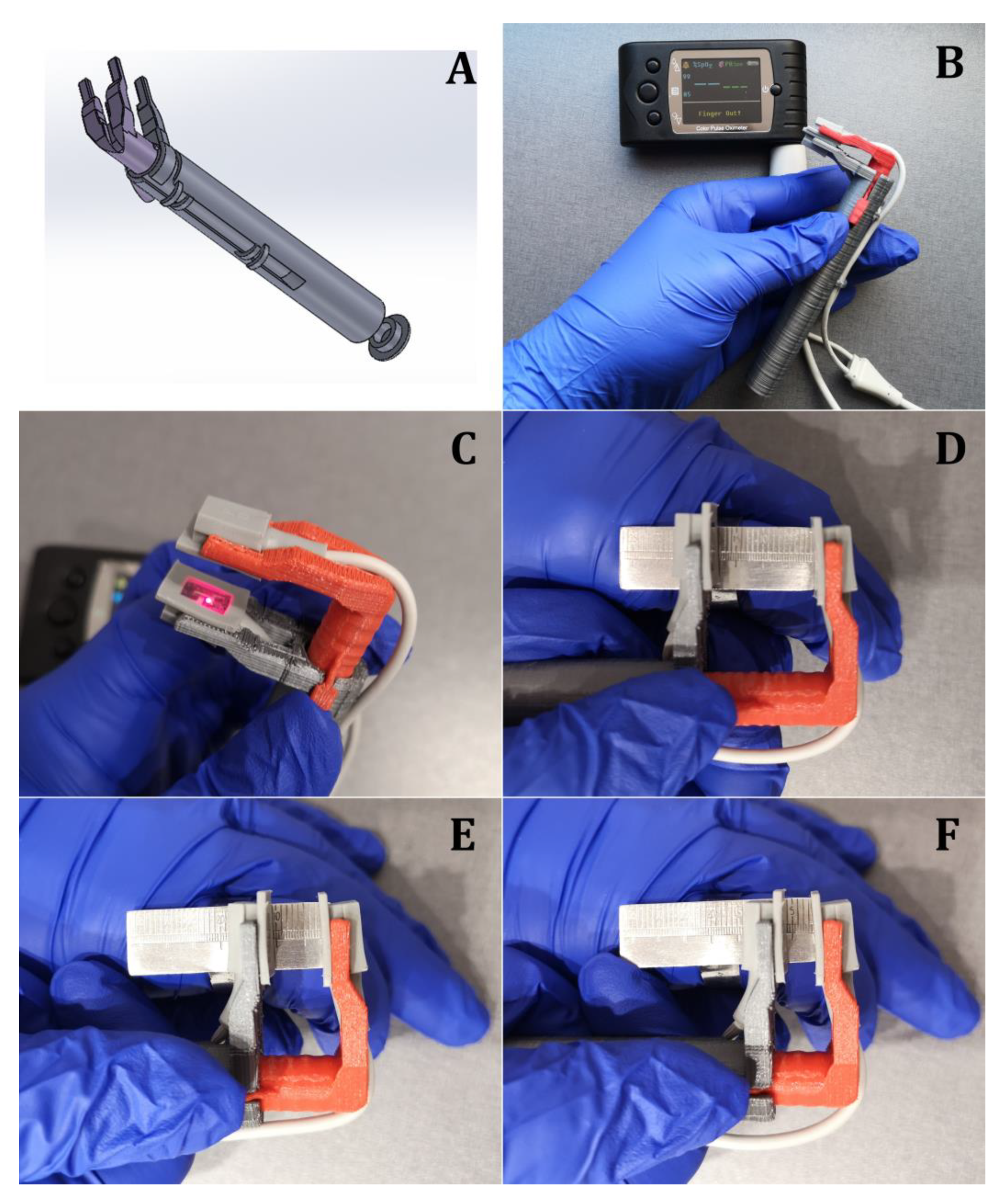
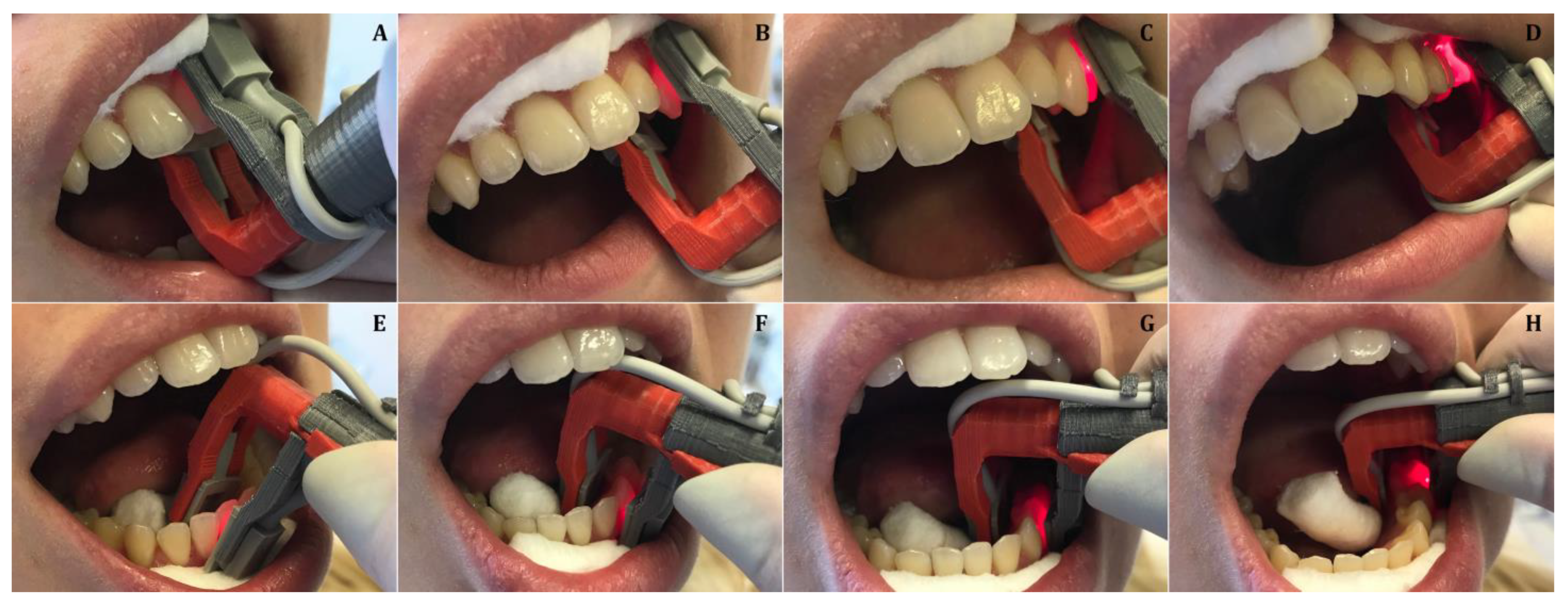
2.1. Development of the Universal Pulse Oximeter Probe Holder
2.2. Statistical Analysis
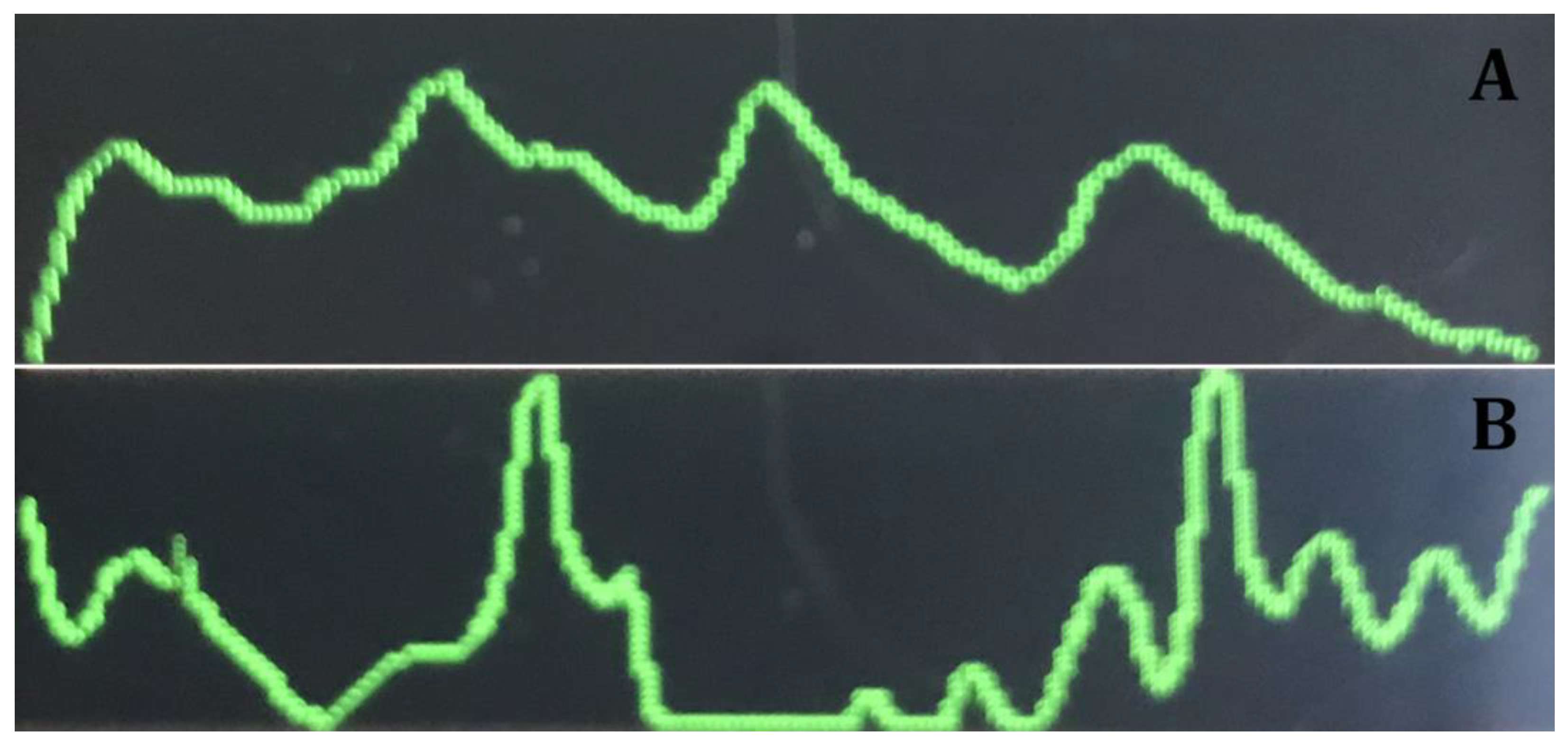
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd-Elmeguid, A.; Yu, D.C. Dental pulp neurophysiology: Part 1. Clinical and diagnostic implications. J. Can. Dent. Assoc. 2009, 75, 55–59. [Google Scholar] [PubMed]
- Abd-Elmeguid, A.; Yu, D.C. Dental pulp neurophysiology: Part 2. Current diagnostic tests to assess pulp vitality. J. Can. Dent. Assoc. 2009, 75, 139–143. [Google Scholar] [PubMed]
- Bhaskar, S.N.; Rappaport, H.M. Dental vitality tests and pulp status. J. Am. Dent. Assoc. 1973, 86, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Bernick, S.; Nedelman, C. Effect of aging on the human pulp. J. Endod. 1975, 1, 88–94. [Google Scholar] [CrossRef]
- Peterson, K.; Söderström, C.; Levy, G.; Kiani-Anaraki, M. Evaluation of the ability of thermal and electrical tests to register pulp vitality. Endod. Dent. Traumatol. 1999, 15, 127–131. [Google Scholar] [CrossRef]
- Zach, L.; Cohen, G. Pulp Response to Externally Applied Heat. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 515–530. [Google Scholar] [CrossRef]
- Johnson, J.V.; Hinds, E.C. Evaluation of teeth vitality after subapical osteotomy. J. Oral Surg. 1969, 27, 256–257. [Google Scholar]
- Kim, S.; Dorscher-Kim, J.E.; Liu, M. Microcirculation of the dental pulp and its autonomic control. Proc. Finn. Dent. Soc. 1989, 85, 279–287. [Google Scholar]
- Gazelius, B.; Lindh-Strömberg, U.; Pettersson, H.; Oberg, P.A. Laser Doppler technique—A future diagnostic tool for tooth pulp vitality. Int. Endod. J. 1993, 26, 8–9. [Google Scholar]
- Matthews, B.; Vongsavan, N. Advantages and limitations of laser Doppler flow meters. Int. Endod. J. 1993, 26, 9–10. [Google Scholar] [CrossRef]
- Ingolfsson, A.R.; Tronstad, L.; Hersh, E.V.; Riva, C.E. Efficacy of laser Doppler flowmetry in determining pulp vitality of human teeth. Endod. Dent. Traumatol. 1994, 10, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Vaghela, D.J.; Sinha, A.A. Pulse oximetry and laser Doppler flowmetry for diagnosis of pulpal vitality. J. Interdiscip. Dent. 2011, 1, 14–21. [Google Scholar] [CrossRef]
- Musselwhite, J.M.; Klitzman, B.; Maixner, W.; Burkes, E.J., Jr. Laser Doppler flowmetry: A clinical test of pulpal vitality. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 411–419. [Google Scholar] [PubMed]
- Akpinar, K.E.; Er, K.; Polat, S.; Polat, N.T. Effect of gingiva on laser doppler pulpal blood flow measurements. J. Endod. 2004, 30, 138–140. [Google Scholar] [CrossRef]
- Polat, S.; Er, K.; Polat, N.T. Penetration depth of laser Doppler flowmetry beam in teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 125–129. [Google Scholar] [CrossRef]
- Gopikrishna, V.; Pradeep, G.; Venkateshbabu, N. Assessment of pulp vitality: A review. Int. J. Paediatr. Dent. 2009, 19, 3–15. [Google Scholar] [CrossRef]
- Noblett, W.C.; Wilcox, L.R.; Scamman, F.; Johnson, W.T.; Diaz-Arnold, A. Detection of pulpal circulation in vitro by pulse oximetry. J. Endod. 1996, 22, 1–5. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Munshi, A.K.; Hegde, A.M. Pulse oximetry: A diagnostic instrument in pulpal vitality testing. J. Clin. Pediatr. Dent. 2002, 26, 141–145. [Google Scholar]
- Anusha, B.; Madhusudhana, K.; Chinni, S.K.; Paramesh, Y. Assessment of Pulp Oxygen Saturation Levels by Pulse Oximetry for Pulpal Diseases -A Diagnostic Study. J. Clin. Diagn. Res. 2017, 11, 36–39. [Google Scholar] [CrossRef]
- Stella, J.P.; Barletta, F.B.; Giovanella, L.B.; Grazziotin-Soares, R.; Tovo, M.F.; Felippe, W.T.; Estrela, C. Oxygen Saturation in Dental Pulp of Permanent Teeth: Difference between Children/Adolescents and Adults. J. Endod. 2015, 41, 1445–1449. [Google Scholar] [CrossRef]
- Estrela, C.; Serpa, G.C.; Alencar, A.H.G.; Bruno, K.F.; Barletta, F.B.; Felippe, W.T.; Estrela, C.R.; Souza, J.B. Oxygen Saturation in the Dental Pulp of Maxillary Premolars in Different Age Groups—Part 1. Braz. Dent. J. 2017, 28, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kahan, R.S.; Gulabivala, K.; Snook, M.; Setchell, D.J. Evaluation of a pulse oximeter and customized probe for pulp vitality testing. J. Endod. 1996, 22, 105–109. [Google Scholar] [CrossRef]
- Goho, C. Pulse oximetry evaluation of vitality in primary and immature permanent teeth. Pediatr. Dent. 1999, 21, 125–127. [Google Scholar] [PubMed]
- Gopikrishna, V.; Kandaswamy, D.; Gupta, T. Assessment of the efficacy of an indigeniously developed pulse oximeter dental sensor holder for pulp vitality testing. Indian J. Dent. Res. 2006, 17, 111–113. [Google Scholar]
- Gopikrishna, V.; Tinagupta, K.; Kandaswamy, D. Comparison of electrical, thermal, and pulse oximetry methods for assessing pulp vitality in recently traumatized teeth. J. Endod. 2007, 33, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, V.; Tinagupta, K.; Kandaswamy, D. Evaluation of efficacy of a new custom-made pulse oximeter dental probe in comparison with the electrical and thermal tests for assessing pulp vitality. J. Endod. 2007, 33, 411–414. [Google Scholar] [CrossRef]
- Estrela, C.; Oliveira, K.S.; Alencar, A.H.G.; Barletta, F.B.; Felippe, W.T. Oxygen Saturation in the Dental Pulp of Maxillary and Mandibular Molars—Part 2. Braz. Dent. J. 2017, 28, 704–709. [Google Scholar] [CrossRef]
- Calil, E.; Caldeira, C.L.; Gavini, G.; Lemos, E.M. Determination of pulp vitality in vivo with pulse oximetry. Int. Endod. J. 2008, 41, 741–746. [Google Scholar]
- Pozzobon, M.H.; De Sousa Vieira, R.; Alves, A.M.; Reyes-Carmona, J.; Teixeira, C.S.; de Souza, B.D.M.; Felippe, W.T. Assessment of pulp blood flow in primary and permanent teeth using pulse oximetry. Dent. Traumatol. 2011, 27, 184–188. [Google Scholar]
- Sadique, M.; Ravi, S.V.; Thomas, K.; Dhanapal, P.; Simon, E.P.; Shaheen, M. Evaluation of efficacy of a pulse oximeter to assess pulp vitality. J. Int. Oral. Health 2014, 6, 70–72. [Google Scholar]
- Sharma, D.S.; Mishra, S.; Banda, N.R.; Vaswani, S. In Vivo Evaluation of Customized Pulse Oximeter and Sensitivity Pulp Tests for Assessment of Pulp Vitality. J. Clin. Pediatr. Dent. 2019, 43, 11–15. [Google Scholar] [CrossRef] [PubMed]
| Jaw | Tooth Type | N | Oxygen Saturation (%) | Range (%) | |
|---|---|---|---|---|---|
| Mean (SD) | Median (25–75%) | ||||
| Maxillary | Incisor | 16 | 93.75 (3.26) | 94.5 (91.0–96.0) | 88–99 |
| Canine | 16 | 91.63 (4.98) | 91.5 (89.0–95.75) | 79–98 | |
| Premolar | 16 | 93.38 (5.76) | 96.0 (89.5–97.75) | 80–99 | |
| Molar | 16 | 92.31 (4.42) | 91.0 (88.25–96.0) | 87–99 | |
| Mandibular | Incisor | 16 | 93.69 (6.22) | 95.5 (91.25–98.75) | 75–99 |
| Canine | 16 | 93.56 (3.6) | 94.0 (91.0–96.5) | 87–99 | |
| Premolar | 16 | 93.94 (4.74) | 96.0 (88.25–98.0) | 86–99 | |
| Molar | 16 | 93.13 (5.82) | 94.0 (88.25–99.0) | 82–99 | |
| Authors/Year | Index Finger Saturation | Tested Teeth/Saturation (Mean) | Statistically Significant Differences between Tested Teeth | |
|---|---|---|---|---|
| 1 | B. Anusha et al./2017 [19] | 98.4% | Max. and mand. front teeth—94.6% | - |
| 2 | Calil et al./2008 [28] | 95% | Max. c. incisor—91.29% Max. canine—90.69% | No |
| 3 | M.H. Pozzobon et al./2011 [29] | 92.85% | Max. front teeth—85.27% | No |
| 4 | M. Sadique et al./2013 [30] | 95.88% | Max. front teeth—85% | - |
| 5 | C. Estrela et al./2017 [27] | 92.89% | Max. molars—83.59% Mand molars—86.89% | Yes No |
| 6 | C. Estrela et al./2017 [21] | 93.7% | Max. premolars—86.2% | - |
| 7 | V. Gopi Krishna et al./2006 [24] | 97.58% | Max. front teeth—79.59% | No |
| 8 | Present study | 97.22% | All types of teeth—93.17% | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabliauskienė, Ž.; Zamaliauskienė, R.; Lodienė, G. Pulp Vitality Testing with a Developed Universal Pulse Oximeter Probe Holder. Medicina 2021, 57, 101. https://doi.org/10.3390/medicina57020101
Grabliauskienė Ž, Zamaliauskienė R, Lodienė G. Pulp Vitality Testing with a Developed Universal Pulse Oximeter Probe Holder. Medicina. 2021; 57(2):101. https://doi.org/10.3390/medicina57020101
Chicago/Turabian StyleGrabliauskienė, Živilė, Roberta Zamaliauskienė, and Greta Lodienė. 2021. "Pulp Vitality Testing with a Developed Universal Pulse Oximeter Probe Holder" Medicina 57, no. 2: 101. https://doi.org/10.3390/medicina57020101
APA StyleGrabliauskienė, Ž., Zamaliauskienė, R., & Lodienė, G. (2021). Pulp Vitality Testing with a Developed Universal Pulse Oximeter Probe Holder. Medicina, 57(2), 101. https://doi.org/10.3390/medicina57020101






