The Dynamics of Inflammatory Markers in Patients with Suspected Acute Appendicitis
Abstract
1. Introduction
2. Materials and Methods
- Catarrhal appendicitis—neutrophils within mucosa and mucosal ulceration, with or without intraluminal neutrophils;
- Secondary changes/periappendicitis—inflammation of serosa and subserosa, infiltration extends no further than outer muscularis propria;
- Phlegmonous appendicitis—neutrophilic infiltration of mucosa, submucosa, and muscularis propria; transmural inflammation; extensive ulceration and intramural abscesses; vascular thrombosis;
- Gangrenous appendicitis—transmural inflammation with areas of necrosis, extensive mucosal ulceration.
- Catarrhal appendicitis—no visible changes;
- Secondary changes/periappendicitis—may appear normal or serosa may be dull, congested, and show exudate;
- Phlegmonous appendicitis—dilated or increased diameter appendix; dull serosa; dilatation and congestion of surface vessels; fibrinopurulent serosal exudate;
- Gangrenous appendicitis—appendiceal wall friable; purple, green, or black.
Statistical Analysis
3. Results
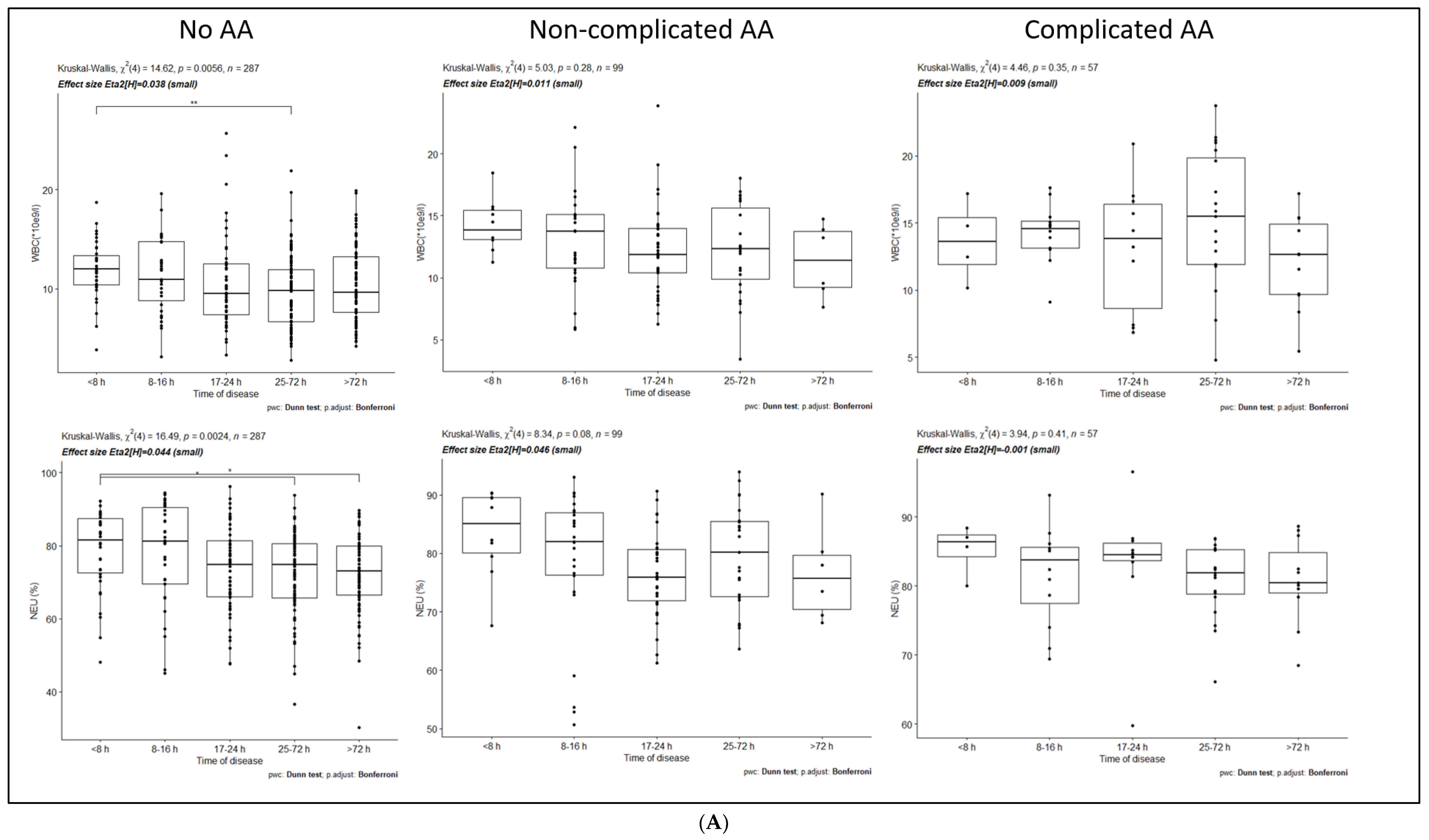
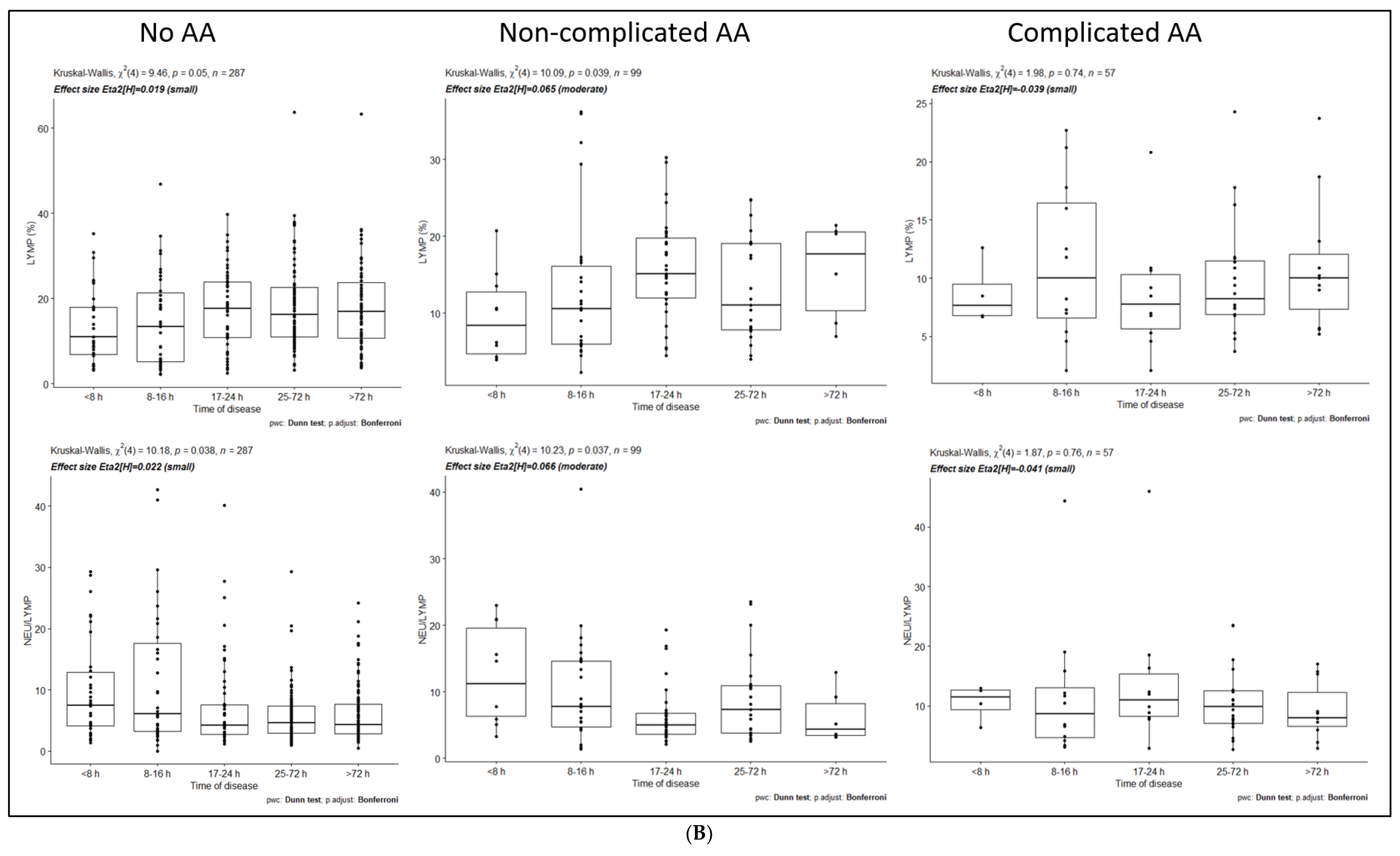
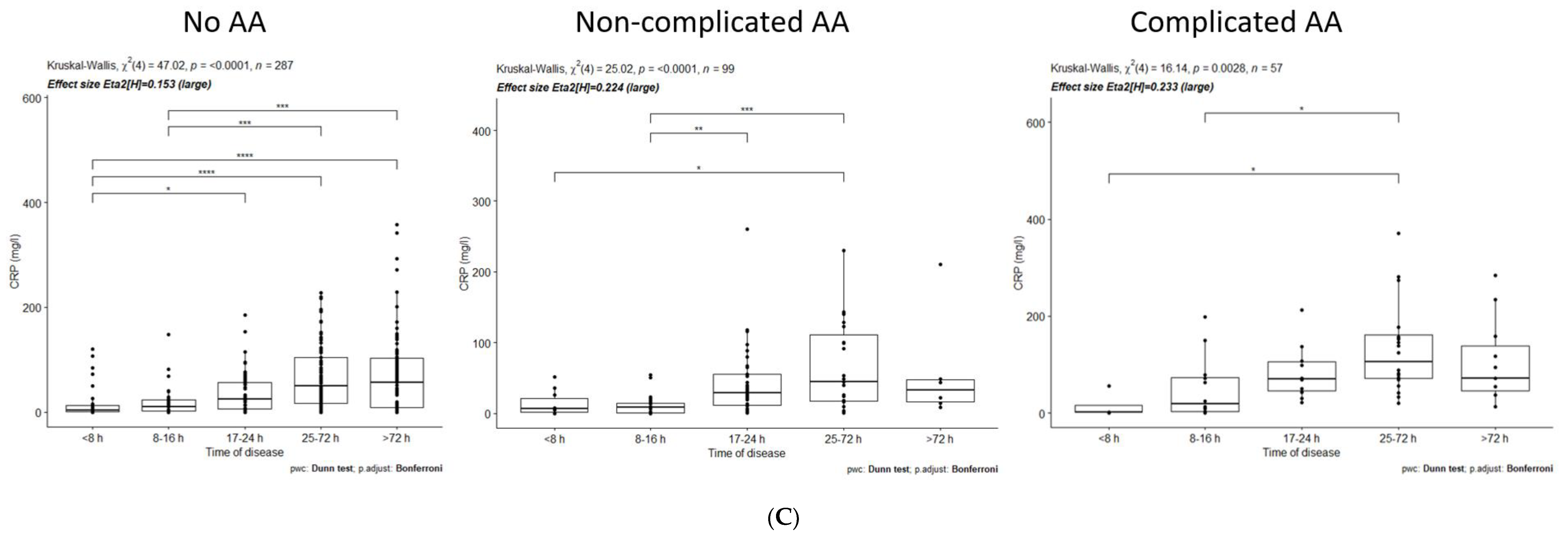
3.1. Dynamics of Inflammatory Markers within No-AA, NAA, and CAA Groups
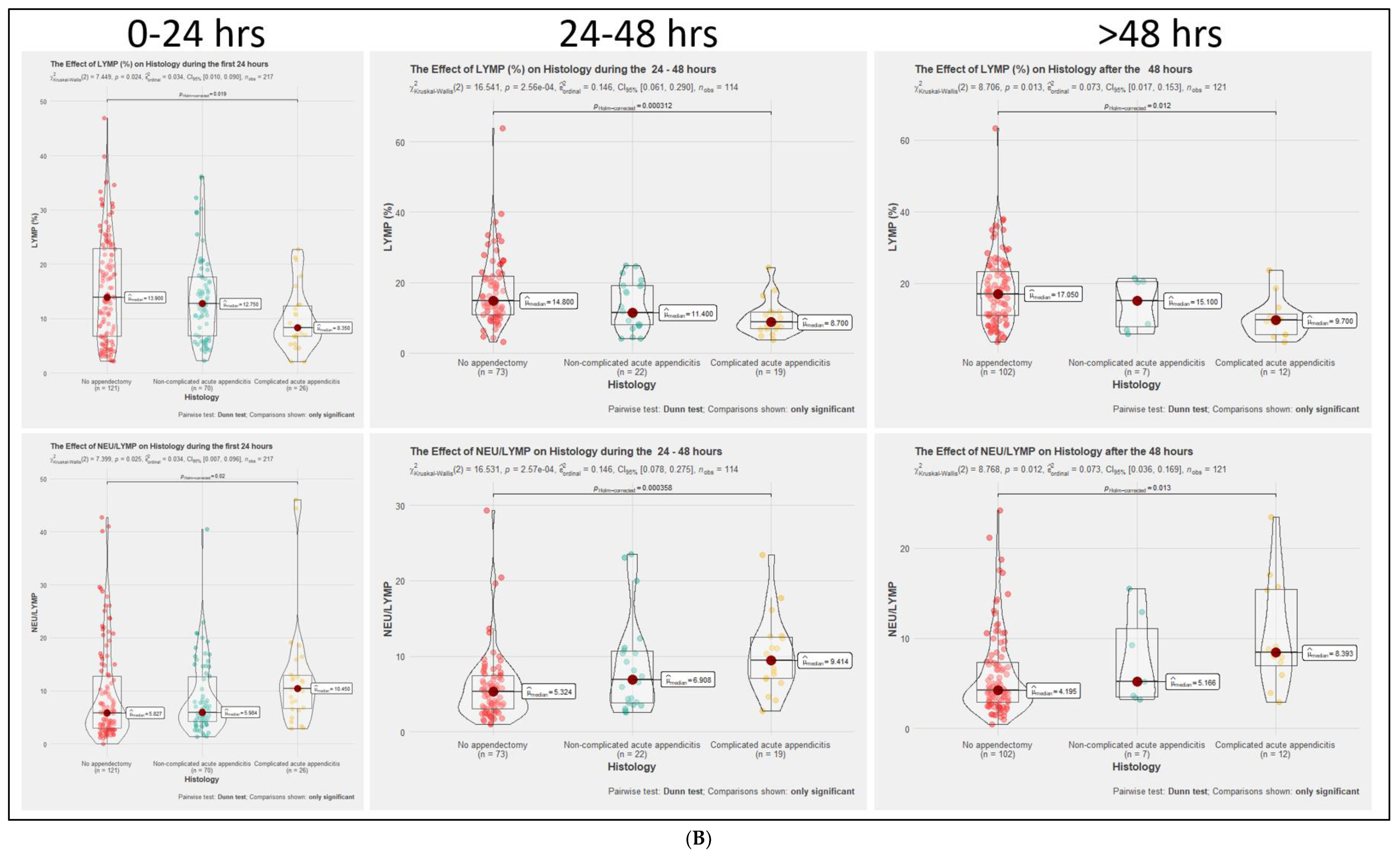
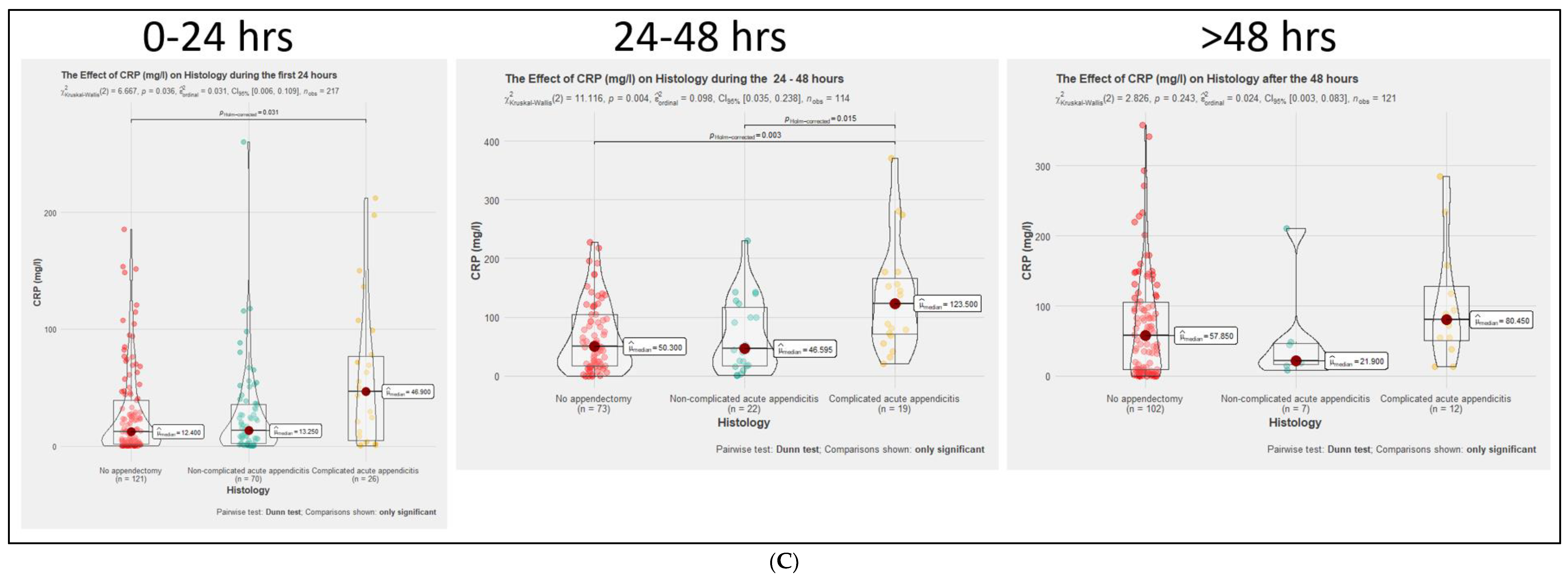
3.2. Comparison of Inflammatory Markers Dynamics between No-AA, NAA, and CAA Groups
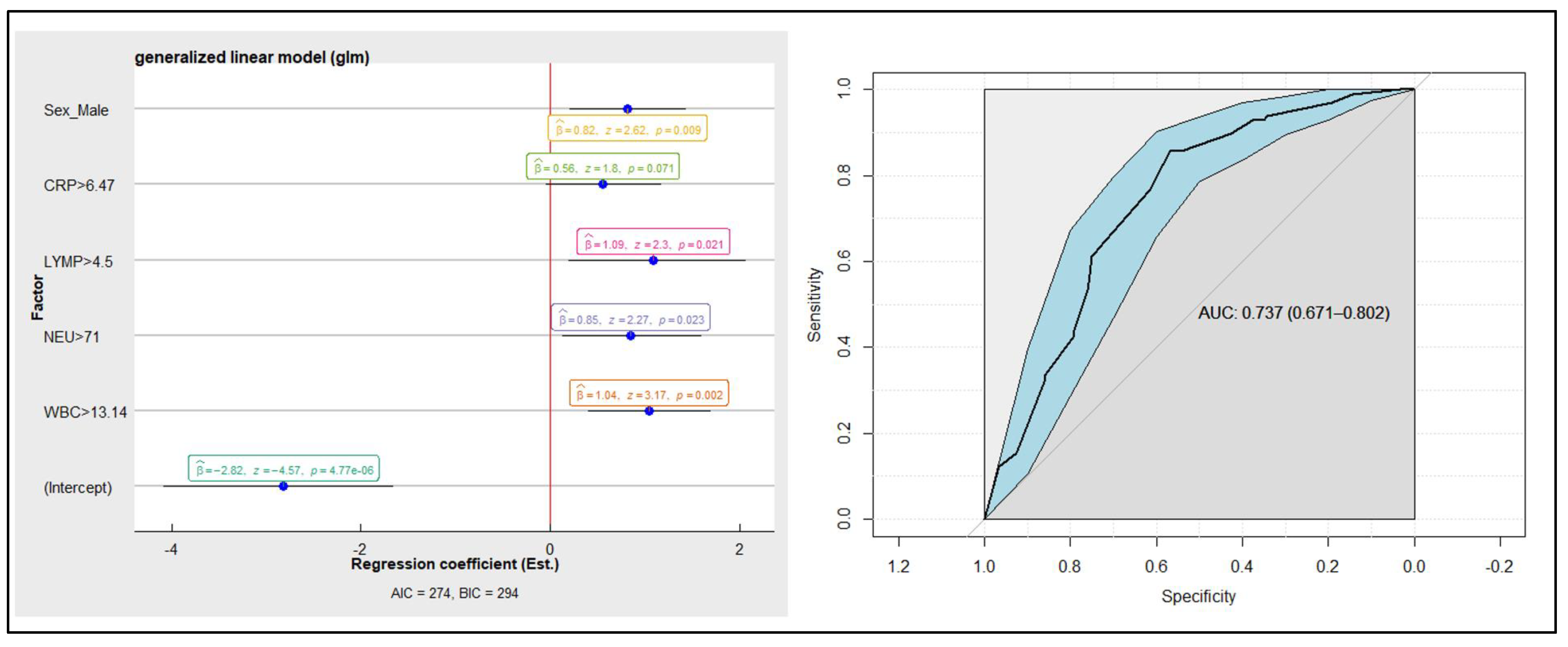
3.3. Linear Logistic Regression Model on Inflammatory Markers and the Diagnosis of AA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wickramasinghe, D.P.; Xavier, C.; Samarasekera, D.N. The Worldwide Epidemiology of Acute Appendicitis: An Analysis of the Global Health Data Exchange Dataset. World J. Surg. 2021, 45, 1999–2008. [Google Scholar] [CrossRef]
- Stewart, B.; Khanduri, P.; McCord, C.; Ohene-Yeboah, M.; Uranues, S.; Vega Rivera, F.; Mock, C. Global Disease Burden of Conditions Requiring Emergency Surgery. Br. J. Surg. 2014, 101, e9–e22. [Google Scholar] [CrossRef]
- Di Saverio, S.; Podda, M.; De Simone, B.; Ceresoli, M.; Augustin, G.; Gori, A.; Boermeester, M.; Sartelli, M.; Coccolini, F.; Tarasconi, A.; et al. Diagnosis and Treatment of Acute Appendicitis: 2020 Update of the WSES Jerusalem Guidelines. World J. Emerg. Surg. 2020, 15, 27. [Google Scholar] [CrossRef]
- Salminen, P.; Tuominen, R.; Paajanen, H.; Rautio, T.; Nordström, P.; Aarnio, M.; Rantanen, T.; Hurme, S.; Mecklin, J.-P.; Sand, J.; et al. Five-Year Follow-up of Antibiotic Therapy for Uncomplicated Acute Appendicitis in the APPAC Randomized Clinical Trial. JAMA 2018, 320, 1259–1265. [Google Scholar] [CrossRef]
- Sallinen, V.; Akl, E.A.; You, J.J.; Agarwal, A.; Shoucair, S.; Vandvik, P.O.; Agoritsas, T.; Heels-Ansdell, D.; Guyatt, G.H.; Tikkinen, K.A.O. Meta-Analysis of Antibiotics versus Appendicectomy for Non-Perforated Acute Appendicitis. Br. J. Surg. 2016, 103, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Ohle, R.; O’Reilly, F.; O’Brien, K.K.; Fahey, T.; Dimitrov, B.D. The Alvarado Score for Predicting Acute Appendicitis: A Systematic Review. BMC Med. 2011, 9, 139. [Google Scholar] [CrossRef]
- Kryzauskas, M.; Danys, D.; Poskus, T.; Mikalauskas, S.; Poskus, E.; Jotautas, V.; Beisa, V.; Strupas, K. Is Acute Appendicitis Still Misdiagnosed? Open Med. 2016, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.O.; Muhammed, A.M. Alternative Diagnosis for Pain in Patients Who Underwent Appendectomies for Normal Appendices and the Incidence of Negative Appendectomies. Ann. Coll. Med. Mosul. 2011, 37, 80–86. [Google Scholar] [CrossRef]
- Liese, J.; Halbinger, T.M.; Ulrich, F.; Bechstein, W.O.; Strey, C.W. Appendicitis—The Balance between Cost Effectiveness and Safety Remains Challenging. Langenbecks Arch. Surg. 2014, 399, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Alhamdani, Y.F.; Rizk, H.A.; Algethami, M.R.; Algarawi, A.M.; Albadawi, R.H.; Faqih, S.N.; Ahmed, E.H.; Abukammas, O.J. Negative Appendectomy Rate and Risk Factors That Influence Improper Diagnosis at King Abdulaziz University Hospital. Mater. Socio-Med. 2018, 30, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lukenaite, B.; Luksaite-Lukste, R.; Mikalauskas, S.; Samuilis, A.; Strupas, K.; Poškus, T. Magnetic Resonance Imaging Reduces the Rate of Unnecessary Operations in Pregnant Patients with Suspected Acute Appendicitis: A Retrospective Study. Ann. Surg. Treat. Res. 2021, 100, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.G.J.; Langen, R.M.R.; Janssen, L.; Verrijth-Wilms, I.M.H.A.; Wouda, S.; Janzing, H.M.J. Does the Use of Diagnostic Imaging Reduce the Rate of Negative Appendectomy? Acta Chir. Belg. 2015, 115, 393–396. [Google Scholar] [CrossRef]
- Coursey, C.A.; Nelson, R.C.; Patel, M.B.; Cochran, C.; Dodd, L.G.; DeLong, D.M.; Beam, C.A.; Vaslef, S. Making the Diagnosis of Acute Appendicitis: Do More Preoperative CT Scans Mean Fewer Negative Appendectomies? A 10-Year Study. Radiology 2010, 254, 460–468. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, X.; Chen, L.; Lu, S. Abdominal Ultrasound and Its Diagnostic Accuracy in Diagnosing Acute Appendicitis: A Meta-Analysis. Front. Surg. 2021, 8, 707160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Suh, C.H.; Yoon, H.M.; Kim, J.R.; Jung, A.Y.; Lee, J.S.; Cho, Y.A. Visibility of Normal Appendix on CT, MRI, and Sonography: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2018, 211, W140–W150. [Google Scholar] [CrossRef]
- Rud, B.; Vejborg, T.S.; Rappeport, E.D.; Reitsma, J.B.; Wille-Jørgensen, P. Computed Tomography for Diagnosis of Acute Appendicitis in Adults. Cochrane Database Syst. Rev. 2019, 2019, CD009977. [Google Scholar] [CrossRef]
- Eng, K.A.; Abadeh, A.; Ligocki, C.; Lee, Y.K.; Moineddin, R.; Adams-Webber, T.; Schuh, S.; Doria, A.S. Acute Appendicitis: A Meta-Analysis of the Diagnostic Accuracy of US, CT, and MRI as Second-Line Imaging Tests after an Initial US. Radiology 2018, 288, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078. [Google Scholar] [CrossRef]
- Luksaite-Lukste, R.; Kliokyte, R.; Samuilis, A.; Jasiunas, E.; Luksta, M.; Strupas, K.; Poskus, T. Conditional CT Strategy—An Effective Tool to Reduce Negative Appendectomy Rate and the Overuse of the CT. J. Clin. Med. 2021, 10, 2456. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.E.B. Meta-Analysis of the Clinical and Laboratory Diagnosis of Appendicitis. Br. J. Surg. 2004, 91, 28–37. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.; Rees, J.; Bell, E.; Hamdan, M.; Platt, T. Normal Inflammatory Markers in Appendicitis: Evidence from Two Independent Cohort Studies. JRSM Short Rep. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Er, S.; Çomçalı, B.; Soykurt, A.; Cavit Yüksel, B.; Tez, M. Diagnosis of Appendicitis in Patients with a Normal White Blood Cell Count; A Cross-Sectional Study. Bull. Emerg. Trauma 2018, 6, 128–132. [Google Scholar] [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Hobbs, N.; Mansour, M. Neutrophil-to-Lymphocyte Ratio Predicts Acute Appendicitis and Distinguishes between Complicated and Uncomplicated Appendicitis: A Systematic Review and Meta-Analysis. Am. J. Surg. 2020, 219, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Shogilev, D.J.; Duus, N.; Odom, S.R.; Shapiro, N.I. Diagnosing Appendicitis: Evidence-Based Review of the Diagnostic Approach in 2014. West. J. Emerg. Med. 2014, 15, 859–871. [Google Scholar] [CrossRef]
- Kabir, S.A.; Kabir, S.I.; Sun, R.; Jafferbhoy, S.; Karim, A. How to Diagnose an Acutely Inflamed Appendix; a Systematic Review of the Latest Evidence. Int. J. Surg. 2017, 40, 155–162. [Google Scholar] [CrossRef]
- Acharya, A.; Markar, S.R.; Ni, M.; Hanna, G.B. Biomarkers of Acute Appendicitis: Systematic Review and Cost–Benefit Trade-off Analysis. Surg. Endosc. 2017, 31, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Poortman, P.; Oostvogel, H.J.M.; Bosma, E.; Lohle, P.N.M.; Cuesta, M.A.; Klerk, E.S.M.d.L.; Hamming, J.F. Improving Diagnosis of Acute Appendicitis: Results of a Diagnostic Pathway with Standard Use of Ultrasonography Followed by Selective Use of CT. J. Am. Coll. Surg. 2009, 208, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Ramarajan, N.; Wang, N.E.; Newman, B.; Rubesova, E.; Mueller, C.M.; Barth, R.A. Effectiveness of a Staged US and CT Protocol for the Diagnosis of Pediatric Appendicitis: Reducing Radiation Exposure in the Age of ALARA. Radiology 2011, 259, 231–239. [Google Scholar] [CrossRef]
- Atema, J.J.; Gans, S.L.; Van Randen, A.; Laméris, W.; van Es, H.W.; van Heesewijk, J.P.M.; van Ramshorst, B.; Bouma, W.H.; ten Hove, W.; van Keulen, E.M.; et al. Comparison of Imaging Strategies with Conditional versus Immediate Contrast-Enhanced Computed Tomography in Patients with Clinical Suspicion of Acute Appendicitis. Eur. Radiol. 2015, 25, 2445–2452. [Google Scholar] [CrossRef]
- Toorenvliet, B.R.; Wiersma, F.; Bakker, R.F.R.; Merkus, J.W.S.; Breslau, P.J.; Hamming, J.F. Routine Ultrasound and Limited Computed Tomography for the Diagnosis of Acute Appendicitis. World J. Surg. 2010, 34, 2278–2285. [Google Scholar] [CrossRef]
- Huckins, D.S.; Copeland, K.; Self, W.; Vance, C.; Hendry, P.; Borg, K.; Gogain, J. Diagnostic Performance of a Biomarker Panel as a Negative Predictor for Acute Appendicitis in Adult ED Patients with Abdominal Pain. Am. J. Emerg. Med. 2017, 35, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Bax, G.; Paterson-Brown, S. White Cell Count and C-Reactive Protein Measurement in Patients with Possible Appendicitis. Ann. R. Coll. Surg. Engl. 2009, 91, 113–115. [Google Scholar] [CrossRef][Green Version]
- Khan, M.N.; Davie, E.; Irshad, K. The Role of White Cell Count and C-Reactive Protein in the Diagnosis of Acute Appendicitis. J. Ayub Med. Coll. Abbottabad JAMC 2004, 16, 17–19. [Google Scholar] [PubMed]
- Soldo, I.; Radisic Biljak, V.; Bakula, B.; Bakula, M.; Simundic, A.-M. The Diagnostic Accuracy of Clinical and Laboratory Parameters in the Diagnosis of Acute Appendicitis in the Adult Emergency Department Population—A Case Control Pilot Study. Biochem. Medica 2018, 28, 030712. [Google Scholar] [CrossRef]
- Yang, H.-R.; Wang, Y.-C.; Chung, P.-K.; Chen, W.-K.; Jeng, L.-B.; Chen, R.-J. Laboratory Tests in Patients with Acute Appendicitis. ANZ J. Surg. 2006, 76, 71–74. [Google Scholar] [CrossRef]
- Von-Mühlen, B.; Franzon, O.; Beduschi, M.G.; Kruel, N.; Lupselo, D. AIR SCORE ASSESSMENT FOR ACUTE APPENDICITIS. Arq. Bras. Cir. Dig. ABCD Braz. Arch. Dig. Surg. 2015, 28, 171–173. [Google Scholar] [CrossRef]
- Sammalkorpi, H.E.; Mentula, P.; Savolainen, H.; Leppäniemi, A. The Introduction of Adult Appendicitis Score Reduced Negative Appendectomy Rate. Scand. J. Surg. 2017, 106, 196–201. [Google Scholar] [CrossRef]
- Chong, C.F.; Adi, M.I.W.; Thien, A.; Suyoi, A.; Mackie, A.J.; Tin, A.S.; Tripathi, S.; Jaman, N.H.; Tan, K.K.; Kok, K.Y.; et al. Development of the RIPASA Score: A New Appendicitis Scoring System for the Diagnosis of Acute Appendicitis. Singapore Med. J. 2010, 51, 220–225. [Google Scholar]
- Kularatna, M.; Lauti, M.; Haran, C.; MacFater, W.; Sheikh, L.; Huang, Y.; McCall, J.; MacCormick, A.D. Clinical Prediction Rules for Appendicitis in Adults: Which Is Best? World J. Surg. 2017, 41, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Anandalwar, S.P.; Callahan, M.J.; Bachur, R.G.; Feng, C.; Sidhwa, F.; Karki, M.; Taylor, G.A.; Rangel, S.J. Use of White Blood Cell Count and Polymorphonuclear Leukocyte Differential to Improve the Predictive Value of Ultrasound for Suspected Appendicitis in Children. J. Am. Coll. Surg. 2015, 220, 1010–1017. [Google Scholar] [CrossRef]
- Burcharth, J.; Pommergaard, H.C.; Rosenberg, J.; Gögenur, I. Hyperbilirubinemia as a Predictor for Appendiceal Perforation: A Systematic Review. Scand. J. Surg. 2013, 102, 55–60. [Google Scholar] [CrossRef]
- Kılıç, M.Ö.; Güldoğan, C.E.; Balamir, İ.; Tez, M. Ischemia-Modified Albumin as a Predictor of the Severity of Acute Appendicitis. Am. J. Emerg. Med. 2017, 35, 92–95. [Google Scholar] [CrossRef]
- Andersson, M.; Rubér, M.; Ekerfelt, C.; Hallgren, H.B.; Olaison, G.; Andersson, R.E. Can New Inflammatory Markers Improve the Diagnosis of Acute Appendicitis? World J. Surg. 2014, 38, 2777–2783. [Google Scholar] [CrossRef]
- Paajanen, H.; Mansikka, A.; Laato, M.; Ristamäki, R.; Pulkki, K.; Kostiainen, S. Novel Serum Inflammatory Markers in Acute Appendicitis. Scand. J. Clin. Lab. Investig. 2002, 62, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.; Thompson, G.C.; Joffe, A.R.; Blackwood, J.; Martin, D.-A.; Brindle, M.; Barkema, H.W.; Jenne, C.N. Cytokines and Chemokines in Pediatric Appendicitis: A Multiplex Analysis of Inflammatory Protein Mediators. Mediat. Inflamm. 2019, 2019, e2359681. [Google Scholar] [CrossRef] [PubMed]
- Nyuwi, K.T.; Singh, C.G.; Khumukcham, S.; Rangaswamy, R.; Ezung, Y.S.; Chittvolu, S.R.; Sharma, A.B.; Singh, H.M. The Role of Serum Fibrinogen Level in the Diagnosis of Acute Appendicitis. J. Clin. Diagn. Res. 2017, 11, PC13–PC15. [Google Scholar] [CrossRef]
- Benito, J.; Acedo, Y.; Medrano, L.; Barcena, E.; Garay, R.P.; Arri, E.A. Usefulness of New and Traditional Serum Biomarkers in Children with Suspected Appendicitis. Am. J. Emerg. Med. 2016, 34, 871–876. [Google Scholar] [CrossRef]
- Yu, C.-W.; Juan, L.-I.; Wu, M.-H.; Shen, C.-J.; Wu, J.-Y.; Lee, C.-C. Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Procalcitonin, C-Reactive Protein and White Blood Cell Count for Suspected Acute Appendicitis. Br. J. Surg. 2013, 100, 322–329. [Google Scholar] [CrossRef]
- Cui, W.; Liu, H.; Ni, H.; Qin, X.; Zhu, L. Diagnostic Accuracy of Procalcitonin for Overall and Complicated Acute Appendicitis in Children: A Meta-Analysis. Ital. J. Pediatr. 2019, 45, 78. [Google Scholar] [CrossRef] [PubMed]

| Df * | Negligible | Small | Medium | Large |
|---|---|---|---|---|
| 1 | 0–0.1 | 0.1–0.3 | 0.3–0.5 | 0.5 or more |
| 2 | 0–0.07 | 0.07–0.21 | 0.21–0.35 | 0.35 or more |
| 3 | 0–0.06 | 0.06–0.17 | 0.17–0.29 | 0.29 or more |
| 4 | 0–0.05 | 0.05–0.15 | 0.15–0.25 | 0.25 or more |
| 5 | 0–0.05 | 0.05–0.13 | 0.13–0.22 | 0.22 or more |
| Overall (n = 453) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Disease | No-AA (n = 297) | NAA (n = 99) | CAA (n = 57) | ||||||
| Time of disease (hours) | ≤24 h | 25–48 h | >48 h | ≤24 h | 25–48 h | >48 h | ≤24 h | 25–48 h | >48 h |
| Age (years) | 28 (18–91) | 38 (19–85) | 40 (18–89) | 30 (18–72) | 31 (22–76) | 32 (21–39) | 48 (19–83) | 49 (21–78) | 48 (23–77) |
| Laboratory tests: | |||||||||
| WBC (*10e9/L) | 11 (3–26) | 10 (3–22) | 10 (4–20) | 13 (6–24) | 12 (3–18) | 11 (8–15) | 14 (7–21) | 16 (5–24) | 13 (5–21) |
| NEU (%) | 78 (45–96) | 76 (45–94) | 73 (30–90) | 80 (51–93) | 79 (64–94) | 78 (68–90) | 85 (60–97) | 82 (66–87) | 81 (68–89) |
| LYMP (%) | 14 (2–47) | 15 (3–64) | 17 (4–63) | 13 (2–36) | 11 (4–24) | 15 (6–21) | 8 (2–23) | 9 (4–24) | 10 (4–24) |
| NEU/LYMP | 6 (0–43) | 5 (1–29) | 4 (0–24) | 6 (1–40) | 7 (3–24) | 5 (3–16) | 10 (3–46) | 9 (3–23) | 8 (3–23) |
| CRP (mg/L) | 12 (0–185) | 50 (0–228) | 58 (0–358) | 13 (0–260) | 47 (0–230) | 22 (9–210) | 47 (1–212) | 124 (20–371) | 80 (13–284) |
| Group | Parameter | Time Period (1) | Time Period (2) | p-Value | Adjusted p-Value |
|---|---|---|---|---|---|
| No-AA | WBC (*10e9/L) | <8 h | 25–72 h | 0 | 0.01 |
| NEU (%) | <8 h | 25–72 h | 0 | 0.03 | |
| <8 h | >72 h | 0 | 0.02 | ||
| LYMP (%) | No statistically significant differences between time intervals | ||||
| NEU/LYMP | No statistically significant differences between time intervals | ||||
| CRP (mg/L) | <8 h | 17–24 h | 0 | 0.05 | |
| <8 h | 25–72 h | 0 | 0 | ||
| <8 h | >72 h | 0 | 0 | ||
| 8–16 h | 25–72 h | 0 | 0 | ||
| 8–16 h | >72 h | 0 | 0 | ||
| NAA | WBC (*10e9/L) | No statistically significant differences between time intervals | |||
| NEU (%) | No statistically significant differences between time intervals | ||||
| LYMP (%) | No statistically significant differences between time intervals | ||||
| NEU/LYMP | No statistically significant differences between time intervals | ||||
| CRP (mg/L) | <8 h | 25–72 h | 0 | 0.04 | |
| 8–16 h | 17–24 h | 0 | 0 | ||
| 8–16 h | 25–72 h | 0 | 0 | ||
| CAA | WBC (*10e9/L) | No statistically significant differences between time intervals | |||
| NEU (%) | No statistically significant differences between time intervals | ||||
| LYMP (%) | No statistically significant differences between time intervals | ||||
| NEU/LYMP | No statistically significant differences between time intervals | ||||
| CRP (mg/L) | <8 h | 25–72 h | 0 | 0.01 | |
| 8–16 h | 25–72 h | 0 | 0.02 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminskas, Ą.A.; Lukšaitė-Lukštė, R.; Jasiūnas, E.; Samuilis, A.; Augustinavičius, V.; Kryžauskas, M.; Strupas, K.; Poškus, T. The Dynamics of Inflammatory Markers in Patients with Suspected Acute Appendicitis. Medicina 2021, 57, 1384. https://doi.org/10.3390/medicina57121384
Kaminskas ĄA, Lukšaitė-Lukštė R, Jasiūnas E, Samuilis A, Augustinavičius V, Kryžauskas M, Strupas K, Poškus T. The Dynamics of Inflammatory Markers in Patients with Suspected Acute Appendicitis. Medicina. 2021; 57(12):1384. https://doi.org/10.3390/medicina57121384
Chicago/Turabian StyleKaminskas, Ąžuolas Algimantas, Raminta Lukšaitė-Lukštė, Eugenijus Jasiūnas, Artūras Samuilis, Vytautas Augustinavičius, Marius Kryžauskas, Kęstutis Strupas, and Tomas Poškus. 2021. "The Dynamics of Inflammatory Markers in Patients with Suspected Acute Appendicitis" Medicina 57, no. 12: 1384. https://doi.org/10.3390/medicina57121384
APA StyleKaminskas, Ą. A., Lukšaitė-Lukštė, R., Jasiūnas, E., Samuilis, A., Augustinavičius, V., Kryžauskas, M., Strupas, K., & Poškus, T. (2021). The Dynamics of Inflammatory Markers in Patients with Suspected Acute Appendicitis. Medicina, 57(12), 1384. https://doi.org/10.3390/medicina57121384







