The Yield of Endoscopy and Histology in the Evaluation of Esophageal Dysphagia: Two Referral Centers’ Experiences
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient’s Selection
2.2. Data Management
2.3. Statistical Analysis
3. Results
3.1. Demographics and Baseline Characteristics
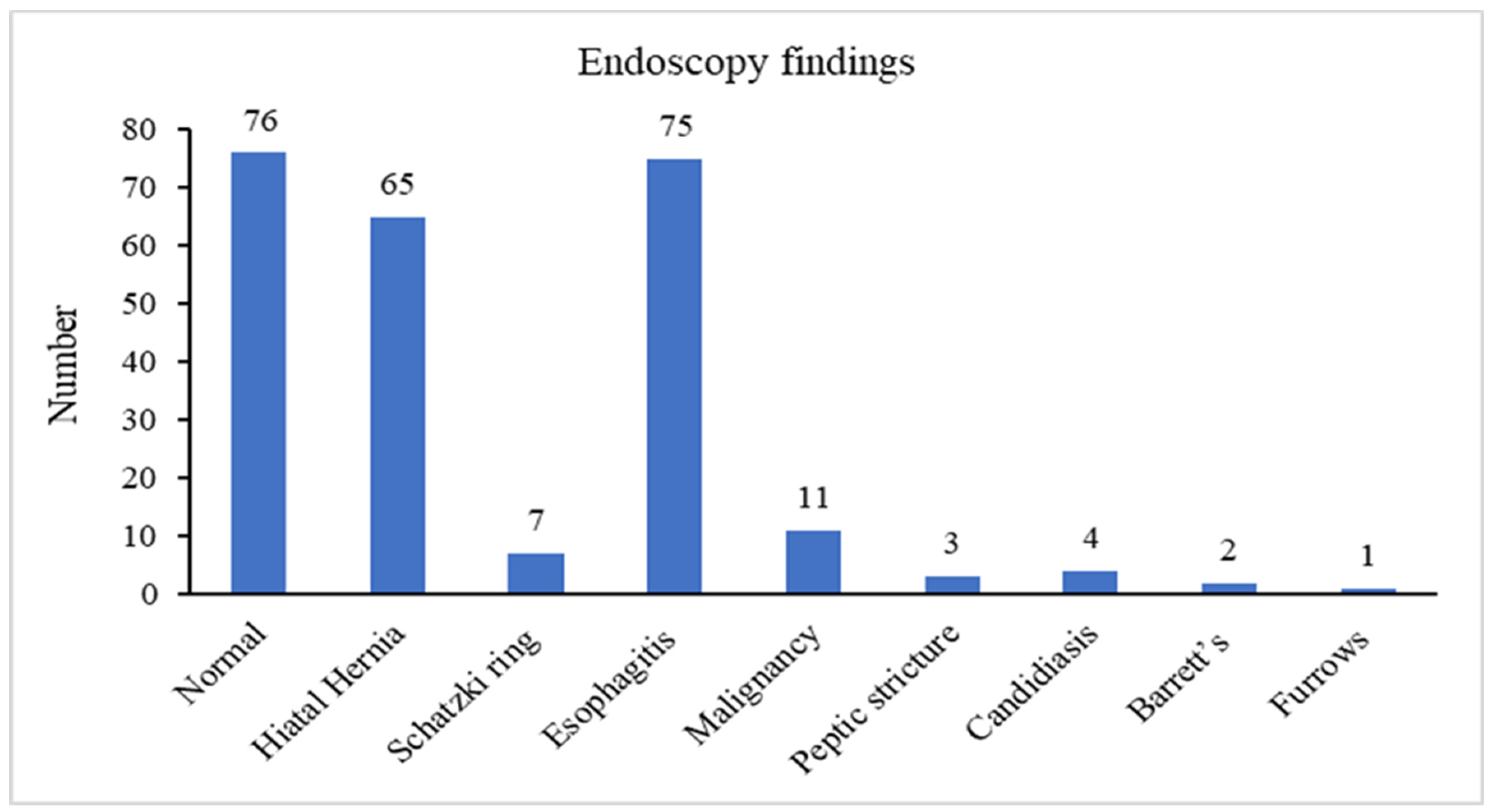
3.2. Endoscopic Findings
3.2.1. Analysis of Normal Versus Pathological Endoscopic Findings
3.2.2. Analysis of Normal versus Barrett’s Esophagus and Neoplastic Endoscopic Findings
3.2.3. Analysis of Pathological Non-Neoplastic vs. Neoplastic Endoscopic Findings
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Wilkins, T.; Gillies, R.A.; Thomas, A.M.; Wagner, P.J. The prevalence of dysphagia in primary care patients: A HamesNet Research Network study. J. Am. Board. Fam. Med. 2007, 20, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Sweis, R. Assessment and management of dysphagia and achalasia. Clin. Med. 2021, 21, 119–123. [Google Scholar] [CrossRef]
- Cook, I.J. Oropharyngeal dysphagia. Gastroenterol. Clin. N. Am. 2009, 38, 411–431. [Google Scholar] [CrossRef]
- Mari, A.; Patel, K.; Mahamid, M.; Khoury, T.; Pesce, M. Achalasia: Insights into diagnostic and therapeutic advances for an ancient disease. Rambam Maimonides Med. J. 2019, 10, e0008. [Google Scholar] [CrossRef]
- Mari, A.; Tsoukali, E.; Yaccob, A. eosinophilic esophagitis in adults: A concise overview of an evolving disease. Korean J. Fam. Med. 2020, 41, 75–83. [Google Scholar] [CrossRef]
- Straumann, A.; Spichtin, H.P.; Bucher, K.A.; Heer, P.; Simon, H.U. Eosinophilic esophagitis: Red on microscopy, white on endoscopy. Digestion 2004, 70, 109–116. [Google Scholar] [CrossRef]
- Peery, A.F.; Cao, H.; Dominik, R.; Shaheen, N.J.; Dellon, E.S. Variable reliability of endoscopic findings with white-light and narrow-band imaging for patients with suspected eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2011, 9, 475–480. [Google Scholar] [CrossRef]
- Kim, H.P.; Vance, R.B.; Shaheen, N.J.; Dellon, E.S. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 988–996.e5. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Molina-Infante, J.; Arias, A.; von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Amil Dias, J.; Bove, M.; Gonzalez-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef]
- Mari, A.; Abu Baker, F.; Mahamid, M.; Khoury, T.; Sbeit, W.; Pellicano, R. Eosinophilic esophagitis: Pitfalls and controversies in diagnosis and management. Minerva Med. 2020, 111, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.D. Dysphagia: Evaluation and treatment. Gastroenterol. Clin. N. Am. 2003, 32, 553–575. [Google Scholar] [CrossRef]
- Richter, J.E. Practical approach to the diagnosis and treatment of esophageal dysphagia. Compr. Ther. 1998, 24, 446–453. [Google Scholar] [PubMed]
- Krishnamurthy, C.; Hilden, K.; Peterson, K.A.; Mattek, N.; Adler, D.G.; Fang, J.C. Endoscopic findings in patients presenting with dysphagia: Analysis of a national endoscopy database. Dysphagia 2012, 27, 101–105. [Google Scholar] [CrossRef]
- Khan, A.N.; Said, K.; Ahmad, M.; Ali, K.; Hidayat, R.; Latif, H. Endoscopic findings in patients presenting with oesophageal dysphagia. J. Ayub Med. Coll. Abbottabad 2014, 26, 216–220. [Google Scholar] [PubMed]
- Agreus, L.; Svardsudd, K.; Talley, N.J.; Jones, M.P.; Tibblin, G. Natural history of gastroesophageal reflux disease and functional abdominal disorders: A population-based study. Am. J. Gastroenterol. 2001, 96, 2905–2914. [Google Scholar] [CrossRef]
- Velanovich, V.; Karmy-Jones, R. Measuring gastroesophageal reflux disease: Relationship between the health-related quality of life score and physiologic parameters. Am. Surg. 1998, 64, 649–653. [Google Scholar]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef]
- Philpott, H.; Sweis, R. Hiatus hernia as a cause of dysphagia. Curr. Gastroenterol. Rep. 2017, 19, 40. [Google Scholar] [CrossRef]
- Layke, J.C.; Lopez, P.P. Esophageal cancer: A review and update. Am. Fam. Physician 2006, 73, 2187–2194. [Google Scholar]
- Kim, B.J.; Cheon, W.S.; Oh, H.C.; Kim, J.W.; Park, J.D.; Kim, J.G. Prevalence and risk factor of erosive esophagitis observed in Korean National Cancer Screening Program. J. Korean Med. Sci. 2011, 26, 642–646. [Google Scholar] [CrossRef][Green Version]
- Lee, D.; Lee, K.J.; Kim, K.M.; Lim, S.K. Prevalence of asymptomatic erosive esophagitis and factors associated with symptom presentation of erosive esophagitis. Scand. J. Gastroenterol. 2013, 48, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Suzuki, H.; Kobayakawa, M.; Inadomi, J.M.; Takayama, M.; Makino, K.; Iwao, Y.; Sugino, Y.; Kanai, T. Association of visceral fat area, smoking, and alcohol consumption with reflux esophagitis and Barrett’s esophagus in Japan. PLoS ONE 2015, 10, e0133865. [Google Scholar] [CrossRef]
- Dellon, E.S.; Hirano, I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef]
- Kumar, S.; Choi, S.; Gupta, S.K. Eosinophilic esophagitis—A primer for otolaryngologists. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 373–380. [Google Scholar] [CrossRef]
| Number of Patients | 209 |
| Average age (years), mean ± SD | 57.1 ± 17.1 |
| Gender, N (%) | |
| Male | 107 (51) |
| Female | 102 (49) |
| Hyperlipidemia | 94 (44.8) |
| Hypertension | 89 (42.4) |
| Chronic renal failure | 10 (4.9) |
| Congestive heart failure | 18 (8.7) |
| Diabetes mellitus | 50 (23.9) |
| Ischemic heart disease | 36 (17) |
| Asthma | 9 (4.4) |
| Rheumatoid arthritis | 3 (1.5) |
| Ankylosing spondylitis | 1 (0.5) |
| Scleroderma | 1 (0.5) |
| Systemic lupus erythematosus | 1 (0.5) |
| Normal Gastroscopy | Any Pathological Findings | p Value | |
|---|---|---|---|
| Number | 74 | 135 | − |
| Age, years (mean) | 53.9 | 58.5 | 0.07 |
| Male gender | 33 (44.6) | 74 (54.8) | 0.08 |
| Smoking | 23 (31.1) | 60 (44.7) | 0.05 |
| Hyperlipidemia | 26 (35.6) | 68 (50) | 0.02 |
| Hypertension | 29 (39.7) | 59 (43.9) | 0.3 |
| Chronic renal failure | 3 (4.1) | 7 (5.3) | 0.3 |
| Congestive heart failure | 10 (13.5) | 8 (6.1) | 0.03 |
| Diabetes mellitus | 19 (25.7) | 31 (22.9) | 0.3 |
| Ischemic heart disease | 14 (18.9) | 21 (15.9) | 0.2 |
| Asthma | 3 (4.1) | 6 (4.5) | 0.4 |
| Rheumatoid arthritis | 2 (2.7) | 1 (0.7) | 0.1 |
| Scleroderma | 0 | 1 (0.7) | 0.2 |
| Systemic lupus erythematosus | 0 | 1 (0.7) | 0.2 |
| Use of PPI > 3-month prior endoscopy | 41 (55.9) | 84 (62.4) | 0.1 |
| Use of statin > 3-month prior endoscopy | 28 (37.5) | 41 (30.6) | 0.2 |
| Normal Gastroscopy | Neoplastic Findings | p Value | |
|---|---|---|---|
| Number | 74 | 13 | − |
| Age, years (mean) | 53.9 | 64 | 0.13 |
| Male gender, N (%) | 33 (44.6) | 6 (46.2) | 0.9 |
| Smoking, N (%) | 23 (31.1) | 6 (46.2) | 0.80 |
| Hyperlipidemia, N (%) | 26 (35.1) | 3 (23.1) | 0.39 |
| Hypertension, N (%) | 29 (39.2) | 6 (46.2) | 0.64 |
| Chronic renal failure, N (%) | 3 (4.1) | 1 (7.7) | 0.56 |
| Congestive heart failure, N (%) | 10 (13.5) | 1 (7.7) | 0.56 |
| Diabetes mellitus, N (%) | 19 (25.7) | 1 (7.7) | 0.16 |
| Ischemic heart disease, N (%) | 13 (17.6) | 3 (23.1) | 0.63 |
| Asthma, N (%) | 3 (4.1) | 1 (7.7) | 0.56 |
| Rheumatoid arthritis, N (%) | 2 (2.7) | 0 | − |
| Scleroderma, N (%) | 0 | 0 | − |
| Systemic lupus erythematosus, N (%) | 0 | 0 | − |
| Use of PPI > 3-month prior endoscopy, N (%) | 38 (51.3) | 3 (23.1) | 0.059 |
| Pathological Endoscopic Findings | |||
|---|---|---|---|
| Neoplastic Findings | Non-Neoplastic Findings | p Value | |
| Number | 13 | 121 | − |
| Age, years (mean) | 54.3 | 58.6 | 0.32 |
| Male gender, N (%) | 6 (46.2) | 68 (56.2) | 0.49 |
| Smoking, N (%) | 6 (46.2) | 53 (43.8) | 0.80 |
| Hyperlipidemia, N (%) | 3 (23.1) | 62 (51.2) | 0.053 |
| Hypertension, N (%) | 5 (38.5) | 53 (43.8) | 0.71 |
| Chronic renal failure, N (%) | 1 (7.7) | 6 (4.9) | 0.67 |
| Congestive heart failure, N (%) | 1 (7.7) | 6 (4.9) | 0.67 |
| Diabetes mellitus, N (%) | 1 (7.7) | 29 (24) | 0.18 |
| Ischemic heart disease, N (%) | 3 (23.1) | 18 (14.9) | 0.44 |
| Asthma, N (%) | 1 (7.7) | 5 (4.1) | 0.56 |
| Rheumatoid arthritis, N (%) | 0 | 1 (0.8) | − |
| Scleroderma, N (%) | 0 | 1 (0.8) | − |
| Systemic lupus erythematosus, N (%) | 0 | 1 (0.8) | − |
| Use of PPI > 3-month prior endoscopy, N (%) | 3 (23.1) | 75 (62) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mari, A.; Abu Baker, F.; Said Ahmad, H.; Omari, A.; Jawabreh, Y.; Abboud, R.; Shahin, A.; Shibli, F.; Sbeit, W.; Khoury, T. The Yield of Endoscopy and Histology in the Evaluation of Esophageal Dysphagia: Two Referral Centers’ Experiences. Medicina 2021, 57, 1336. https://doi.org/10.3390/medicina57121336
Mari A, Abu Baker F, Said Ahmad H, Omari A, Jawabreh Y, Abboud R, Shahin A, Shibli F, Sbeit W, Khoury T. The Yield of Endoscopy and Histology in the Evaluation of Esophageal Dysphagia: Two Referral Centers’ Experiences. Medicina. 2021; 57(12):1336. https://doi.org/10.3390/medicina57121336
Chicago/Turabian StyleMari, Amir, Fadi Abu Baker, Helal Said Ahmad, Ali Omari, Yazed Jawabreh, Rand Abboud, Amir Shahin, Fahmi Shibli, Wisam Sbeit, and Tawfik Khoury. 2021. "The Yield of Endoscopy and Histology in the Evaluation of Esophageal Dysphagia: Two Referral Centers’ Experiences" Medicina 57, no. 12: 1336. https://doi.org/10.3390/medicina57121336
APA StyleMari, A., Abu Baker, F., Said Ahmad, H., Omari, A., Jawabreh, Y., Abboud, R., Shahin, A., Shibli, F., Sbeit, W., & Khoury, T. (2021). The Yield of Endoscopy and Histology in the Evaluation of Esophageal Dysphagia: Two Referral Centers’ Experiences. Medicina, 57(12), 1336. https://doi.org/10.3390/medicina57121336








