Parasite-Induced Th2 Polarization—An Unusual Cause of Paediatric Hepatic Abscess
Abstract
1. Introduction
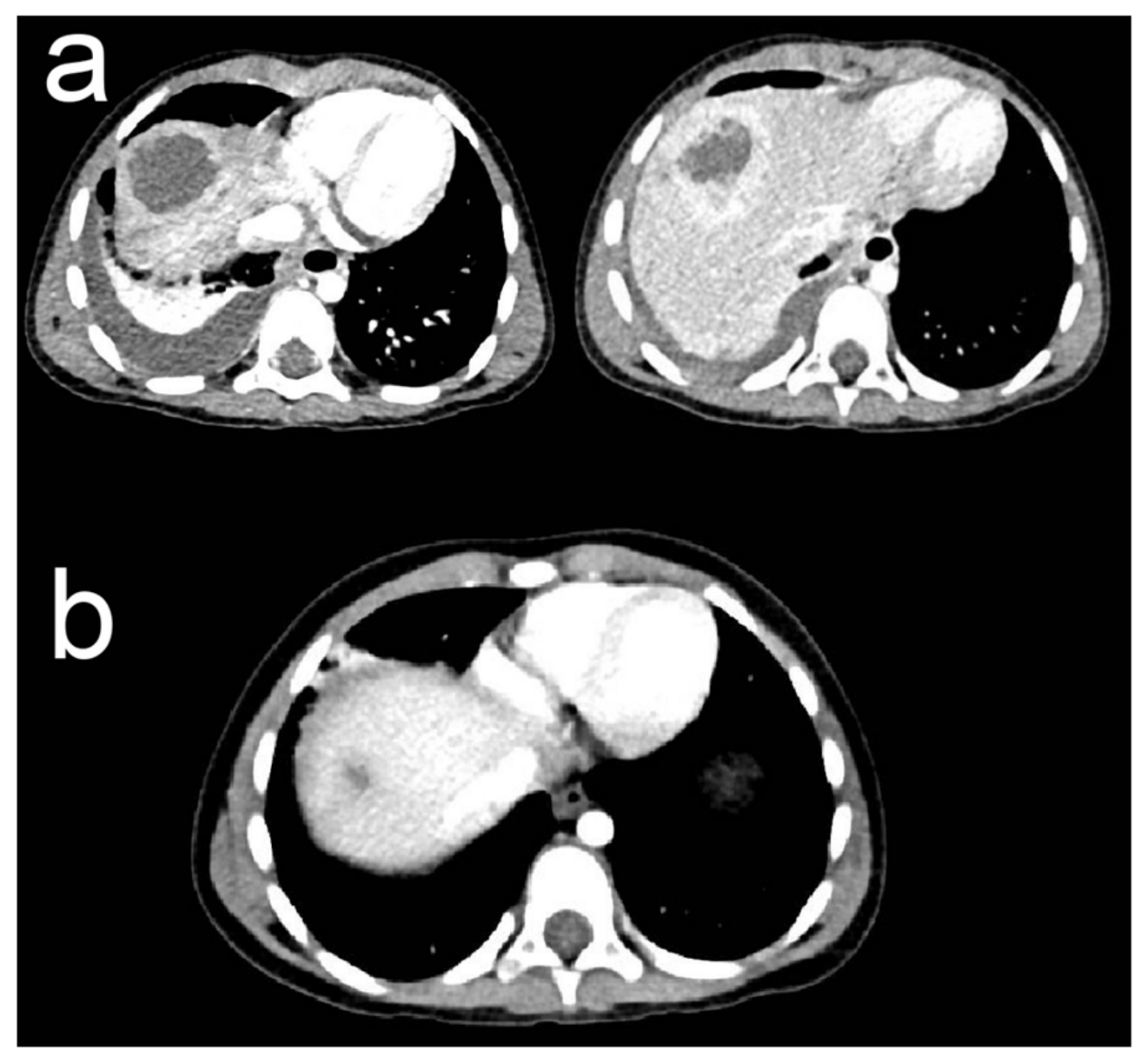
2. Case Presentation
3. Discussion
3.1. Hepatic Abscess
3.2. Case Particularities
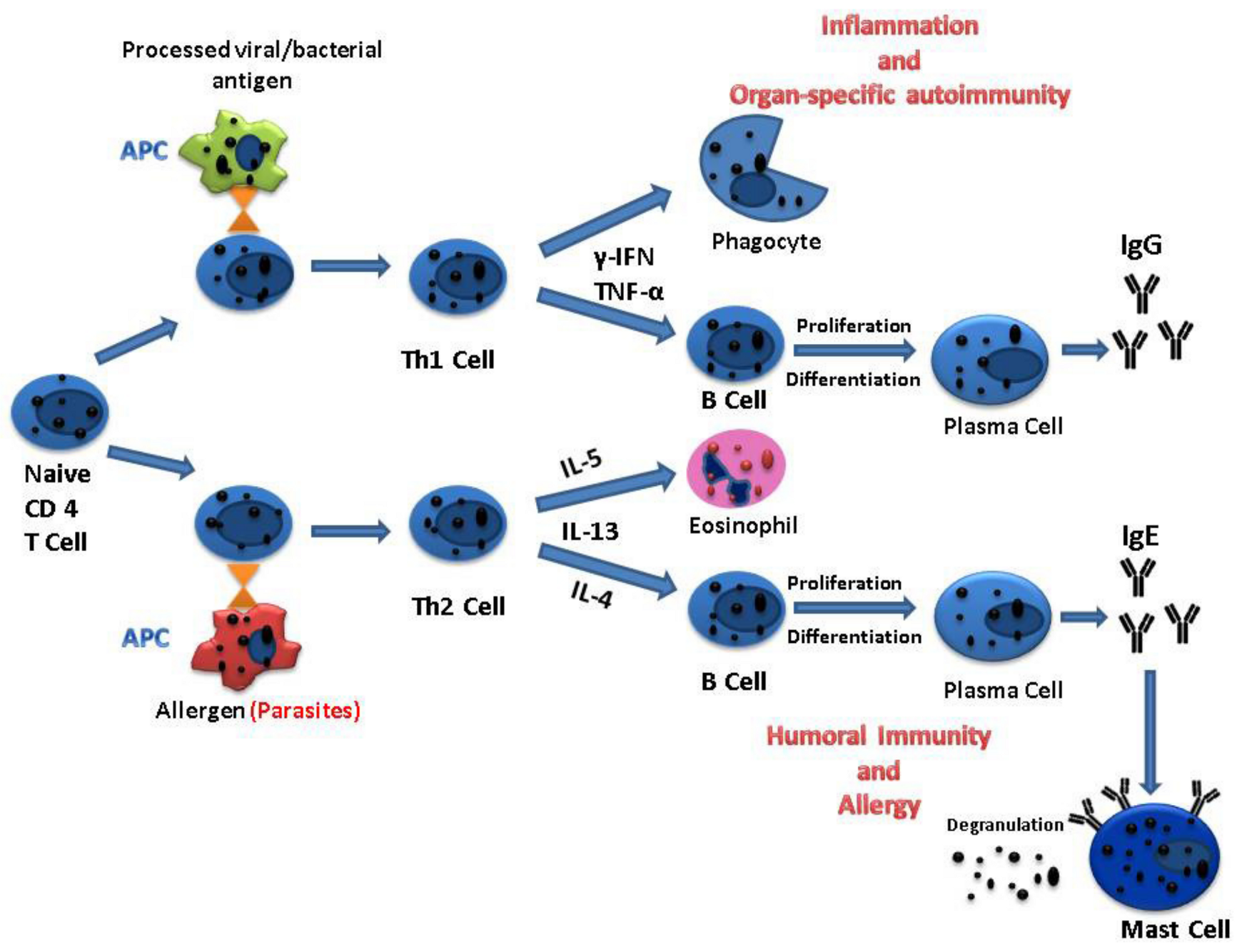
3.3. Parasitic Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanni, L.A.; Lopez, P.B.; Porto, S.O. Solitary Pyogenic Liver Abscess in Children. Am. J. Dis. Child. 1978, 132, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Kouassi-Dria, A.S.; Moh, N.E.; Aké, Y.L.; Midekor-Gonebo, K.; Keita, B.; Bonny-Obro, R.; Aguehounde, C. Liver Abscess of Children in Côte-d’Ivoire: Retrospective Analysis of a Series of 30 Cases. Ann. Pediatr. Surg. 2018, 14, 51–55. [Google Scholar] [CrossRef]
- Pineiro-Carrero, V.M.; Andres, J.M. Morbidity and Mortality in Children with Pyogenic Liver Abscess. Am. J. Dis. Child. 1960 1989, 143, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srinivasan, S.; Sharma, A.K. Pyogenic Liver Abscess in Children--South Indian Experiences. J. Pediatr. Surg. 1998, 33, 417–421. [Google Scholar] [CrossRef]
- Yeh, P.-J.; Chen, C.-C.; Lai, M.-W.; Yeh, H.-Y.; Chao, H.-C. Pediatric Liver Abscess: Trends in the Incidence, Etiology, and Outcomes Based on 20-Years of Experience at a Tertiary Center. Front. Pediatr. 2020, 8, 111. [Google Scholar] [CrossRef]
- Mishra, K.; Basu, S.; Roychoudhury, S.; Kumar, P. Liver Abscess in Children: An Overview. World J. Pediatr. WJP 2010, 6, 210–216. [Google Scholar] [CrossRef]
- Seidel, J. Diagnosis and Management of Amebic Liver Abscess in Children. West. J. Med. 1984, 140, 932–933. [Google Scholar]
- Galgamuwa, L.S.; Iddawela, D.; Dharmaratne, S.D. Prevalence and Intensity of Ascaris Lumbricoides Infections in Relation to Undernutrition among Children in a Tea Plantation Community, Sri Lanka: A Cross-Sectional Study. BMC Pediatr. 2018, 18, 13. [Google Scholar] [CrossRef]
- Nishiura, H.; Imai, H.; Nakao, H.; Tsukino, H.; Changazi, M.A.; Hussain, G.A.; Kuroda, Y.; Katoh, T. Ascaris Lumbricoides among Children in Rural Communities in the Northern Area, Pakistan: Prevalence, Intensity, and Associated Socio-Cultural and Behavioral Risk Factors. Acta Trop. 2002, 83, 223–231. [Google Scholar] [CrossRef]
- Blumenthal, D.S.; Schultz, M.G. Incidence of Intestinal Obstruction in Children Infected with Ascaris Lumbricoides. Am. J. Trop. Med. Hyg. 1975, 24, 801–805. [Google Scholar] [CrossRef]
- Stephenson, L.S.; Latham, M.C.; Kinoti, S.N.; Kurz, K.M.; Brigham, H. Improvements in Physical Fitness of Kenyan Schoolboys Infected with Hookworm, Trichuris Trichiura and Ascaris Lumbricoides Following a Single Dose of Albendazole. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 277–282. [Google Scholar] [CrossRef]
- Nokes, C.; Bundy, D.A. Does Helminth Infection Affect Mental Processing and Educational Achievement? Parasitol. Today 1994, 10, 14–18. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Hon, K.L. Human Ascariasis: An Updated Review. Recent Pat. Inflamm. Allergy Drug Discov. 2020, 14, 133–145. [Google Scholar] [CrossRef]
- Hendricks, M.K.; Moore, S.W.; Millar, A.J.W. Epidemiological Aspects of Liver Abscesses in Children in the Western Cape Province of South Africa. J. Trop. Pediatr. 1997, 43, 103–105. [Google Scholar] [CrossRef]
- Wani, N.A.; Shah, O.J.; Naqash, S.H. Postoperative Biliary Ascariasis: Presentation and Management--Experience. World J. Surg. 2000, 24, 1143–1145. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the Host Immune System by Helminth Parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef]
- Kassaw, M.W.; Abebe, A.M.; Tlaye, K.G.; Zemariam, A.B.; Abate, B.B. Prevalence and Risk Factors of Intestinal Parasitic Infestations among Preschool Children in Sekota Town, Waghimra Zone, Ethiopia. BMC Pediatr. 2019, 19, 437. [Google Scholar] [CrossRef]
- Akhondi, H.; Sabih, D.E. Liver Abscess. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sharma, M.P.; Kumar, A. Liver Abscess in Children. Indian J. Pediatr. 2006, 73, 813–817. [Google Scholar] [CrossRef]
- Grabau, M.; Pandya, S.; Nanjappa, S.; Shenoy, R.; Aslam, S.; Greene, J. Liver Abscess in Patients With Leukemia and Prolonged Neutropenia. Infect. Dis. Clin. Pract. 2017, 25, 193–198. [Google Scholar] [CrossRef]
- Bari, S.; Sheikh, K.A.; Malik, A.A.; Wani, R.A.; Naqash, S.H. Percutaneous Aspiration versus Open Drainage of Liver Abscess in Children. Pediatr. Surg. Int. 2007, 23, 69–74. [Google Scholar] [CrossRef]
- Medina Andrade, L.; Aguilar, L.; Duarte, E.; Rodríguez Rodríguez, C.; Mendoza, A. Liver Abscess Secondary to Ascaris Lumbricoides: Case Report. Arch. Clin. Gastroenterol. 2016, 2, 80–82. [Google Scholar] [CrossRef]
- Javid, G.; Wani, N.A.; Gulzar, G.M.; Khan, B.A.; Shah, A.H.; Shah, O.J.; Khan, M. Ascaris-Induced Liver Abscess. World J. Surg. 1999, 23, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, A.E.; López, M.C.; Ricaurte, O.; Castillo, B.; Murcia, M.I.; Nicholls, R.S.; Duque, S.; Orozco, L.C. Liver Abscess Caused by Ascaris Lumbricoides: Case Report. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Espinoza, J.A.; Vásquez-Ciriaco, S.; Doña-Jaimes, R.; Aragon-Soto, R.; Velazco-Budar, C.; López-Martínez, E. Parasitosis in the Bile Duct, Report of 3 Cases and Literature Review. Rev. Médica Hosp. Gen. México 2018, 81, 18–23. [Google Scholar] [CrossRef]
- Lublin, M.; Bartlett, D.L.; Danforth, D.N.; Kauffman, H.; Gallin, J.I.; Malech, H.L.; Shawker, T.; Choyke, P.; Kleiner, D.E.; Schwartzentruber, D.J.; et al. Hepatic Abscess in Patients with Chronic Granulomatous Disease. Ann. Surg. 2002, 235, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Leto, T.L.; Gallin, J.I.; Malech, H.L.; Holland, S.M. Genetic, Biochemical, and Clinical Features of Chronic Granulomatous Disease. Medicine 2000, 79, 170–200. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Akers, J.Y.; Prakash, V.; Oliver, T.I. Amebic Liver Abscess. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Halliez, M.C.M.; Buret, A.G. Extra-Intestinal and Long Term Consequences of Giardia Duodenalis Infections. World J. Gastroenterol. 2013, 19, 8974–8985. [Google Scholar] [CrossRef] [PubMed]
- Bari, S.; Sheikh, K.A.; Ashraf, M.; Hussain, Z.; Hamid, A.; Mufti, G.N. Ascaris Liver Abscess in Children. J. Gastroenterol. 2007, 42, 236–240. [Google Scholar] [CrossRef]
- Khuroo, M.S. Ascariasis. Gastroenterol. Clin. N. Am. 1996, 25, 553–577. [Google Scholar] [CrossRef]
- Davidson, C.S. Tropical and Geographical Medicine. Edited by Kenneth S. Warren and Adel A. F. Mahmoud. 1,175 Pp. + Xvii. New York: McGraw-Hill, 1984. Hepatology 1984, 4, 981. [Google Scholar] [CrossRef]
- Pawłowski, Z.S. Ascariasis. Clin. Gastroenterol. 1978, 7, 157–178. [Google Scholar] [CrossRef]
- Misra, V.; Debnath, S.; Misra, S.P.; Singh, P.A. Significance of Charcot Leyden Crystals in Hepatic Aspirates. J. Cytol. Indian Acad. Cytol. 2009, 26, 77–79. [Google Scholar] [CrossRef]
- Freeman, A.F.; Holland, S.M. The Hyper-IgE Syndromes. Immunol. Allergy Clin. N. Am. 2008, 28, 277–291, viii. [Google Scholar] [CrossRef]
- Lambertucci, J.R.; Rayes, A.A.; Serufo, J.C.; Nobre, V. Pyogenic Abscesses and Parasitic Diseases. Rev. Inst. Med. Trop. São Paulo 2001, 43, 67–74. [Google Scholar] [CrossRef]
- Dawson, H.; Solano-Aguilar, G.; Beal, M.; Beshah, E.; Vangimalla, V.; Jones, E.; Botero, S.; Urban, J.F. Localized Th1-, Th2-, T Regulatory Cell-, and Inflammation-Associated Hepatic and Pulmonary Immune Responses in Ascaris Suum-Infected Swine Are Increased by Retinoic Acid. Infect. Immun. 2009, 77, 2576–2587. [Google Scholar] [CrossRef]
- Nutman, T.B. Looking beyond the Induction of Th2 Responses to Explain Immunomodulation by Helminths. Parasite Immunol. 2015, 37, 304–313. [Google Scholar] [CrossRef]
- Dong, C.; Flavell, R.A. Cell Fate Decision: T-Helper 1 and 2 Subsets in Immune Responses. Arthritis Res. Ther. 2000, 2, 179. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Rook, G.A. The Role of TNF-Alpha in T-Cell-Mediated Inflammation Depends on the Th1/Th2 Cytokine Balance. Immunology 1994, 82, 591–595. [Google Scholar]
- Allen, J.E.; Maizels, R.M. Immunology of Human Helminth Infection. Int. Arch. Allergy Immunol. 1996, 109, 3–10. [Google Scholar] [CrossRef]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role Played by Th2 Type Cytokines in IgE Mediated Allergy and Asthma. Lung India 2010, 27, 66–71. [Google Scholar] [CrossRef]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-Histaminergic Itch. Immunity 2019, 50, 1163–1171.e5. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, E.; Bazin, H. Elevation of Total Serum IgE in Rats Following Helminth Parasite Infection. Nature 1974, 251, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; Feddema, L.; Quinn, E.H. Non-Specific Potentiation of IgE by Parasitic Infections in Man. Int. Arch. Allergy Appl. Immunol. 1979, 58, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Qualizza, R.; Losappio, L.M.; Furci, F. A Case of Atopic Dermatitis Caused by Ascaris Lumbricoides Infection. Clin. Mol. Allergy 2018, 16, 10. [Google Scholar] [CrossRef]
- Medeiros, D.; Silva, A.R.; Rizzo, J.A.; Motta, M.E.; Sarinho, E. Total IgE Level Is Associated with Anti-Ascaris Specific IgE but Not with Stool Parasites or Blood Eosinophilia in Young Allergic Patients in a Tropical Region (Recife-Brazil). J. Allergy Clin. Immunol. 2006, 117, S200. [Google Scholar] [CrossRef]
- Tagle C, M.T.; Melys G, A.; Castillo M, A.; Norambuena R, X.; Quezada L, A. Hyper IgE Syndrome: Three Case Reports. Rev. Chil. Pediatría 2014, 85, 328–336. [Google Scholar] [CrossRef][Green Version]
- Fan, H.; Huang, L.; Yang, D.; Lin, Y.; Lu, G.; Xie, Y.; Yu, J.; Zhang, D. Pediatric Hyperimmunoglobulin E Syndrome: A Case Series of 4 Children in China. Medicine 2018, 97, e0215. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ieşanu, M.-I.; Cliveti, R.; Anghel, M.; Stoicescu, M.-M.; Boboc, C.; Ioan, A.; Galoş, F. Parasite-Induced Th2 Polarization—An Unusual Cause of Paediatric Hepatic Abscess. Medicina 2021, 57, 1322. https://doi.org/10.3390/medicina57121322
Ieşanu M-I, Cliveti R, Anghel M, Stoicescu M-M, Boboc C, Ioan A, Galoş F. Parasite-Induced Th2 Polarization—An Unusual Cause of Paediatric Hepatic Abscess. Medicina. 2021; 57(12):1322. https://doi.org/10.3390/medicina57121322
Chicago/Turabian StyleIeşanu, Mara-Ioana, Ramona Cliveti, Mălina Anghel, Mihai-Mirel Stoicescu, Cătălin Boboc, Andreea Ioan, and Felicia Galoş. 2021. "Parasite-Induced Th2 Polarization—An Unusual Cause of Paediatric Hepatic Abscess" Medicina 57, no. 12: 1322. https://doi.org/10.3390/medicina57121322
APA StyleIeşanu, M.-I., Cliveti, R., Anghel, M., Stoicescu, M.-M., Boboc, C., Ioan, A., & Galoş, F. (2021). Parasite-Induced Th2 Polarization—An Unusual Cause of Paediatric Hepatic Abscess. Medicina, 57(12), 1322. https://doi.org/10.3390/medicina57121322





