The Immunomodulatory Function of Vitamin D, with Particular Reference to SARS-CoV-2
Abstract
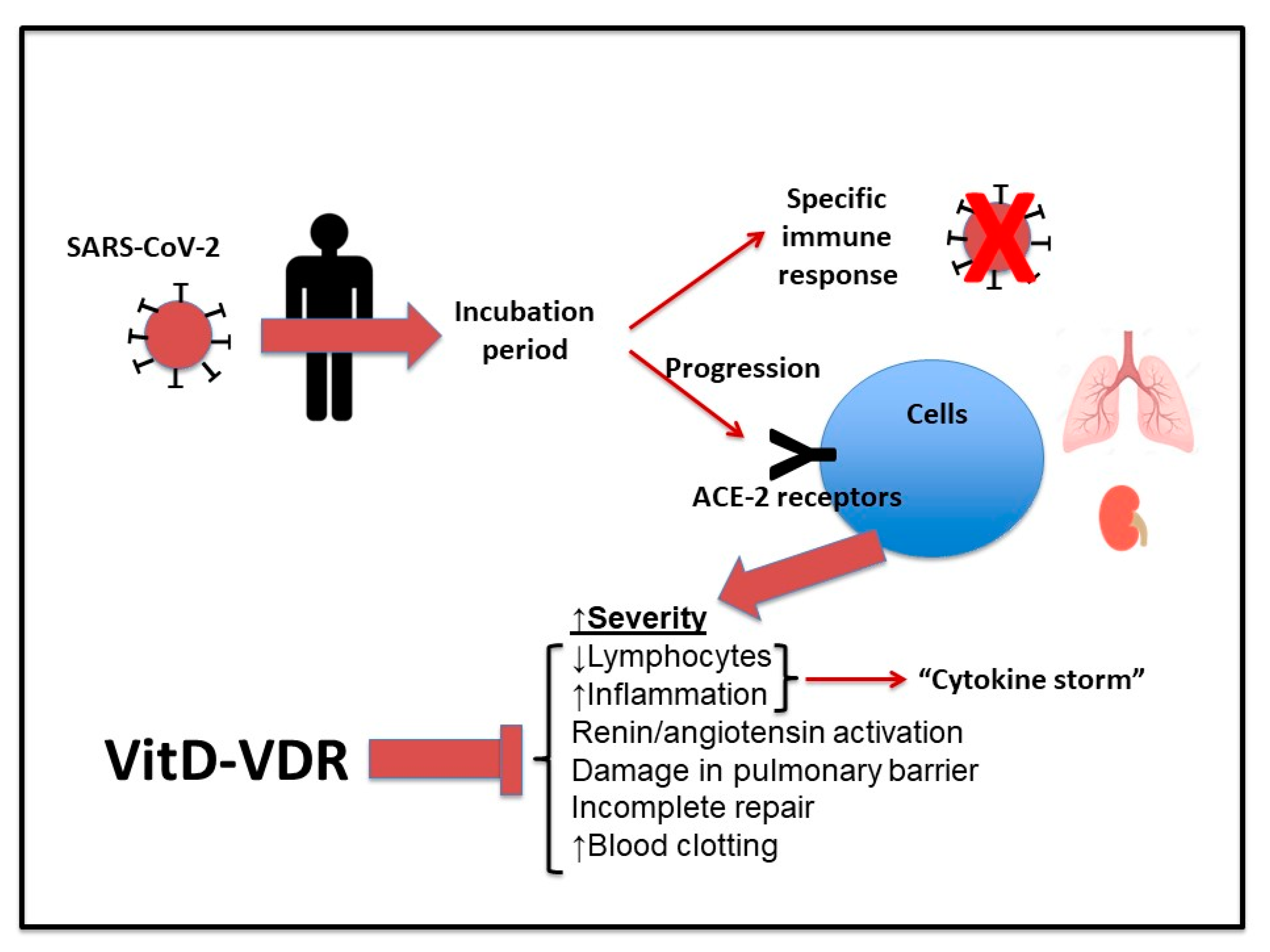
:1. Introduction
2. Immune Response against SARS-CoV-2
3. Inflammatory Response in COVID-19
4. Cytokine Response in Inflammation
5. Vitamin D
5.1. Vitamin D against Infection
5.2. Vitamin D and COVID-19
5.3. Vitamin D and the Inflammatory Response
6. Why Vitamin D Could Act as Adjuvant in the Vaccination Protocol in COVID-19
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Fox, B.A.; Urba, W.J.; Anderson, A.C.; Atkins, M.B.; Borden, E.C.; Brahmer, J.R.; Butterfield, L.H.; Cesano, A.; Chen, D.C.; et al. Insights from immuno-oncology: The Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J. Immunother. Cancer 2020, 8, e000878. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Y.; Kong, W.P.; Huang, Y.; Roberts, A.; Murphy, B.R.; Subbarao, K.; Nabel, G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004, 428, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel Coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, Y.; Siddiqui, P.; Jiang, S. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem. Biophys. Res. Commun. 2004, 325, 445–452. [Google Scholar] [CrossRef]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell. Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Forni, G.; Mantovani, A.; Commission of Accademia Nazionale dei Lincei, Rome. Covid-19 vaccines: Where we stand and challenges ahead. Cell. Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Pereira, N.L.; Ahmad, F.; Byku, M.; Cummins, N.W.; Morris, A.A.; Owens, A.; Tuteja, S.; Cresci, S. COVID-19: Understanding inter-individual variability and implications for precision medicine. Mayo. Clin. Proc. 2021, 96, 446–463. [Google Scholar] [CrossRef]
- Bartlett, B.L.; Tyring, S.K. Safety and efficacy of vaccines. Dermatol. Ther. 2009, 22, 97–103. [Google Scholar] [CrossRef]
- Lei, W.T.; Shih, P.C.; Liu, S.J.; Lin, C.-Y.; Yeh, T.-L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1175. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Mariani, J.; Tajer, C.; Antonietti, L.; Inserra, F.; Ferder, L.; Manucha, W. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Study protocol of a multicentre, randomized, controlled clinical trial (CARED TRIAL). Trials 2021, 22, 111. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [Green Version]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Bruggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Reviews 2020, 53, 38–42. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 9–14. [Google Scholar] [CrossRef]
- Dastoli, S.; Bennardo, L.; Patruno, C.; Nisticò, S.P. Are erythema multiforme and urticaria related to a better outcome of COVID-19? Dermatol. Ther. 2020, 33, e13681. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and adaptive immune memory: An evolutionary continuum in the host’s response to pathogens. Cell. Host. Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Bennardo, L.; Nisticò, S.P.; Dastoli, S.; Provenzano, E.; Napolitano, M.; Silvestri, M.; Passante, M.; Patruno, C. Erythema multiforme and COVID-19: What do we know? Medicina 2021, 57, 828. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E.; Corry, D.B. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 2020, 22, 165–167. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Altmann, D.M. Adaptive immunity to SARS-CoV-2. Oxf. Open Immunol. 2020, 1, iqaa003. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; Izquierdo-Useros, N.; Ávila-Nieto, C.; Pradenas, E.; Clotet, B.; Blanco, J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem. Biophys. Res. Commun. 2021, 538, 187–191. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.H.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Hu, W.C.; Howell, J.C.; Ozturk, T.; Benameur, K.; Bassit, L.C.; Ramonell, R.; Cashman, K.S.; Pirmohammed, S.; Roback, J.D.; Marconi, V.C.; et al. Antibody profiles according to mild or severe SARS-CoV-2 infection, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020, 26, 2974–2978. [Google Scholar] [CrossRef]
- Premkumar, L.; Segovia-Chumbez, B.; Jadi, R.; Martínez, D.R.; Raut, R.; Markmann, A.; Cornaby, C.; Bartelt, L.; Weiss, S.; Park, Y.; et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020, 5, eabc8413. [Google Scholar] [CrossRef]
- Chia, W.N.; Tan, C.W.; Foo, R.; Kang, A.E.; Peng, Y.; Sivalingam, V.; Tiu, C.; Ong, X.M.; Zhu, F.; Young, B.E.; et al. Serological differentiation between COVID-19 and SARS infections. Emerg. Microbes. Infect. 2020, 9, 1497–1505. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological roles of mast cells: Collegium Internationale Allergologicum update 2019. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Pagliuca, A.; D’Ascanio, M.; Innammorato, M.; De Vitis, C.; Mancini, R.; Giovagnoli, S.; Facchiano, F.; Sposato, B.; Anibaldi, P.; et al. Circulating vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir. Res. 2021, 22, 76. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M. Immunology; Academic Press: New York, NY, USA, 2017. [Google Scholar]
- Córdova, A.; Alvarez de Mon, M. Inmunidad en el Deporte; Gymnos: Madrid, Spain, 2001. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sánchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-cytokine therapeutics and infections. Vaccine 2003, 21, S24–S34. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Pontelli, M.C.; Castro, I.A.; Martins, R.B.; Veras, F.P.; La Serra, L.; Nascimento, D.C.; Cardoso, R.S.; Rosales, R.; Lima, T.M.; Souza, J.P.; et al. Infection of human lymphomononuclear cells by SARS-Cov-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, Y.; Zhou, Z.; Ye, F.; Huang, B.; Huang, Y.; Ma, J.; Zuo, Q.; Tan, X.; Xie, J.; et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: A randomized, open-label, parallel-group trial. Int. J. Infect. Dis. 2020, 99, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunologic features in severe and moderate forms of Coronavirus disease. J. Clin. Investig. 2020, 130, 137244. [Google Scholar] [CrossRef] [Green Version]
- Lagunas-Rangel, F.A.; Chávez-Valencia, V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020, 92, 1789–1790. [Google Scholar] [CrossRef]
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Benvenuto, D.; Giovanetti, M.; Angeletti, S.; Ciccozzi, M.; Pascarella, S. Sars-CoV-2 envelope and membrane proteins: Structural differences linked to virus characteristics? BioMed Res. Int. 2020, 2020, 4389089. [Google Scholar] [CrossRef] [PubMed]
- Astuti, I. Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.; Walsh, N. Exercise Immunology; Routledge (Taylor-Francis Group): London, UK, 2013. [Google Scholar]
- Steenblock, C.; Todorov, V.; Kanczkowski, W.; Eisenhofer, G.; Schedl, A.; Wong, M.L.; Licinio, J.; Bauer, M.; Young, A.H.; Gainetdinov, R.R.; et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol. Psychiatry 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Kempuraj, D.; Mentor, S.; Thangavel, R.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Dubova, I.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer’s disease. Front. Cell. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minihan, E.; Gavin, B.; Kelly, B.D.; McNicholas, F. Covid-19, mental health and psychological first aid. Ir. J. Psychol. Med. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Regueiro, J.; López, C.; González, S.; Martínez, E. Inmunología. Biologíay Patología del Sistema Inmunitario; Editorial Medica Panamericana: Madrid, Spain, 2010. [Google Scholar]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regu-latory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the regulation of inflammatory responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A. Interferon gamma: Biology and role in pathogenesis. Adv. Immunol. 1996, 62, 61–130. [Google Scholar] [CrossRef]
- Viti, A.; Muscettola, M.; Paulesu, L.; Bocci, V.; Almi, A. Effect of exercise on plasma interferon levels. J. Appl. Physiol. 1985, 59, 426–428. [Google Scholar] [CrossRef]
- Ruggiero, V.; Tavernier, J.; Fiers, W.; Baglioni, C. Induction of synthesis of tumor necrosis factor alpha by interferon gamma. J. Immunol. 1986, 136, 2445–2450. [Google Scholar]
- Lowenstein, C.J.; Snyder, S.H. Nitric oxide, a novel biologic messenger. Cell 1992, 70, 705–707. [Google Scholar] [CrossRef]
- Córdova, A. Fisiología Dinámica; Elsevier: Barcelona, Spain, 2003. [Google Scholar]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and innate and adaptive immunity. Vitam Horm 2011, 86, 23–62. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Soruri, A.; Gieseler, R.K.; Peters, J.H. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993, 38, 535540. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 2. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid. Biochem. Mol. Biol. 2020, 202, 105719. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public. Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Balla, M.; Merugu, G.P.; Konala, V.M.; Sangani, V.; Kondakindi, H.; Pokal, M.; Gayam, V.; Adapa, S.; Naramala, S.; Malayala, S.V. Back to basics: Review on vitamin D and respiratory viral infections including COVID-19. J. Community Hosp. Intern. Med. Perspect. 2020, 29, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.; Hou, A.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acutelung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Sadarangani, S.P.; Whitaker, J.A.; Poland, G.A. “Let there be light”: The role of vitamin D in the immune response to vaccines. Expert. Rev. Vaccines 2015, 14, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Kallas, M.; Green, F.; Hewison, M.; White, C.; Kline, G. Rare causes of calcitriol-mediated hypercalcemia: A case report and literature review. J. Clin. Endocrinol. Metab. 2010, 95, 3111–3117. [Google Scholar] [CrossRef] [Green Version]
- Hewison, M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010, 321, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.; Manansala, J.S.; Abdulrahman, H.A.; Nasrallah, G.K.; Smatti, M.K.; Younes, N.; Althani, A.A.; Yassine, H.M. Immune modulatory effects of vitamin D on viral infections. Nutrients 2020, 12, 2879. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef]
- Kast, J.I.; McFarlane, A.J.; Głobińska, A.; Sokolowska, M.; Wawrzyniak, P.; Sanak, M.; Schwarze, J.; Akdis, C.A.; Wanke, K. Respiratory syncytial virus infection influences tight junction integrity. Clin. Exp. Immunol. 2017, 190, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Curiel, M.; Cabello, A.; Arboiro-Pinel, R.; Mansur, J.L.; Heili-Frades, S.; Mahillo-Fernandez, I.; Herrero-González, A.; Andrade-Poveda, M. The relationship between 25(OH) vitamin D levels and COVID-19 onset and disease course in Spanish patients. J. Steroid. Biochem. Mol. Biol. 2021, 212, 105928. [Google Scholar] [CrossRef]
- Malek Mahdavi, A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev. Med. Virol. 2020, 30, e2119. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell. Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Kohlmeier, M. Avoidance of vitamin D deficiency to slow the COVID-19 pandemic. BMJ Nutr. Prev. Health 2020, 3, 67–73. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y.C. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013, 27, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.X.; Yang, J.X.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parekh, D.; Gao-Smith, F.; Thickett, D.R.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 177, 113955. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, J.M.; Herencia, C.; De Oca, A.M.; Muñoz-Castañeda, J.R.; Rodríguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Peralbo-Santaella, E.; Camargo, A.; Canalejo, A.; Rodriguez, M.; et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016, 30, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. Vitamin D: Newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol. Metab. 2010, 21, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Quesada, J.A.; López-Pineda, A.; Gil-Guillén, V.F.; Arriero-Marín, J.M.; Gutiérrez, F.; Carratala-Munuera, C. Período de incubación de la COVID-19: Revisión sistemática y metaanálisis [Incubation period of COVID-19: A systematic review and meta-analysis]. Rev. Clin. Esp. 2021, 221, 109–117. [Google Scholar] [CrossRef] [PubMed]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolo, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25- hydroxyvitamin D (25[OH]D) levels in patients hospitalised with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020, 93, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020, 5, e137799. [Google Scholar] [CrossRef]
- Yang, A.P.; Li, H.M.; Tao, W.Q.; Yang, X.J.; Wang, M.; Yang, W.J.; Liu, J.P. Infection with SARS-CoV-2 causes abnormal laboratory results of multiple organs in patients. Aging 2020, 12, 10059–10069. [Google Scholar] [CrossRef]
- Álvarez-Mon, M.; Ortega, M.A.; Gasullaet, O.; Fortuny-Profitós, J.; Mazaira-Font, F.A.; Saurina, P.; Monserrat, J.; Plana, M.N.; Troncoso, D.; Moreno, J.S.; et al. A predictive model and risk factors for case fatality of COVID-19. J. Pers. Med. 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports health benefits of vitamin D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef] [Green Version]
- De la Puente Yagüe, M.; Collado, L.; Ciudad-Cabañas, M.J.; Cuadrado-Cenzual, M.A. Role of vitamin D in athletes and their performance: Current concepts and new trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef] [Green Version]
- Córdova, A.; Monserrat, J.; Villa, G.; Reyes, E.; Soto, M.A. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J. Sports Sci. 2006, 24, 565–573. [Google Scholar] [CrossRef]
- Córdova, A.; Sureda, A.; Pons, A.; Alvarez-Mon, M. Modulation of TNF-α, TNF-α receptors and IL-6 after treatment with AM3 in professional cyclists. J. Sports Med. Phys. Fitness 2015, 55, 345–351. [Google Scholar] [PubMed]
- Steinacker, J.M.; Lormes, W.; Reissnecker, S.; Liu, Y. New aspects of the hormone and cytokine response to training. Eur. J. Appl. Physiol. 2004, 91, 382–391. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco-Calvo, J.; Córdova-Martínez, A.; Caballero-García, A.; Fernandez-Lázaro, C.I. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: A systematic review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera-Quintanar, L.; Funes, L.; Herranz-López, M.; Martínez-Peinado, P.; Pascual-García, S.; Sempere, J.M.; Boix-Castejón, M.; Córdova, A.; Pons, A.; Micol, V.; et al. Antioxidant supplementation modulates neutrophil inflammatory response to exercise-induced stress. Antioxidants 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Tauler, P.; Maestre, I.; Aguiló, A.; Córdova, A.; Tur, J.A.; Roche, E.; Pons, A. Intense physical ac-tivity enhances neutrophil antioxidant gene expression. Immunocytochemistry evidence for catalase secretion. Free Rad. Res. 2007, 41, 874–883. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, J.J. The role of NF-kappaB in the regulation of cell stress responses. Int. Immunopharmacol. 2002, 2, 1509–1520. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [Green Version]
- Kanikarla-Marie, P.; Jain, S.K. 1,25(OH)2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregula-tion of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis). J. Steroid. Biochem. Mol. Biol. 2016, 159, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Kammüler, M.E. Recombinant human interleukin-6: Safety issues of a pleiotropic growth factor. Toxicology 1995, 105, 91–107. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, P.M.F.; Allain, T.J. Muscle strength and vitamin D in older people. Gerontology 2006, 52, 335–338. [Google Scholar] [CrossRef]
- Dalakas, M.C. Immunotherapy of myositis: Issues, concerns and future prospects. Nature. Rev. Rheumatol. 2010, 6, 129–137. [Google Scholar] [CrossRef]
- Marantes, I.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J., 3rd; Amin, S. Is vitamin D a determinant of muscle mass and strength? J. Bone Miner. Res. 2011, 26, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord. 2012, 13, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, B. Vitamin D and athletic performance: The potential role of muscle. Asian J. Sports Med. 2011, 2, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceglia, L. Vitamin D and skeletal muscle tissue and function. Mol. Aspects Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Hu, X.; Greenfield, H.; Fraser, D.R. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J. Nutr. 2009, 139, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [Green Version]
- Salminen, M.; Saaristo, P.; Salonoja, M.; Vaapio, S.; Vahlberg, T.; Lamberg-Allardt, C.; Aarnio, P.; Kivelä, S.L. Vitamin D status and physical function in older Finnish people: A one-year follow-up study. Arch. Gerontol. Geriatr. 2015, 61, 419–424. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1alpha,25-Dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [Green Version]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, C.M.; Bojunga, J.; Ramos-López, E.; von Wagner, M.; Hassler, A.; Vermehren, J.; Herrmann, E.; Badenhoop, K.; Zeuzem, S.; Sarrazin, C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 2011, 54, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Greiller, C.L.; Mein, C.A.; Hoti, M.; Bakhsoliani, E.; Telcian, A.G.; Simpson, A.; Barnes, N.C.; Curtin, J.A.; Custovic, A.; et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 2018, 120, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Germolec, D.R.; Frawley, R.P.; Evans, E. Markers of inflammation. Methods Mol. Biol. 2010, 598, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.; Lam, C.W.; Wu, A.K.; Ip, W.K.; Lee, N.L.; Chan, I.H.; Lit, L.C.; Hui, D.S.; Chan, M.H.; Chung, S.S.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, S.; Fu, Y.; Gao, Z.; Long, H.; Ren, H.-W.; Zuo, Y.; Wang, J.; Li, H.; Xu, Q.B.; et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin. Infect. Dis. 2020, 71, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.Y.; Visic, D.; Daynes, R.A. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: Alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine 2000, 18, 2753–2767. [Google Scholar] [CrossRef]
- Van der Stede, Y.; Cox, E.; Van den Broeck, W.; Goddeeris, B.M. Enhanced induction of the IgA response in pigs by calcitriol after intramuscular immunization. Vaccine 2001, 19, 1870–1878. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Stabel, J.R.; Goff, J.P. 1,25-dihydroxyvitamin D3 enhances milk antibody titers to Escherichia coli J5 vaccine. J Dairy Sci. 1999, 82, 1904–1909. [Google Scholar] [CrossRef]
- Kriesel, J.D.; Spruance, J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 1999, 17, 1883–1888. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Weeratna, R.D. Novel adjuvant systems. Curr. Drug Targets Infect. Disord. 2001, 1, 263–271. [Google Scholar] [CrossRef]
- Lalor, M.K.; Floyd, S.; Gorak-Stolinska, P.; Weir, R.E.; Blitz, R.; Branson, K.; Fine, P.E.; Dockrell, H.M. BCG vaccination: A role for vitamin D? PLoS ONE 2011, 6, e16709. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.P.; Dragunsky, E.M.; Chumakov, K.M. 1,25-dihydroxyvitamin D3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J. Infect. Dis. 2006, 193, 598–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daynes, R.A.; Enioutina, E.Y.; Butler, S.; Mu, H.H.; McGee, Z.A.; Araneo, B.A. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect. Immun. 1996, 64, 1100–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Benskin, L.L. A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front. Public Health 2020, 8, 513. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-García, A.; Noriega, D.C.; Bello, H.J.; Roche, E.; Córdova-Martínez, A. The Immunomodulatory Function of Vitamin D, with Particular Reference to SARS-CoV-2. Medicina 2021, 57, 1321. https://doi.org/10.3390/medicina57121321
Caballero-García A, Noriega DC, Bello HJ, Roche E, Córdova-Martínez A. The Immunomodulatory Function of Vitamin D, with Particular Reference to SARS-CoV-2. Medicina. 2021; 57(12):1321. https://doi.org/10.3390/medicina57121321
Chicago/Turabian StyleCaballero-García, Alberto, David C. Noriega, Hugo J. Bello, Enrique Roche, and Alfredo Córdova-Martínez. 2021. "The Immunomodulatory Function of Vitamin D, with Particular Reference to SARS-CoV-2" Medicina 57, no. 12: 1321. https://doi.org/10.3390/medicina57121321
APA StyleCaballero-García, A., Noriega, D. C., Bello, H. J., Roche, E., & Córdova-Martínez, A. (2021). The Immunomodulatory Function of Vitamin D, with Particular Reference to SARS-CoV-2. Medicina, 57(12), 1321. https://doi.org/10.3390/medicina57121321








