Toxocariasis as a Rare Parasitic Complication of a Transthoracic Spine Surgery Procedure
Abstract
:1. Introduction
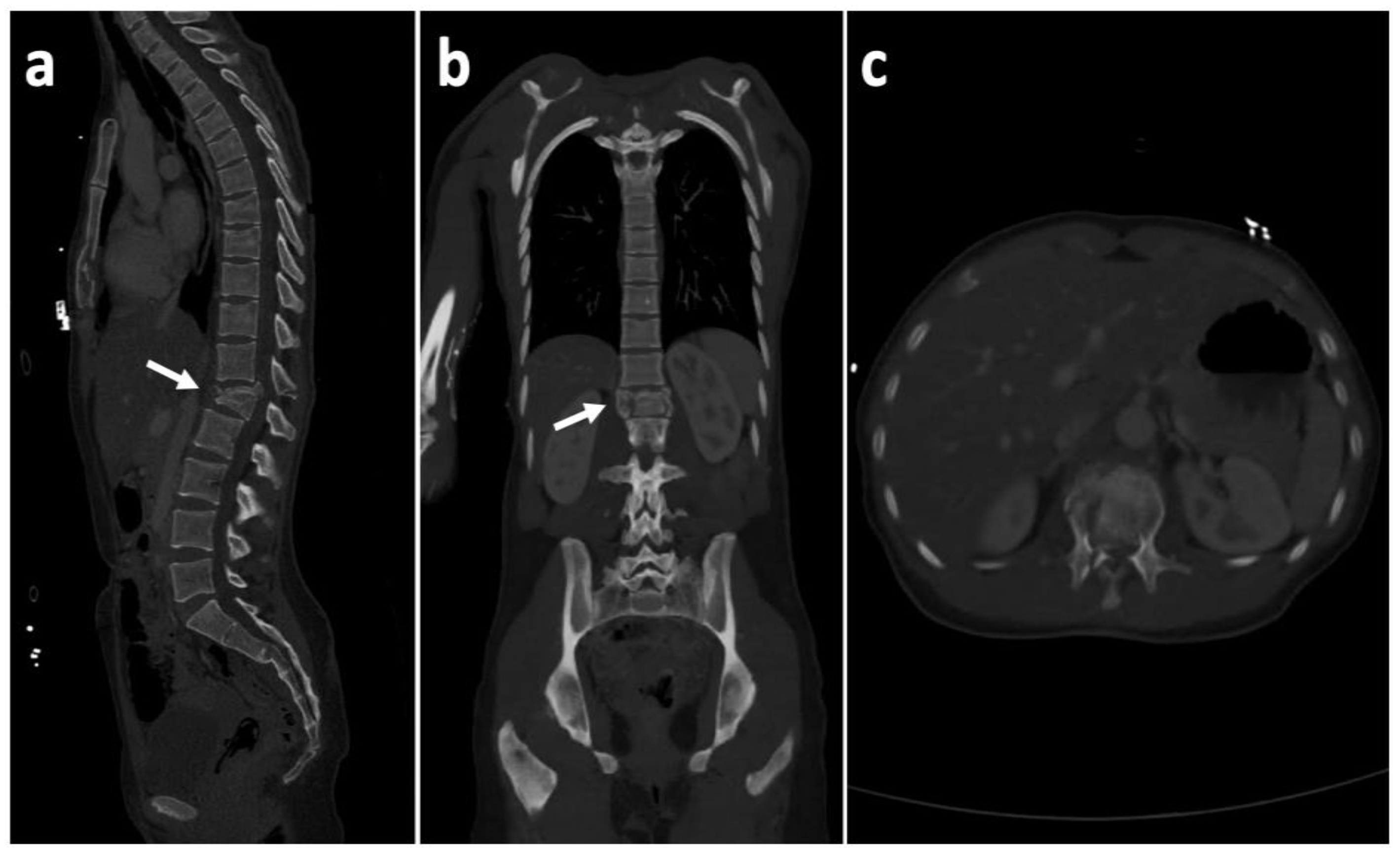
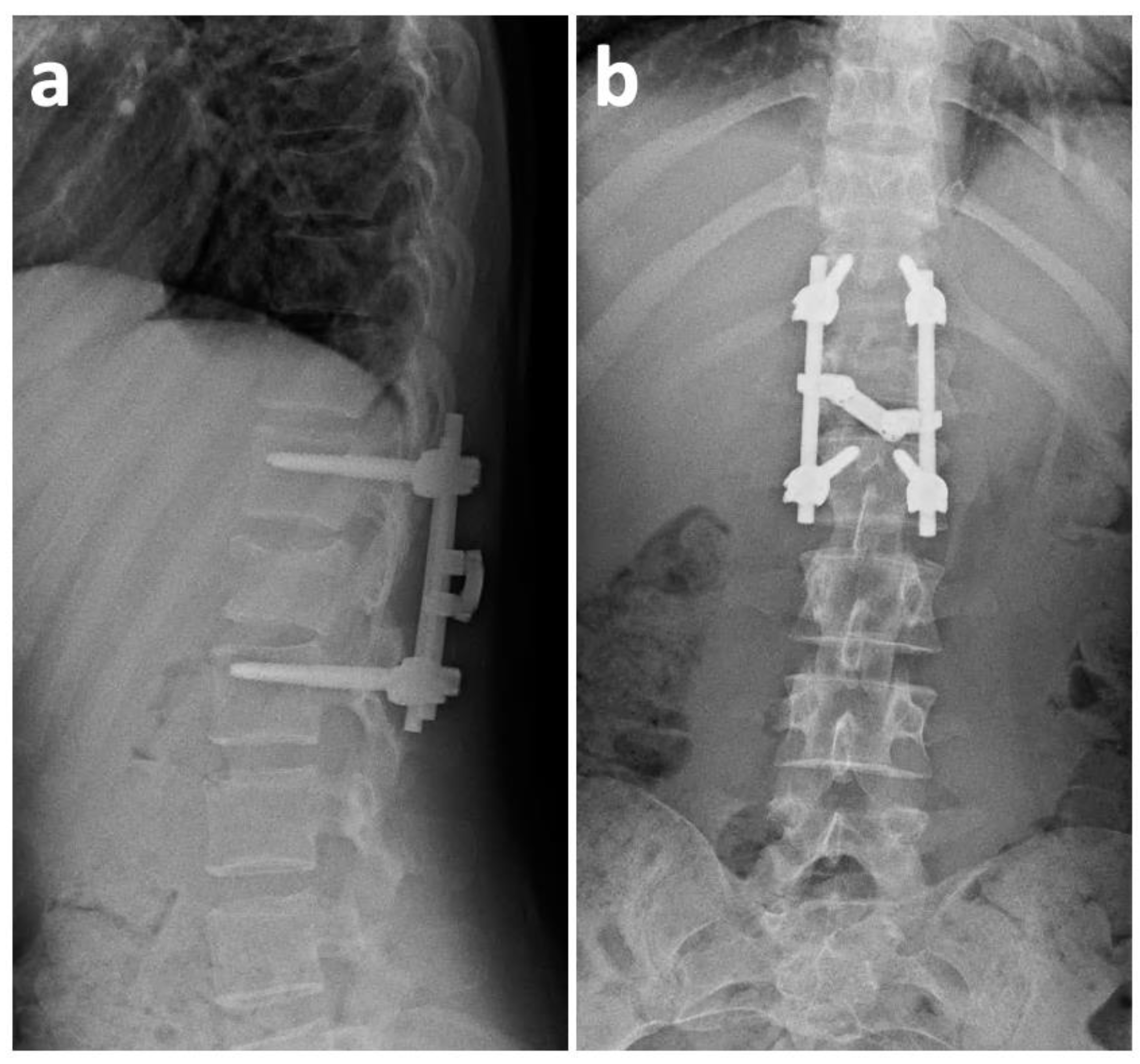
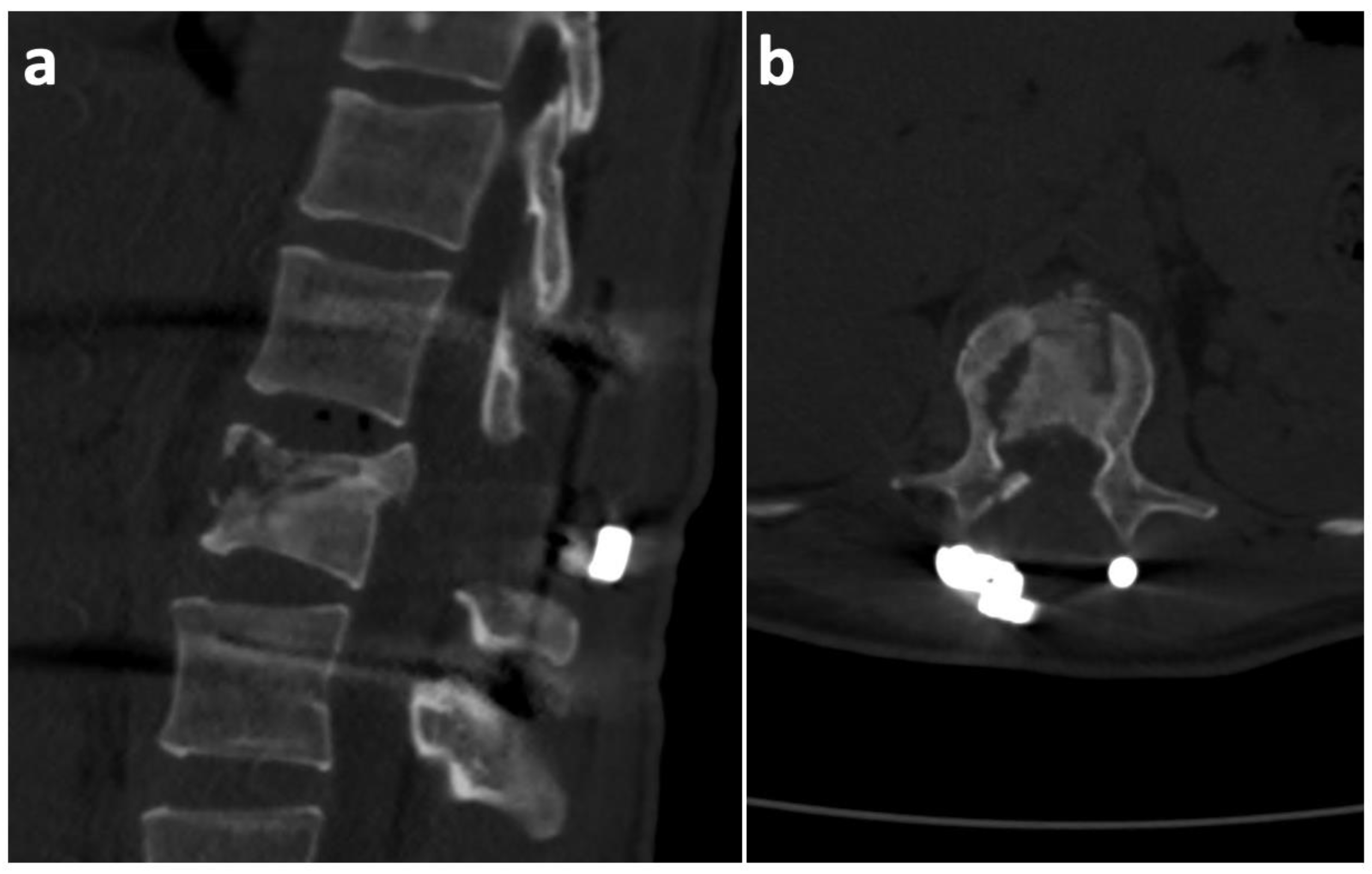
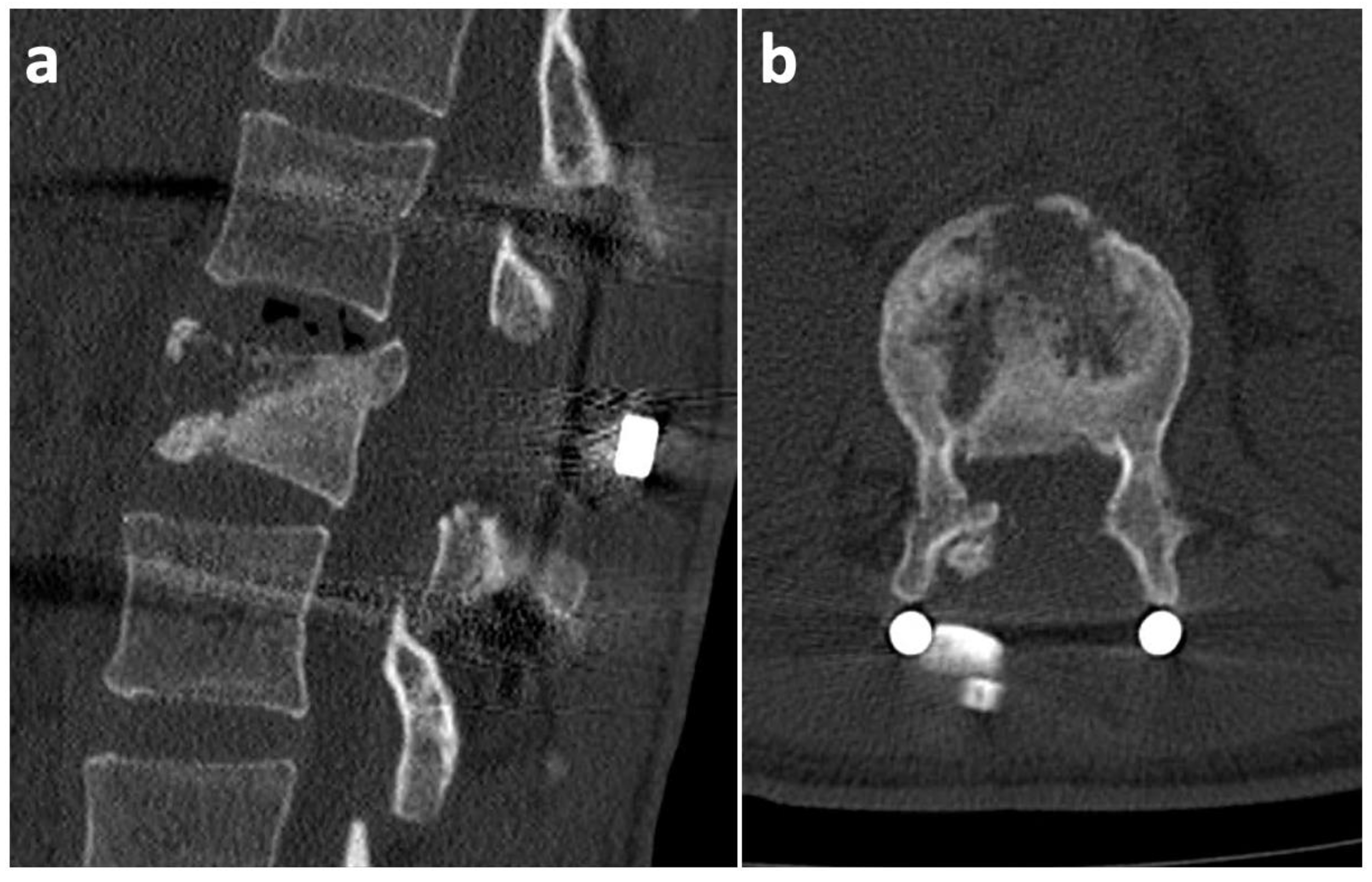
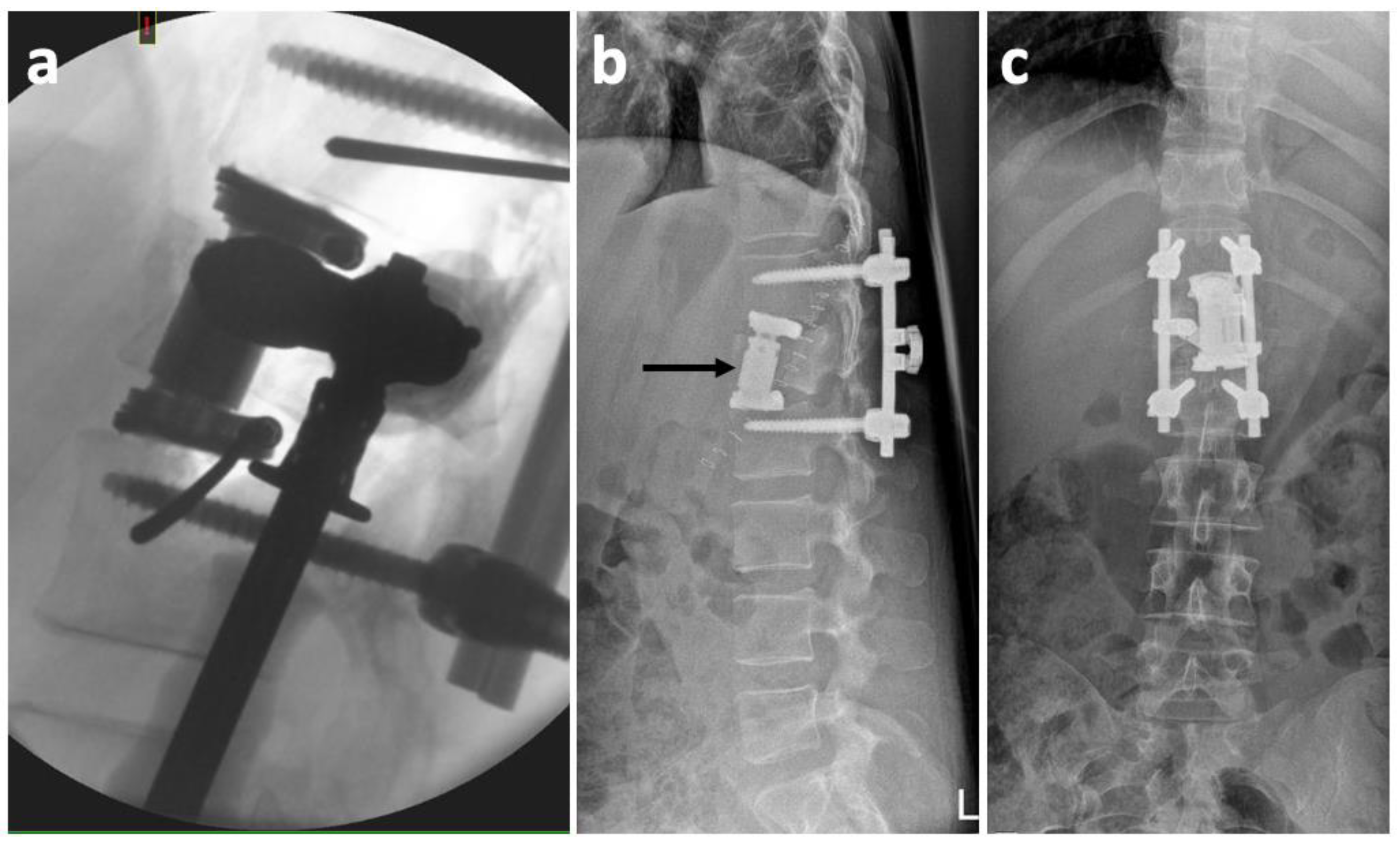
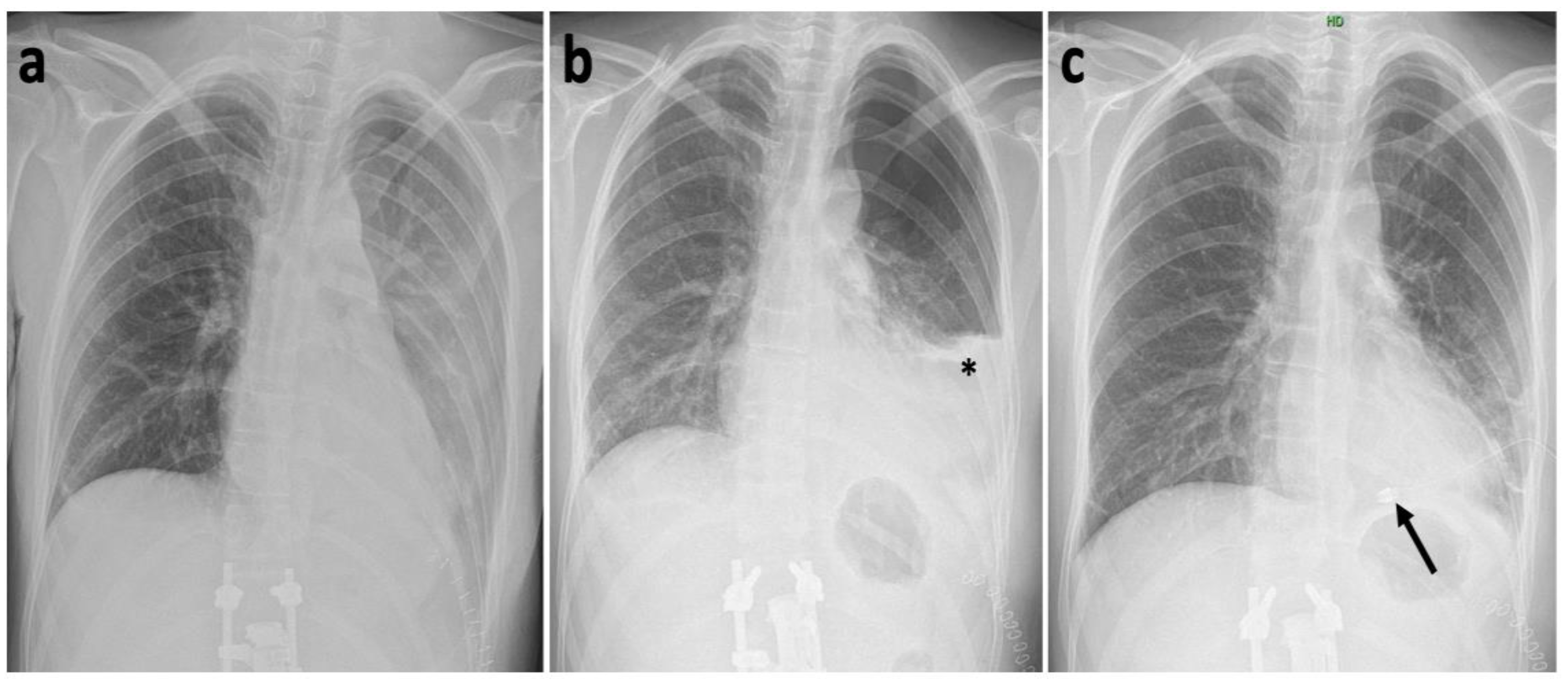
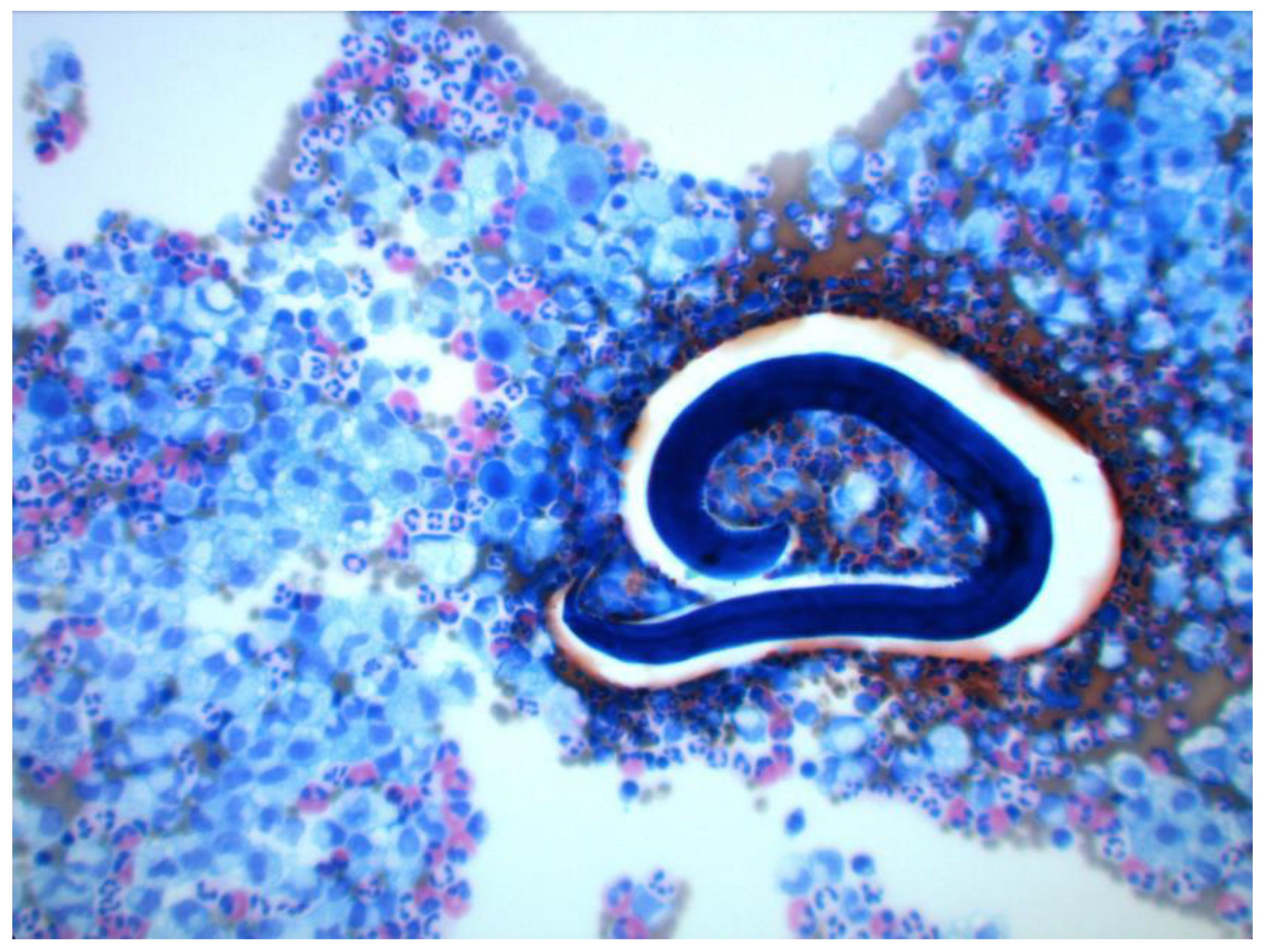
2. Case History
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chieffi, P.P.; Lescano, S.A.Z.; e Fonseca, G.R.; dos Santos, S.V. Human Toxocariasis: 2010 to 2020 Contributions from Brazilian Researchers. Res. Rep. Trop. Med. 2021, 12, 81–91. [Google Scholar] [CrossRef]
- Fialho, P.M.M.; Correa, C.R.S. A Systematic Review of Toxocariasis: A Neglected but High-Prevalence Disease in Brazil. Am. J. Trop. Med. Hyg. 2016, 94, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Skulinova, K.; Novak, J.; Kasny, M.; Kolarova, L. Seroprevalence of Larval Toxocarosis in the Czech Republic. Acta Parasitol. 2019, 65, 68–76. [Google Scholar] [CrossRef]
- Mitsuhashi, Y.; Naitou, K.; Yamauchi, S.; Naruse, H.; Matsuoka, Y.; Nakamura-Uchiyama, F.; Hiromatsu, K. A case of the myelitis due to Toxocara canis infection complicated with cervical spondylosis. No Shinkei Geka Neurol. Surg. 2006, 34, 1149–1154. [Google Scholar]
- Bohm, A.; Salmon-Rousseau, A.; Gentil, A.; Dalle, F. Myelitis and tenosynovitis attributed to toxocariasis. Jt. Bone Spine 2019, 86, 405–406. [Google Scholar] [CrossRef]
- Wilder, H.C. Nematode endophthalmitis. Trans.—Am. Acad. Ophthalmol. Otolaryngol. 1950, 55, 99–109. [Google Scholar]
- Dent, D.H.; Nichols, R.L.; Beaver, P.C.; Carrera, G.M.; Staggers, R.J. Visceral larva migrans with case report. Am. J. Pathol. 1956, 32, 777–803. [Google Scholar]
- Neafie, R.C.; Connor, D.H. Visceral larva migrans. In Pathology of Tropical and Extraordinary Diseases; Binford, C.H., Connor, D.H., Eds.; Armed Forces Institute of Pathology: Washington, DC, USA, 1976; pp. 433–436. [Google Scholar]
- Gass, J.D.M.; Braunstein, R.A. Further Observations Concerning the Diffuse Unilateral Subacute Neuroretinitis Syndrome. Arch. Ophthalmol. 1983, 101, 1689–1697. [Google Scholar] [CrossRef]
- Janecek, E.; Waindok, P.; Bankstahl, M.; Strube, C. Abnormal neurobehaviour and impaired memory function as a consequence of Toxocara canis- as well as Toxocara cati-induced neurotoxocarosis. PLoS Negl. Trop. Dis. 2017, 11, e0005594. [Google Scholar] [CrossRef]
- Magnaval, J.-F.; Glickman, L.T.; Dorchies, P.; Morassin, B. Highlights of human toxocariasis. Korean J. Parasitol. 2001, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dubná, S.; Langrová, I.; Jankovská, I.; Vadlejch, J.; Pekár, S.; Nápravník, J.; Fechtner, J. Contamination of soil with Toxocara eggs in urban (Prague) and rural areas in the Czech Republic. Vet. Parasitol. 2007, 144, 81–86. [Google Scholar] [CrossRef]
- Woodhall, D.M.; Fiore, A.E. Toxocariasis: A Review for Pediatricians. J. Pediatr. Infect. Dis. Soc. 2014, 3, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Fillaux, J.; Magnaval, J.F. Laboratory diagnosis of human toxocariasis. Vet. Parasitol. 2013, 193, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Shen, C.; Huh, S.; Sohn, W.-M.; Choi, M.-H.; Hong, S.-T. Serodiagnosis of Toxocariasis by ELISA Using Crude Antigen of Toxocara canis Larvae. Korean J. Parasitol. 2013, 51, 433–439. [Google Scholar] [CrossRef]
- Jacquier, P.; Gottstien, B.; Stingelin, Y.; Eckert, J. Immunodiagnosis of toxocarosis in humans: Evaluation of a new enzyme-linked immunosorbent assay kit. J. Clin. Microbiol. 1991, 29, 1831–1835. [Google Scholar] [CrossRef] [Green Version]
- Kelbich, P.; Hejčl, A.; Staněk, I.; Svítilová, E.; Hanuljaková, E.; Sameš, M. Principles of the cytological-energy analysis of the extravascular body fluids. Biochem. Mol. Biol. J. 2017, 3, 1–3. [Google Scholar]
- Kelbich, P.; Malý, V.; Matuchová, I.; Čegan, M.; Staněk, I.; Král, J.; Karpjuk, O.; Moudrá-Wünschová, I.; Kubalík, J.; Hanuljaková, E.; et al. Cytological-energy analysis of pleural effusions. Ann. Clin. Biochem. Int. J. Lab. Med. 2019, 56, 630–637. [Google Scholar] [CrossRef]
- Matuchova, I.; Kelbich, P.; Kubalik, J.; Hanuljakova, E.; Stanek, I.; Maly, V.; Karpjuk, O.; Krejsek, J. Cytological-energy analysis of pleural effusions with predominance of neutrophils. Ther. Adv. Respir. Dis. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Faciszewski, T.; Winter, R.B.; Lonstein, J.E.; Denis, F.; Johnson, L. The Surgical and Medical Perioperative Complications of Anterior Spinal Fusion Surgery in the Thoracic and Lumbar Spine in Adults. Spine 1995, 20, 1592–1599. [Google Scholar] [CrossRef]
- Ikard, R.W. Methods and Complications of Anterior Exposure of the Thoracic and Lumbar Spine. Arch. Surg. 2006, 141, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Lubelski, D.; Abdullah, K.G.; Steinmetz, M.P.; Masters, F.; Benzel, E.C.; Mroz, T.E.; Shin, J.H. Lateral Extracavitary, Costotransversectomy, and Transthoracic Thoracotomy Approaches to the Thoracic Spine. J. Spinal Disord. Tech. 2013, 26, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Willhuber, G.C.; Elizondo, C.; Slullitel, P. Analysis of Postoperative Complications in Spinal Surgery, Hospital Length of Stay, and Unplanned Readmission: Application of Dindo-Clavien Classification to Spine Surgery. Glob. Spine J. 2018, 9, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Strube, C.; Raulf, M.K.; Springer, A.; Waindok, P.; Auer, H. Chapter Nineteen—Seroprevalence of human toxocarosis in Europe: A review and meta-analysis. In Advances in Parasitology; Bowman, D.D., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 109, pp. 375–418. [Google Scholar]
- Rostami, A.; Riahi, S.M.; Holland, C.V.; Taghipour, A.; Khalili-Fomeshi, M.; Fakhri, Y.; Omrani, V.F.; Hotez, P.J.; Gasser, R.B. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007809. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soukup, J.; Cerny, J.; Cegan, M.; Kelbich, P.; Novotny, T. Toxocariasis as a Rare Parasitic Complication of a Transthoracic Spine Surgery Procedure. Medicina 2021, 57, 1328. https://doi.org/10.3390/medicina57121328
Soukup J, Cerny J, Cegan M, Kelbich P, Novotny T. Toxocariasis as a Rare Parasitic Complication of a Transthoracic Spine Surgery Procedure. Medicina. 2021; 57(12):1328. https://doi.org/10.3390/medicina57121328
Chicago/Turabian StyleSoukup, Jan, Jan Cerny, Martin Cegan, Petr Kelbich, and Tomas Novotny. 2021. "Toxocariasis as a Rare Parasitic Complication of a Transthoracic Spine Surgery Procedure" Medicina 57, no. 12: 1328. https://doi.org/10.3390/medicina57121328
APA StyleSoukup, J., Cerny, J., Cegan, M., Kelbich, P., & Novotny, T. (2021). Toxocariasis as a Rare Parasitic Complication of a Transthoracic Spine Surgery Procedure. Medicina, 57(12), 1328. https://doi.org/10.3390/medicina57121328






