Abstract
Backgroundand objectives: Patients with BRAF-mutated metastatic colorectal cancer have considerably poorer responses to conventional systemic treatment. The real-world effects of triplet therapy with BRAF, mitogen-activated protein kinase kinase, and epidermal growth factor receptor inhibitors in Asia have not been well-reported. Materials and Methods: This single-center case series included patients with BRAF-mutated metastatic colorectal cancer undergoing triplet therapy after failure of prior systemic treatment from 2016 to 2020. The primary outcome was progression-free survival, and secondary outcomes were overall survival, response rate, disease control rate, and adverse events. Results: Nine eligible patients with BRAF-mutated metastatic colorectal cancer receiving triplet therapy were enrolled, with a median follow-up time of 14.5 months (range, 1–26). Most patients (88.8%) had two or more prior systemic treatments, and the triplet regimen was mainly dabrafenib, trametinib, and panitumumab. The overall response rate and disease control rate were 11.1% and 33.3%, respectively. Median progression-free survival and overall survival were 2.9 and 7.4 months, respectively, and a trend toward better overall survival was found with left-sided metastatic colorectal cancer compared with right-sided disease (9.2 vs. 6.9 months, p = 0.093). Adverse events were mostly Grade 1–2, including nausea, hypertension, gastrointestinal symptoms, and skin disorders. Conclusions: In this single-center case series, triplet therapy with BRAF, mitogen-activated protein kinase kinase, and epidermal growth factor receptor inhibitors in BRAF-mutated metastatic colorectal cancer had an acceptable safety profile and reasonable efficacy.
1. Introduction
Cases of metastatic colorectal cancer (mCRC) comprise approximately one-fourth of all colorectal cancer (CRC) cases at initial diagnosis, and an additional 20% of CRC patients may also present subsequent metachronous metastasis despite treatment [1,2]. Progress has been made in various treatment strategies, including surgery, cytotoxic chemotherapy, target therapy, and immunotherapy. RAS wild type mCRC is still a treatment challenge, especially when other resistant gene alterations are present.
Along with RAS [3,4] and microsatellite instability [5,6], the BRAF V600E mutation [7,8] is a well-known biomarker that has an impact on mCRC survival and may affect the response of systemic and targeted therapies. Although the BRAF mutation is only detected in 5%–10% of all cases, mCRC patients who are microsatellite-stable with the BRAF V600E mutation have worse survival and response to anti-epidermal growth factor receptor (EGFR) agents [9,10]. However, resistance to anti-EGFR agents may be overcome with BRAF inhibitors [11,12], which may be beneficial in patients with progressive mCRC after the failure of first-line treatment.
The combination of a BRAF inhibitor and anti-EGFR agent, with and without a mitogen-activated protein kinase kinase (MEK) inhibitor, has been evaluated in several studies as a promising regimen for mCRC after first-line standard treatment [11,13,14]. The phase III BEACON trial demonstrated that the triplet regimen, which consists of a BRAF inhibitor, anti-EGFR agent, and MEK inhibitor, significantly improved overall survival (OS) and progression-free survival (PFS) compared with the control group (chemotherapy plus anti-EGFR agent) [11]. Another ongoing single-arm trial (ANCHOR CRC, a phase II study of first-line triple therapy with cetuximab, encorafenib, and binimetinib) also showed a favorable response rate [15]. However, real-world data on the triplet regimen as a later line of systemic treatment in Asian patients, is still lacking due to the scarcity of such patients. Thus, this case series aimed to report the clinical outcomes and safety of triplet therapy in mCRC patients with BRAF V600E mutations after the failure of at least first-line chemotherapy.
2. Methods
2.1. Patient Eligibility
This case series was a single-center study conducted at our hospital. Eligible cases were identified through medical chart review from April 2016 to April 2020. Patients were included if they met all the following criteria: (1) recurrence or progressive disease after first-line chemotherapy plus target therapy, with or without surgery; (2) at least one metastatic focus found in an imaging study; (3) pathologic examination of the tumor specimen revealing a BRAF V600E mutation; and (4) receiving triplet therapy as the second or later line of systemic treatment. Eligible cases were enrolled for this study until April 2020. This study was approved by the institutional review board of our hospital [KMUHIRB-2012-03-02(II)].
2.2. Analysis of BRAF Mutation, RAS Mutation, and Status of Microsatellite Stability
BRAF V600E mutation analysis was performed using direct deoxyribonucleic acid (DNA) sequencing from formalin-fixed, paraffin-embedded CRC tissue samples according to our previous study [16]. After deparaffinization and air-drying, DNA was isolated using the proteinase K and QIAamp DNA Micro Kit (QIAGEN). A high-resolution melting analysis was undertaken using the LightCycler 480 System Gene Scanning Assay. The primers used, which were specific for the BRAF V600E mutation, were designed using Primer3 free software. The forward and reverse primer sequences were 5′-CATAATGCTTGCTCTGATAGGAAA-3′ and 5′-TCAGCACATCTCAGGGCCAAA-3′, respectively. All the primers were produced with standard molecular biology quality (Protech Technology Enterprise Co., Ltd., Taipei, Taiwan). RAS mutations were identified through direct DNA sequencing, the procedure for which was described in detail in our previous study [17]. Both KRAS and NRAS mutation statuses were examined in the patients. The presence of a deficient mismatch repair gene (dMMR) was determined by immunohistochemical staining of CRC tissue specimens. Loss of at least one mismatch repair protein (MLH-1, MSH-2, MSH-6, or PMS-2) was deemed indicative of the presence of dMMR [18].
2.3. Systemic Treatment and Outcome Assessment
In this case series, all eligible patients received the triplet regimen, which comprised the BRAF inhibitor dabrafenib (Novartis Pharmaceuticals, Basel, Switzerland), the MEK inhibitor trametinib (Novartis Pharmaceuticals, Basel, Switzerland), and the anti-EGFR agent panitumumab (Amgen Inc., Thousand Oaks, CA, USA) or cetuximab (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA), after progressive disease was treated with at least second-line systemic treatment, including chemotherapy plus target therapy. The dosages were as follows: dabrafenib, 150 mg orally, twice per day; trametinib, 2 mg orally, once per day; panitumumab, 6 mg/kg every two weeks intravenously; and cetuximab, 400 mg/m2 loading, then 500 mg/m2 biweekly, intravenously. The patients attended regular follow-up visits at outpatient clinics every 2 weeks to evaluate symptoms and adverse events by the visiting staff and study nurses. When the patients were hospitalized for treatment or any other reason, the visiting staff and study nurses would be informed to allow assessment. The adverse events were recorded and graded during each cycle based on the National Cancer Institute Common Terminology Criteria for Adverse Events (Version 4.3; http://ctep.cancer.gov/reporting/ctc.html). Symptomatic treatments were provided for milder (grade 1-2) adverse events without interruption of systemic therapy, and the triplet therapy would be temporarily withheld for more severe adverse events (grade 3). Triplet therapy was only resumed if the adverse events were not life-threatening, and the patient got substantial improvement. The treatment response was typically assessed after 8–12 weeks of treatment by computed tomography, magnetic resonance imaging, or positron emission tomography according to the criteria of the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) [19]. The median follow-up period was 14.5 (range, 1–26) months.
The primary outcome of this study was PFS, and secondary outcomes were OS, response rate (RR), disease control rate (DCR), and adverse events (AEs) of treatment. PFS was defined as the time from the initiation of the triplet regimen to the first radiological progression or tumor-related death, whichever came first. OS was defined as the time from the initiation of the triplet regimen to death due to any cause. DCR was represented as the percentage of patients with complete response, partial response, or stable disease as their best response.
2.4. Statistics
SPSS (Version 20.0; SPSS, Chicago, IL, USA) was used for all data analyses. The continuous variables were compared with Wilcoxon’s signed-rank test, and categorical variables were compared using the Chi-square test. The Kaplan–Meier method was used to calculate PFS and OS, and a log-rank test was used to compare time-to-event distributions by clinical and molecular factors. Statistical significance was set at p < 0.05.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Baseline Characteristics of Included Patients
This case series included nine patients (4 had primary tumors on the right side: 2 in ascending colon and 2 in transverse colon; and 5 on the left side: 3 in descending colon cancer and 2 in sigmoid colon) with BRAF V600E-mutated mCRC who underwent triplet therapy. Their baseline characteristics are displayed in Table 1. All patients had tumors with wild-type KRAS/NRAS and moderate to poor differentiation. dMMR was noted in two of the six analyzed patients. Most patients received panitumumab, dabrafenib, and trametinib as the triplet regimen, but one patient used cetuximab instead of panitumumab. In addition, triplet therapy was exclusively used as third-line or later treatment in all but one patient, for whom the therapy was initiated after the failure of first-line therapy. Most patients (77.7%) had liver metastases, and in nearly half of them (44.4%), at least three organs were involved at the time of treatment. No significant differences in baseline characteristics were observed between left-sided and right-sided mCRC.

Table 1.
Baseline characteristics of all included patients with BRAF-mutated mCRC receiving triplet therapy, stratified by tumor sidedness.
3.2. Response Rate and Survival Analysis
Among the patients who underwent triplet therapy, only one patient had a partial response, and another two had stable disease (Table 2). All other patients had disease progression despite treatment (RR, 11.1%; DCR, 33.3%). The median PFS and OS were 2.9 months and 7.4 months, respectively (Figure 1A,B). No specific clinical or molecular factors were found to be significantly associated with favorable DCR or OS. However, a trend toward improved OS was found in left-sided mCRC compared with right-sided disease (9.2 vs. 6.9 months, p = 0.093) and patients with disease control. Median survival was not reached for patients with partial response or stable disease, and the median OS was 5.2 months for those with progressive disease (p = 0.069, Figure 2). In one patient with initial partial response after triplet therapy, PFS time persisted for 26 months until the last follow-up. In two patients with stable disease after triplet therapy, one had disease progression 3 months later and died, and the other patient achieved a PFS of 19 months without further systemic treatment.

Table 2.
dMMR status, treatment responses, and survival of each patient with BRAF-mutated mCRC receiving triplet therapy.
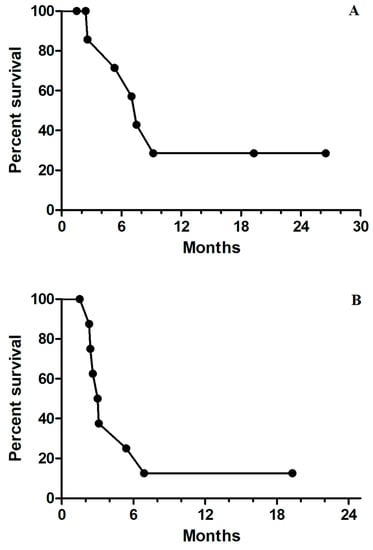
Figure 1.
(A) Kaplan–Meier survival curves for median progression-free survival of 2.9 months for all nine patients; (B) Kaplan–Meier survival curves for median overall survival of 7.4 months for all nine patients.
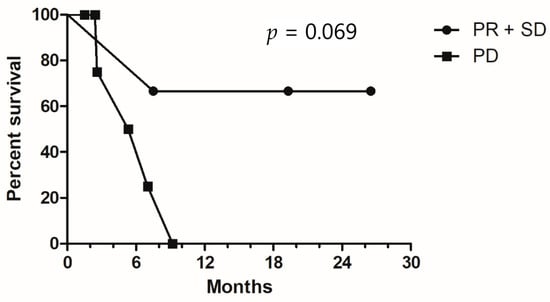
Figure 2.
Kaplan–Meier survival curves for overall survival, stratified by disease control status. PR, partial response; SD, stable disease; PD, progressive disease.
3.3. Adverse Events
The adverse events in patients who received triplet therapy are summarized in Table 3. Triplet therapy was generally well-tolerated, and most adverse events were Grades 1–2. The most frequent adverse events were liver function abnormality (66.6%), hypertension (66.6%), and dermatitis (66.6%), followed by nausea (44.4%) and skin rash (44.4%). The most frequent severe events (Grade 3) were nausea (22%), hypertension (22%), dermatitis (22%), and diarrhea (11%). Of note, one patient developed blurred vision during the second month of triplet therapy, which gradually improved following the completion of systemic treatment and conservative management. No patient experienced grade 4 adverse events.

Table 3.
Adverse events in all patients receiving triplet therapy for BRAF-mutated mCRC.
4. Discussion
In this case series, we demonstrated the real-world experience of using triplet therapy for BRAF-mutated mCRC as later lines of salvage therapy in Asian patients. Our findings suggest that triplet therapy appears to be well-tolerated and patients with initial disease control and longer PFS might gain considerable survival benefit, although most patients in our study still experienced disease progression.
The clinical efficacy of triplet therapy in BRAF-mutated mCRC has been demonstrated in two large clinical trials by Corcoran et al. [14] and Kopetz et al. (the BEACON trial) [11], and further trials are ongoing [15,20]. The trial by Corcoran et al. was a phase I trial using dabrafenib, panitumumab, and trametinib as triplet therapy, which was in line with our study’s regimen. Triplet therapy resulted in a 21% RR, and median PFS and OS were 4.2 and 9.1 months, respectively. Nevertheless, the BEACON trial showed that triplet therapy (encorafenib, binimetinib, and cetuximab) had a 26% RR, and median PFS and OS were 4.3 months and 9.0 months, respectively; by contrast, the control group had only a 2% RR, and median PFS and OS were 1.5 months and 5.4 months, respectively. Of note, these two trials included a considerable portion of patients who failed to respond to first-line treatment; by contrast, in the current study, most patients previously underwent at least second-line systemic treatment. Although a direct comparison between our study and those mentioned above was not possible, the RR and survival in the current study seem acceptable.
Another compelling question is whether primary tumor location affects the outcome in BRAF-mutated mCRC treated with triplet therapy. Although the role of tumor location in the prognosis of BRAF-mutated mCRC remains controversial [16,21], it may have some impact with the concomitant use of target therapy such as bevacizumab or cetuximab [22]. Several studies have demonstrated that first-line bevacizumab plus chemotherapy resulted in a superior prognosis for right-sided BRAF-mutated mCRC [16,23,24,25]. Conversely, left-sided mCRC had more favorable outcomes when treated with anti-EGFR agents than did right-sided tumors [22,26], which was consistent with our observation. Further studies exploring the impact of the primary tumor side on the prognostic outcomes of BRAF-mutated mCRC treated with anti-EGFR agents may be quite valuable.
Several factors may have contributed to the discrepancies related to treatment response and survival between this study and others. First, our study included mostly patients who underwent two or more prior systemic treatments, a factor that has been found to be associated with a worse RR [27,28]. Second, the difference between clinical trials and real-world practice may lead to some bias in objective evaluation. Other clinical factors, such as the presence of dMMR [29], differences in ethnicity, and different regimens, as well as the genetic alteration patterns of the BRAF mutation [30], might also influence the outcomes. These factors warrant a higher case enrollment and detailed analysis to clarify the best candidates for triplet therapy as salvage therapy among BRAF-mutated mCRC patients.
Regarding adverse events with triplet therapy, the most common AEs, including gastrointestinal and dermatologic disorders, were similar to those in previous clinical trials [11,14]. Our study did not observe any cases that required dose escalation or discontinuation due to side effects; one patient developed blurred vision, but the cycle of triplet therapy was maintained after two weeks until this symptom subsided. A previous study demonstrated that MEK inhibitors can induce retinopathy [31]. The onset is typically rapid in the first week of treatment but often resolves gradually, even without drug interruption. Thus, although this unique adverse event must be carefully monitored, it typically does not cause a serious sequela.
To the best of our knowledge, this is the first real-world study of triplet therapy in BRAF-mutated mCRC in Asian patients. In this study, we demonstrated an acceptable safety profile for triplet therapy, and we expect prolonged survival when initial disease control is obtained, even with two or more failures of prior systemic treatments. However, the limited case number precluded a robust subgroup analysis, and more data are necessary to explore the predictive factors of the prognosis of triplet therapy for BRAF-mutated mCRC in real-world practice.
In summary, this single-center case series demonstrated that triplet therapy with BRAF and MEK inhibitors and an anti-EGFR agent had an acceptable safety profile and reasonable efficacy for BRAF-mutated mCRC. Further studies enrolling more patients are needed to identify potential treatment responses and improve the efficacy of the treatment regimen.
Author Contributions
Conceptualization, J.-H.Y., Y.-P.L. and J.-Y.W.; methodology, H.-L.T., Y.-C.C. and C.-C.L.; formal analysis, J.-H.Y. and P.-J.C.; investigation, W.-C.S. and C.-W.H.; data curation, C.-W.H. and T.-K.C.; project administration, H.-L.T.; funding acquisition, J.-Y.W.; writing—original draft preparation, J.-H.Y.; writing—review and editing, J.-Y.W. and Y.-P.L.; supervision, J.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants through funding from the Ministry of Science and Technology (MOST 109-2314-B-037-035, MOST 109-2314-B-037-040, MOST 109-2314-B-037-046-MY3, MOST110-2314-B-037-097) and the Ministry of Health and Welfare (MOHW109-TDU-B-212-134026, MOHW109-TDU-B-212-114006, MOHW110-TDU-B-212-1140026) and funded by the health and welfare surcharge of on tobacco products, and the Kaohsiung Medical University Hospital (KMUH109-9R32, KMUH109-9R33, KMUH109-9R34, KMUH109-9M30, KMUH109-9M31, KMUH109-9M32, KMUH109-9M33, KMUHSA10903, KMUHSA11013, KMUH-DK(C)110010, KMUH-DK(B)110004-3) and KMU Center for Cancer Research (KMU-TC109A04-1) as well as and a KMU Center for Liquid Biopsy and Cohort Research Center Grant (KMU-TC109B05), Kaohsiung Medical University. In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative, Academia Sinica, Taiwan, R.O.C.
Institutional Review Board Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of [KMUHIRB-2012-03-02(II)].
Informed Consent Statement
Individual consent for this retrospective analysis was waived.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. The authors have no conflict of interest to declare.
References
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Dahabreh, I.J.; Terasawa, T.; Castaldi, P.J.; Trikalinos, T.A. Systematic review: Anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann. Intern. Med. 2011, 154, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tougeron, D.; Lecomte, T.; Pagès, J.C.; Villalva, C.; Collin, C.; Ferru, A.; Tourani, J.M.; Silvain, C.; Levillain, P.; Karayan-Tapon, L. Effect of low-frequency KRAS mutations on the response to anti-EGFR therapy in metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Nazemalhosseini Mojarad, E.; Kashfi, S.M.; Mirtalebi, H.; Taleghani, M.Y.; Azimzadeh, P.; Savabkar, S.; Pourhoseingholi, M.A.; Jalaeikhoo, H.; Aghdaei, H.A.; Kuppen, P.J.K.; et al. Low Level of Microsatellite Instability Correlates with Poor Clinical Prognosis in Stage II Colorectal Cancer Patients. J. Oncol. 2016, 2016, 2196703. [Google Scholar]
- Yuan, Z.X.; Wang, X.Y.; Qin, Q.Y.; Chen, D.F.; Zhong, Q.H.; Wang, L.; Wang, J.P. The prognostic role of BRAF mutation in metastatic colorectal cancer receiving anti-EGFR monoclonal antibodies: A meta-analysis. PLoS ONE 2013, 8, e65995. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.E.; Johnson, B.; Kugathasan, L.; Morris, V.K.; Raghav, K.; Swanson, L.; Lim, H.J.; Renouf, D.J.; Gill, S.; Wolber, R.; et al. Population-based Screening for BRAF (V600E) in Metastatic Colorectal Cancer Reveals Increased Prevalence and Poor Prognosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4599–4605. [Google Scholar] [CrossRef]
- Modest, D.P.; Ricard, I.; Heinemann, V.; Hegewisch-Becker, S.; Schmiegel, W.; Porschen, R.; Stintzing, S.; Graeven, U.; Arnold, D.; von Weikersthal, L.F.; et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1746–1753. [Google Scholar] [CrossRef]
- Gavin, P.G.; Colangelo, L.H.; Fumagalli, D.; Tanaka, N.; Remillard, M.Y.; Yothers, G.; Kim, C.; Taniyama, Y.; Kim, S.I.; Choi, H.J.; et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: An assessment of their prognostic and oxaliplatin predictive value. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 6531–6541. [Google Scholar] [CrossRef] [Green Version]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, R.B.; Atreya, C.E.; Falchook, G.S.; Kwak, E.L.; Ryan, D.P.; Bendell, J.C.; Hamid, O.; Messersmith, W.A.; Daud, A.; Kurzrock, R.; et al. Combined BRAF and MEK Inhibition with Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4023–4031. [Google Scholar] [CrossRef] [Green Version]
- Yaeger, R.; Cercek, A.; O’Reilly, E.M.; Reidy, D.L.; Kemeny, N.; Wolinsky, T.; Capanu, M.; Gollub, M.J.; Rosen, N.; Berger, M.F.; et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 1313–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, R.B.; André, T.; Atreya, C.E.; Schellens, J.H.M.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; Siena, S.; Middleton, G.; et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov. 2018, 8, 428–443. [Google Scholar] [CrossRef] [Green Version]
- Grothey, A.; Tabernero, J.; Taieb, J.; Yaeger, R.; Yoshino, T.; Maiello, E.; Fernandez, E.E.; Casado, A.R.; Ross, P.; André, T.; et al. LBA-5 ANCHOR CRC: A single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann. Oncol. 2020, 31, S242–S243. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Chang, T.-K.; Su, W.-C.; Huang, C.-W.; Tsai, H.-L.; Chen, Y.-C.; Li, C.-C.; Chen, P.-J.; Yin, T.-C.; Ma, C.-J.; et al. UGT1A1 Polymorphism for Irinotecan Dose Escalation in Patients with BRAF-Mutated Metastatic Colorectal Cancer Treated with First-Line Bevacizumab and FOLFIRI. J. Oncol. 2021, 2021, 6686517. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Huang, C.-W.; Chen, Y.-T.; Ma, C.-J.; Tsai, H.-L.; Chang, T.-K.; Su, W.-C.; Hsu, W.-H.; Kuo, C.-H.; Wang, J.-Y. Effect of KRAS and NRAS mutations on the prognosis of patients with synchronous metastatic colorectal cancer presenting with liver-only and lung-only metastases. Oncol. Lett. 2020, 20, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.M.; Bellcross, C.; Bittner, C.C.; Freivogel, M.E.; Haidle, J.L.; Kaurah, P.; Leininger, A.; Palaniappan, S.; Steenblock, K.; Vu, T.M.; et al. Genetic counseling considerations in the evaluation of families for Lynch syndrome—A review. J. Genet. Couns. 2011, 20, 5–19. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotani, D.; Bando, H.; Masuishi, T.; Komatsu, Y.; Yamaguchi, K.; Nakajima, T.; Satoh, T.; Nishina, T.; Esaki, T.; Nomura, S.; et al. BIG BANG study: A multicenter phase II study of the MEK inhibitor binimetinib + BRAF inhibitor encorafenib + anti-EGFR antibody cetuximab in patients with BRAF non-V600E mutated metastatic colorectal cancer (EPOC 1703). Ann. Oncol. 2018, 29, viii201–viii202. [Google Scholar] [CrossRef]
- Loupakis, F.; Intini, R.; Cremolini, C.; Orlandi, A.; Sartore-Bianchi, A.; Pietrantonio, F.; Pella, N.; Spallanzani, A.; Dell’Aquila, E.; Scartozzi, M.; et al. A validated prognostic classifier for V600EBRAF-mutated metastatic colorectal cancer: The ‘BRAF BeCool’ study. Eur. J. Cancer 2019, 118, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Brulé, S.; Jonker, D.; Karapetis, C.; O’Callaghan, C.; Moore, M.; Wong, R.; Tebbutt, N.; Underhill, C.; Yip, D.; Zalcberg, J.; et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J. Cancer 2015, 51, 1405–1414. [Google Scholar] [CrossRef]
- Ulivi, P.; Scarpi, E.; Chiadini, E.; Marisi, G.; Valgiusti, M.; Capelli, L.; Gardini, A.C.; Monti, M.; Ruscelli, S.; Frassineti, G.L.; et al. Right- vs. Left-Sided Metastatic Colorectal Cancer: Differences in Tumor Biology and Bevacizumab Efficacy. Int. J. Mol. Sci. 2017, 18, 1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venook, A.P.; Niedzwiecki, D.; Innocenti, F.; Fruth, B.; Greene, C.; O’Neil, B.H.; Shaw, J.E.; Atkins, J.N.; Horvath, L.E.; Polite, B.N.; et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2016, 34 (Suppl. 15), 3504. [Google Scholar] [CrossRef]
- Stintzing, S.; Heinrich, K.; Tougeron, D.; Modest, D.P.; Schwaner, I.; Euker, J.; Pihusch, R.; Stauch, M.; Kaiser, F.; Kahl, C.; et al. Randomized study to investigate FOLFOXIRI plus either bevacizumab or cetuximab as first-line treatment of BRAF V600E-mutant mCRC: The phase-II FIRE-4.5 study (AIO KRK-0116). J. Clin. Oncol. 2021, 39 (Suppl. 15), 3502. [Google Scholar] [CrossRef]
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.; Thant, C.N.; De Stanchina, E.; et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600–Mutant Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 7089–7097. [Google Scholar] [CrossRef] [Green Version]
- Morris, V.; Overman, M.J.; Jiang, Z.-Q.; Garrett, C.; Agarwal, S.; Eng, C.; Kee, B.; Fogelman, D.; Dasari, A.; Wolff, R.; et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin. Colorectal Cancer 2014, 13, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Huijberts, S.; Grothey, A.; Yaeger, R.; Cuyle, P.J.; Elez, E.; Fakih, M.; Montagut, C.; Peeters, M.; Yoshino, T.; et al. Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients with BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-in Results from the Phase III BEACON Colorectal Cancer Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1460–1469. [Google Scholar] [CrossRef]
- Fanelli, G.N.; Pozzo, C.A.D.; Depetris, I.; Schirripa, M.; Brignola, S.; Biason, P.; Balistreri, M.; Santo, L.D.; Lonardi, S.; Munari, G.; et al. The heterogeneous clinical and pathological landscapes of metastatic Braf-mutated colorectal cancer. Cancer Cell Int. 2020, 20, 30. [Google Scholar] [CrossRef]
- Middleton, G.; Yang, Y.; Campbell, C.D.; André, T.; Atreya, C.E.; Schellens, J.H.M.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; et al. BRAF-Mutant Transcriptional Subtypes Predict Outcome of Combined BRAF, MEK, and EGFR Blockade with Dabrafenib, Trametinib, and Panitumumab in Patients with Colorectal Cancer. Clin. Cancer Res. 2020, 26, 2466. [Google Scholar] [CrossRef] [Green Version]
- Booth, A.E.C.; Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Smith, J.R.; Sorich, M.J. Risk factors for MEK-associated retinopathy in patients with advanced melanoma treated with combination BRAF and MEK inhibitor therapy. Ther. Adv. Med. Oncol. 2020, 12, 1758835920944359. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).