Drug-Induced Esophageal Ulcer in Adolescent Population: Experience at a Single Medical Center in Central Taiwan
Abstract
:1. Introduction
2. Materials and Methods
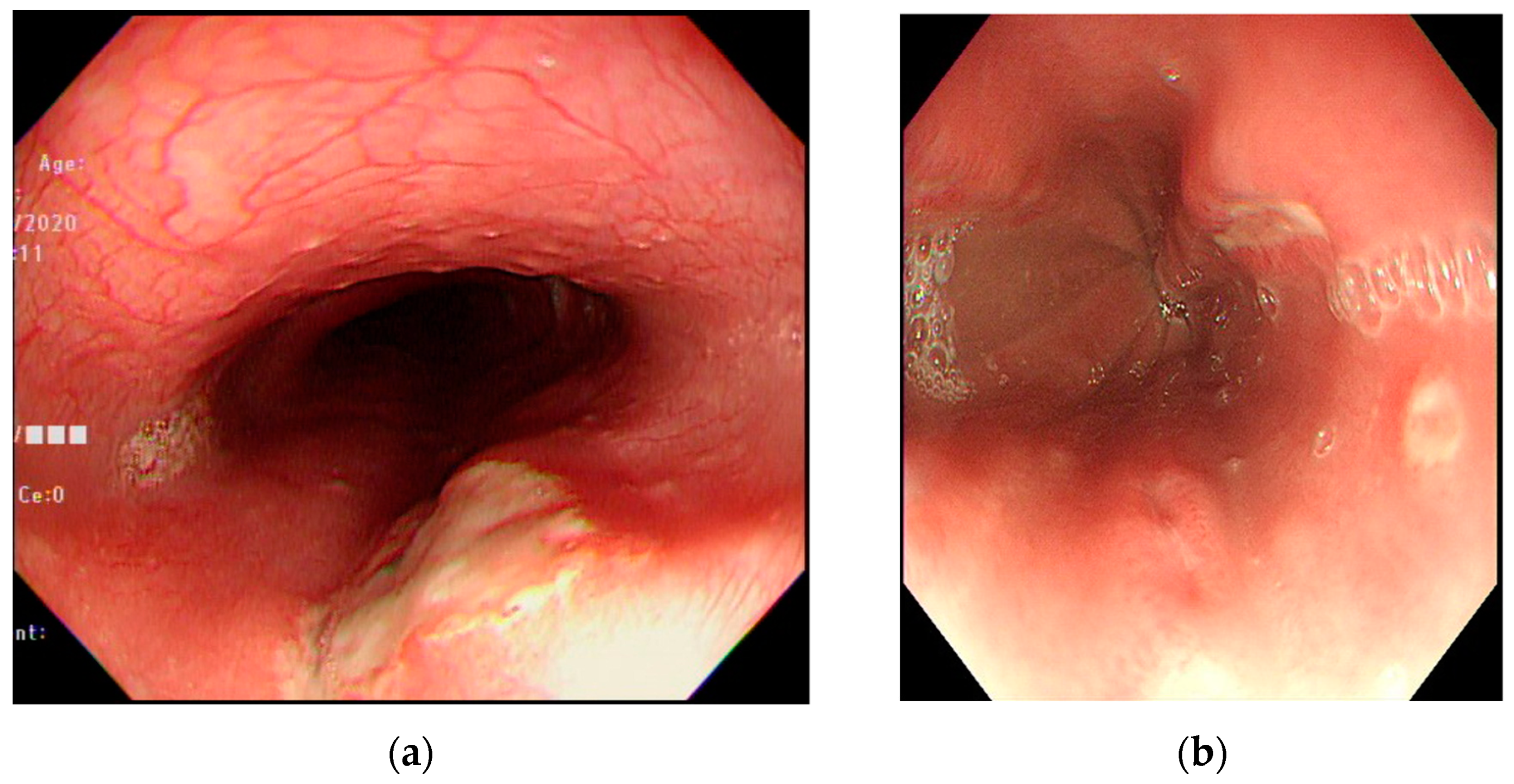
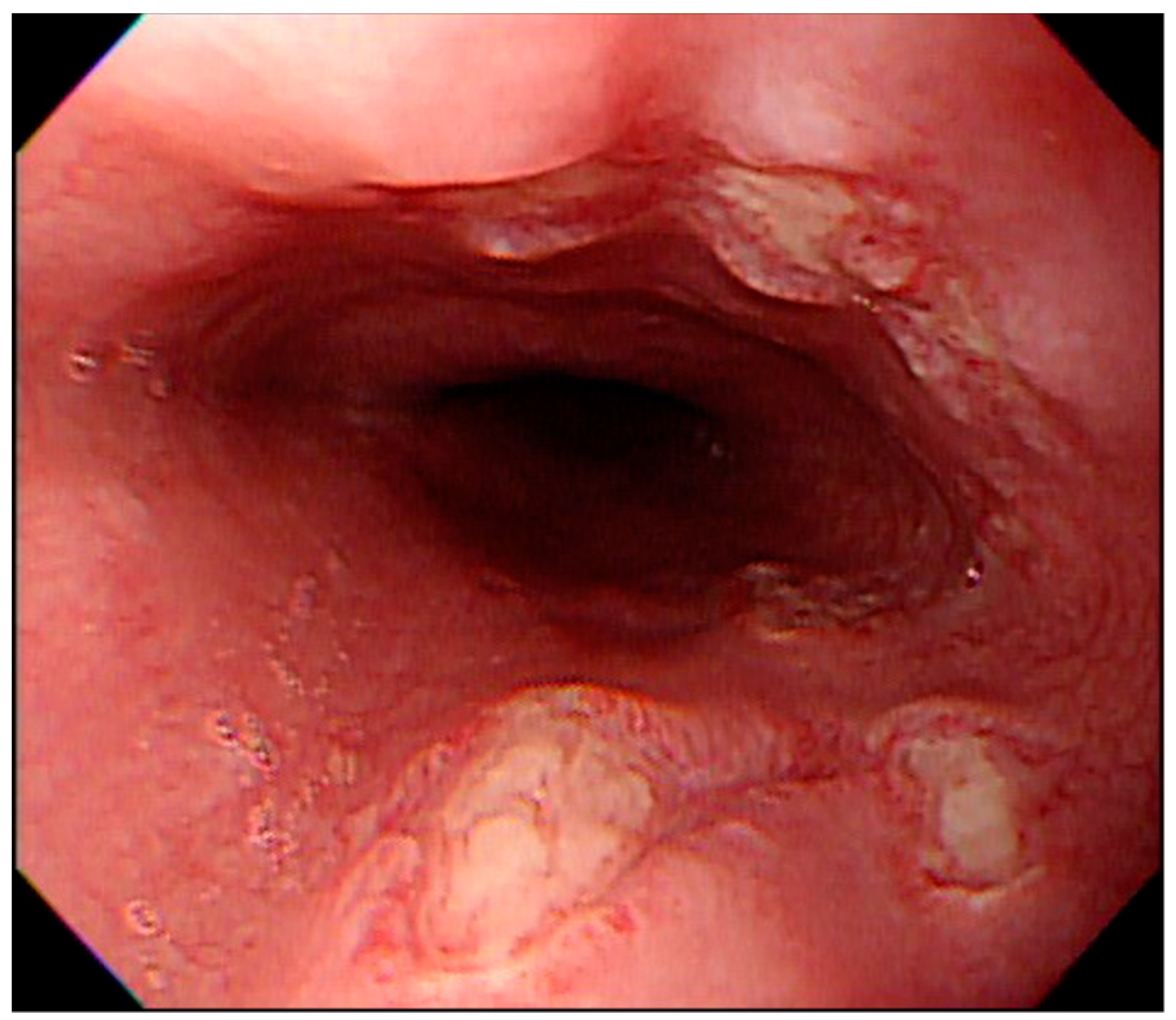
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kikendall, J.W.; Friedman, A.C.; Oyewole, M.A.; Fleischer, D.; Johnson, L.F. Pill-induced esophageal injury. Case reports and review of the medical literature. Dig. Dis. Sci. 1983, 28, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dağ, M.S.; Öztürk, Z.A.; Akın, I.; Tutar, E.; Çıkman, Ö.; Gülşen, M.T. Drug-induced esophageal ulcers: Case series and the review of the literature. Turk. J. Gastroenterol. 2014, 25, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zografos, G.N.; Georgiadou, D.; Thomas, D.; Kaltsas, G.; Digalakis, M. Drug-induced esophagitis. Dis. Esophagus. 2009, 22, 633–637. [Google Scholar] [CrossRef]
- Bordea, M.A.; Pirvan, A.; Sarban, C.; Margescu, C.; Leucuta, D.; Samasca, G.; Miu, N. Pill-induced erosive esophagitis in children. Clujul Med. 2014, 87, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Komatsu, K.; Harada, Y. Medication-induced esophagitis in children. Gastroenterol. Jpn. 1990, 25, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jeong, J.B.; Kim, J.W.; Koh, S.J.; Kim, B.G.; Lee, K.L.; Chang, M.S.; Im, J.P.; Kang, H.W.; Shin, C.M. Clinical and endoscopic characteristics of drug-induced esophagitis. World J. Gastroenterol. 2014, 20, 10994–10999. [Google Scholar] [CrossRef]
- Gebauer, K. Acne in adolescents. Aust. Fam. Physician 2017, 46, 892–895. [Google Scholar]
- Habeshian, K.A.; Cohen, B.A. Current issues in the treatment of acne vulgaris. Pediatrics 2020, 145 (Suppl. 2), S225–S230. [Google Scholar] [CrossRef]
- Hanisah, A.; Omar, K.; Shah, S.A. Prevalence of acne and its impact on the quality of life in school-aged adolescents in Malaysia. J. Prim. Health Care 2009, 1, 20–25. [Google Scholar] [CrossRef]
- Kilkenny, M.; Stathakis, V.; Hibbert, M.E.; Patton, G.; Caust, J.; Bowes, G. Acne in Victorian adolescents: Associations with age, gender, puberty and psychiatric symptoms. J. Paediatr. Child. Health 1997, 33, 430–433. [Google Scholar] [CrossRef]
- Chen, W.; Chang, M.H. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr. Neonatol. 2010, 51, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Jaspersen, D. Drug-induced oesophageal disorders: Pathogenesis, incidence, prevention and management. Drug Saf. 2000, 22, 237–249. [Google Scholar] [CrossRef]
- Kadayifci, A.; Gulsen, M.T.; Koruk, M.; Savas, M.C. Doxycycline-induced pill esophagitis. Dis. Esophagus 2004, 17, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ovartlarnporn, B.; Kulwichit, W.; Hiranniramol, S. Medication-induced esophageal injury: Report of 17 cases with endoscopic documentation. Am. J. Gastroenterol. 1991, 86, 748–750. [Google Scholar]
- Bestari, M.B.; Agustanti, N.; Abdurachman, S.A. Clindamycin-induced esophageal injury: Is it an underdiagnosed entity? Clin. Med. Insights Case Rep. 2019, 12, 1179547619884055. [Google Scholar] [CrossRef] [Green Version]
- Kaewdech, A.; Pattarapuntakul, T.; Sripongpun, P. Amoxycillin-clavulanic acid-induced esophageal ulcer: An unusual cause. Case Rep. Gastroenterol. 2020, 14, 472–476. [Google Scholar] [CrossRef]
- Gallego-Pérez, B.; Martínez-Crespo, J.J.; García-Belmonte, D.; Marín-Bernabé, C.M. Úlcera esofágica por comprimido de L-Arginina: Causa no comunicada previamente de esofagitis por comprimidos (L-Arginine pill induced esophageal ulcer: Causes not reported previously esophagitis for pills). Farm. Hosp. 2014, 38, 487–489. [Google Scholar]
- O’Donnell, C.; Tandon, P.; Govardhanam, V.; Habal, F. Pill-induced esophagitis from intake of dietary supplements. ACG Case Rep. J. 2019, 6, e00106. [Google Scholar] [CrossRef]
- Parra, P.R.; González-Cruz, M.Á.; Ferreiro-Marin, A.; Casaubón-Garcín, P.R. L-arginine-induced esophagitis, report of six cases. Reporte de seis casos de esofagitis inducida por L-arginina. Bol. Med. Hosp. Infant. Mex. 2020, 77, 38–41. [Google Scholar] [CrossRef]
- Bonavina, L.; DeMeester, T.R.; McChesney, L.; Schwizer, W.; Albertucci, M.; Bailey, R.T. Drug-induced esophageal strictures. Ann. Surg. 1987, 206, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-M.; Wong, K.-S.; Huang, Y.-C.; Lai, S.-H.; Tsao, K.-C.; Lin, Y.-J.; Lin, T.-Y. Macrolide-resistant Mycoplasma pneumoniae in children in Taiwan. J. Infect. Chemother. 2013, 19, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yun, K.W.; Lee, H.J.; Choi, E.H. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev. Anti-Infect. Ther. 2018, 16, 23–34. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value |
|---|---|
| Age (years), mean | 15.6 |
| Sex (N), Male/Female | 18/14 |
| Symptoms [N (%)] | |
| Chest pain | 27 (84.4) |
| Odynophagia | 14 (43.8) |
| Epigastric pain | 12 (37.5) |
| Dysphagia | 5 (15.6) |
| Duration from onset to visit (days) | 6.1 |
| Duration from onset to endoscopy (days) | 8.0 |
| Drug | N (%) |
|---|---|
| Doxycycline | 18 (56.3) |
| Nonsteroidal anti-inflammatory drugs | 6 (18.8) |
| Dicloxacillin | 3 (9.4) |
| Amoxicillin | 1 (3.1) |
| Clindamycin | 1 (3.1) |
| L-Arginine | 1 (3.1) |
| Unknown | 2 (6.2) |
| Features | N (%) | |
|---|---|---|
| Location | Upper-third | 5 (15.6) |
| Middle-third | 25 (78.1) | |
| Lower-third | 8 (25.0) | |
| Ulcer characteristics | Single | 9 (28.1) |
| Multiple | 23 (71.9) | |
| Kissing ulcers | 17 (53.1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-W.; Chen, A.-C.; Wu, S.-F. Drug-Induced Esophageal Ulcer in Adolescent Population: Experience at a Single Medical Center in Central Taiwan. Medicina 2021, 57, 1286. https://doi.org/10.3390/medicina57121286
Hu S-W, Chen A-C, Wu S-F. Drug-Induced Esophageal Ulcer in Adolescent Population: Experience at a Single Medical Center in Central Taiwan. Medicina. 2021; 57(12):1286. https://doi.org/10.3390/medicina57121286
Chicago/Turabian StyleHu, Shu-Wei, An-Chyi Chen, and Shu-Fen Wu. 2021. "Drug-Induced Esophageal Ulcer in Adolescent Population: Experience at a Single Medical Center in Central Taiwan" Medicina 57, no. 12: 1286. https://doi.org/10.3390/medicina57121286
APA StyleHu, S.-W., Chen, A.-C., & Wu, S.-F. (2021). Drug-Induced Esophageal Ulcer in Adolescent Population: Experience at a Single Medical Center in Central Taiwan. Medicina, 57(12), 1286. https://doi.org/10.3390/medicina57121286






