Reconstruction of a Lower Polar Artery for Kidney Transplantation Using Donor Ovarian Vein: Case Report with Literature Review
Abstract
1. Introduction
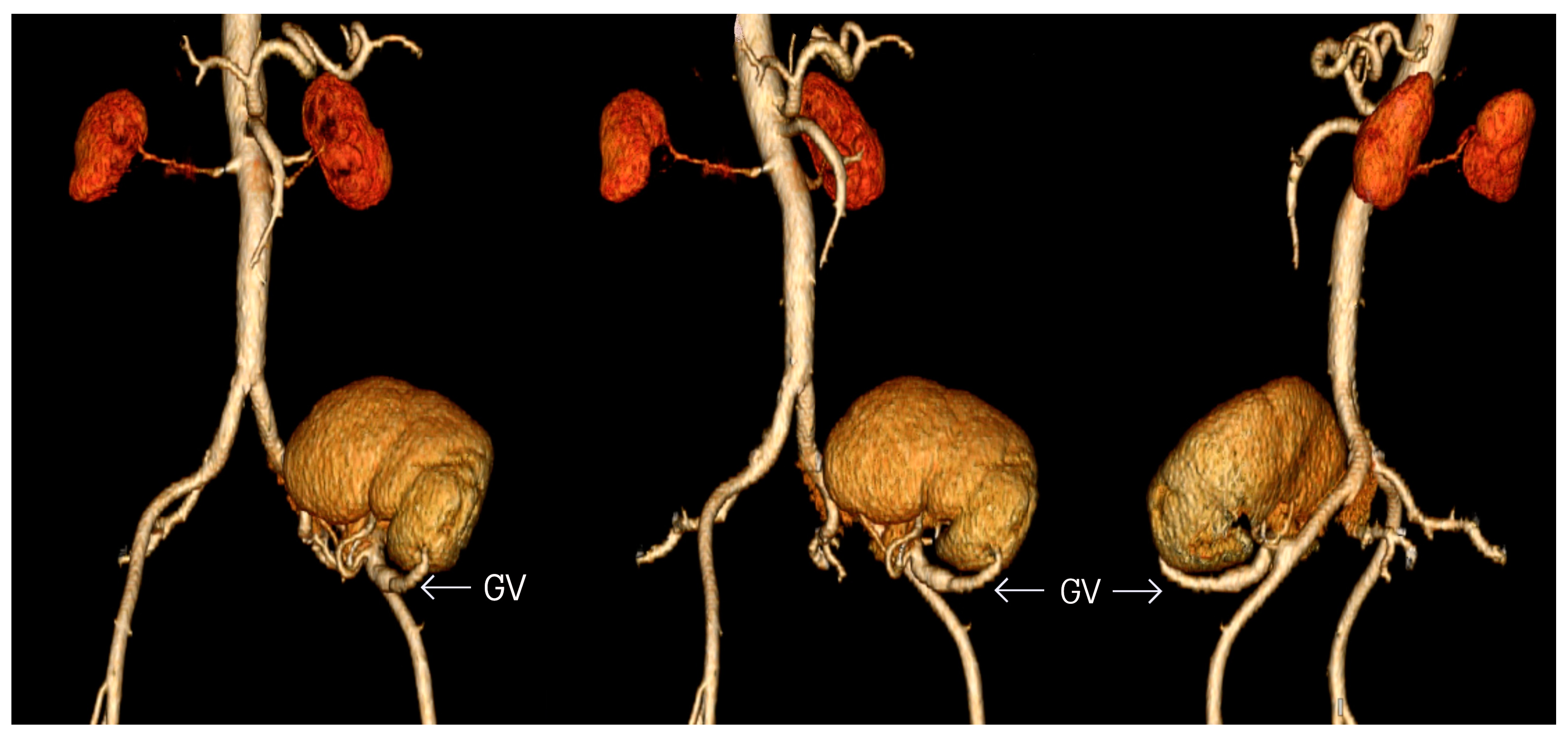
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADPKD | Autosomal dominant polycystic kidney disease |
| CT | Computed tomography |
| DGF | Delayed graft function |
| DGV | Donor gonadal vein |
| eGFR | Glomerular filtration rate |
| ESRD | End-stage renal disease |
| GV | Gonadal vein |
| IPA | Inferior polar artery |
| LUHS | Lithuanian university of health sciences |
| MPGN | Membranoproliferative glomerulonephritis |
| NA | Not available |
| PTFE | Polytetrafluoroethylene |
| RA | Renal artery |
| RV | Renal vein |
| U | Ureter |
Appendix A
| Adult |
| Anastomosis, surgical/methods* |
| Case reports |
| Cold ischemia |
| Computed tomography Angiography |
| Female |
| Follow-up studies |
| Gonads/blood supply |
| Graft survival |
| Humans |
| Kidney failure, chronic/surgery |
| Kidney function Tests |
| Kidney transplantation/methods* |
| Lithuania |
| Nephrectomy/complications |
| Ovary/blood supply* |
| Postoperative period |
| Renal artery/injuries* |
| Tissue donors |
| Treatment outcome |
| Vascular grafting/methods* |
| Warm ischemia |
References
- Timsit, M.O.; Kleinclauss, F.; Thuret, R. Histoire chirurgicale de la transplantation rénale. Progrès Urol. 2016, 26, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Copelan, A.; George, D.; Kapoor, B.; Nghiem, H.V.; Lorenz, J.M.; Erly, B.; Wang, W. Iatrogenic-related transplant injuries: The role of the interventional radiologist. Semin. Interv. Radiol. 2015, 32, 133–155. [Google Scholar]
- Panthier, F.; Lareyre, F.; Audouin, M.; Raffort, J. Pelvi-ureteric junction obstruction related to crossing vessels: Vascular anatomic variations and implication for surgical approaches. Int. Urol. Nephrol. 2018, 50, 385–394. [Google Scholar] [CrossRef]
- Gulas, E.; Wysiadecki, G.; Cecot, T.; Majos, A.; Stefańczyk, L.; Topol, M.; Polguj, M. Accessory (multiple) renal arteries—Differences in frequency according to population, visualizing techniques and stage of morphological development. Vascular 2016, 24, 531–537. [Google Scholar] [CrossRef]
- Felix, W. Mesonephric arteries (aa. mesonephrica). In Manual of Human Embryology; Keibel, F., Mall, F.P., Eds.; Lippincott: Philadelphia, PA, USA, 1912; pp. 820–825. [Google Scholar]
- Merklin, R.J.; Michels, N.A. The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: A statistical analysis based on 185 dissections and reviews of the literature. J. Int. Coll. Surg. 1958, 29, 41–76. [Google Scholar] [PubMed]
- Ashraf, H.S.; Hussain, I.; Siddiqui, A.A.; Ibrahim, M.N.; Khan, M.U. The outcome of living related kidney transplantation with multiple renal arteries. Saudi J. Kidney Dis. Transpl. 2012, 4, 615–619. [Google Scholar] [CrossRef]
- Sagban, T.A.; Baur, B.; Schelzig, H.; Grabitz, K.; Duran, M. Vascular challenges in renal transplantation. Ann. Transplant. 2014, 19, 464–471. [Google Scholar]
- Novick, A.C.; Magnusson, M.; Braun, W.E. Multiple-artery renal transplantation: Emphasis on extracorporeal methods of donor arterial reconstruction. J. Urol. 1979, 122, 731–735. [Google Scholar] [CrossRef]
- Bessede, T.; Droupy, S.; Hammoudi, Y.; Bedretdinova, D.; Durrbach, A.; Charpentier, B.; Benoit, G. Surgical prevention and management of vascular complications of kidney transplantation. Transpl. Int. 2012, 25, 994–1001. [Google Scholar] [CrossRef]
- Afriansyah, A.; Rasyid, N.; Rodjani, A.; Wahyudi, I.; Mochtar, C.A.; Susalit, E.; Hamid, A.R.A.H. Laparoscopic procurement of single versus multiple artery kidney allografts: Meta-analysis of comparative studies. Asian J. Surg. 2019, 42, 61–70. [Google Scholar] [CrossRef]
- Helanterä, I.; Ibrahim, H.N.; Lempinen, M.; Finne, P. Donor age, cold ischemia time, and delayed graft function. Clin. J. Am. Soc. Nephrol. 2020, 15, 813–821. [Google Scholar] [CrossRef]
- Santos Dias, F.; Metelo Coimbra, C.; Roncon-Albuquerque, R., Jr. Renal Warm Ischemia in Organ Donors After Circulatory Death. Exp. Clin. Transplant. 2021, 19, 179–189. [Google Scholar] [CrossRef]
- Kuipers, T.G.; Hellegering, J.; El Moumni, M.; Krikke, C.; Haveman, J.W.; Berger, S.P.; Leuvenink, H.G.; Pol, R.A. Kidney temperature course during living organ procurement and transplantation. Transpl. Int. 2017, 30, 162–169. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Oberhuber, R.; Cardini, B.; Weiss, S.; Ulmer, H.; Bösmüller, C.; Schneeberger, S.; Pratschke, J.; Öllinger, R. The faster the better: Anastomosis time influences patient survival after deceased donor kidney transplantation. Transpl. Int. 2015, 28, 535–543. [Google Scholar] [CrossRef]
- Veeramani, M.; Jain, V.; Ganpule, A.; Sabnis, R.B.; Desai, M.R. Donor gonadal vein reconstruction for extension of the transected renal vessels in living renal transplantation. Indian J. Urol. 2010, 26, 314–316. [Google Scholar] [PubMed]
- Antonopoulos, I.M.; Yamaçake, K.G.R.; Oliveira, L.M.; Piovesan, A.C.; Kanashiro, H.; Nahas, W.C. Revascularization of living-donor kidney transplant with multiple arteries: Long-term outcomes using the inferior epigastric artery. Urology 2014, 84, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, T.; Futamura, K.; Okada, M.; Yamamoto, T.; Tsujita, M.; Goto, N.; Narumi, S.; Watarai, Y.; Kobayashi, T. Impact of arterial reconstruction with recipient’s own internal iliac artery for multiple graft arteries on living donor kidney transplantation: Strobe study. Medicine 2015, 94, 1–7. [Google Scholar] [CrossRef]
- Oertl, A.J.; Jonas, D.; Oremek, G.M.; Jones, J. Saphenous Vein Interposition as a Salvage Technique for Complex Vascular Situations During Renal Transplantation. Transplant. Proc. 2007, 39, 140–142. [Google Scholar] [CrossRef]
- Levy, M.M.; Kiang, W.; Johnson, J.M.; Myers, S.I. Saphenous vein graft aneurysm with graft-enteric fistula after renal artery bypass. J. Vasc. Surg. 2008, 48, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Abou-Elela, A.; Morsy, A.; Salah, M.; Foda, A. The use of the inferior epigastric artery for accessory lower polar artery revascularization in live donor renal transplantation. Int. Urol. Nephrol. 2008, 40, 283–287. [Google Scholar] [CrossRef]
- Kamel, M.H.; Thomas, A.A.; Mohan, P.; Hickey, D.P. Renal vessel reconstruction in kidney transplantation using a polytetrafluoroethylene (PTFE) vascular graft. Nephrol. Dial. Transplant. 2007, 22, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Vento, V.; Kuntz, S.; Steinmetz, L.; Georg, Y.; Thaveau, F.; Heim, F.; Chakfe, N. Current status on vascular substitutes. J. Cardiovasc. Surg. 2020, 61, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Uysal, E.; Yuzbasioglu, M.F.; Ikidag, M.A.; Dokur, M.; Gurer, O.A. Successful elongation of a short graft renal artery by a gonadal vein. Exp. Clin. Transplant. 2017, 15, 467–469. [Google Scholar] [PubMed][Green Version]
- Tomizawa, M.; Hori, S.; Nishimura, N.; Omori, C.; Nakai, Y.; Miyake, M.; Yoneda, T.; Fujimoto, K. Arterial reconstruction using the donor’s gonadal vein in living renal transplantation with multiple renal arteries: A case report and a literature review. BMC Nephrol. 2020, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chatzizacharias, N.A.; Muthusami, A.S.R.; Sullivan, M.; Sinha, S.; Brockmann, J. Use of gonadal vein interposition graft for implantation of polar artery in live donor renal transplantation. Transplantation 2010, 90, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Aliasgari, M.; Shakhssalim, N.; Dadkhah, F.; Ghadian, A.; Moghaddam, S.M.H. Donor nephrectomy with and without preservation of gonadal vein while dissecting the ureter. Urol. J. 2008, 5, 168–172. [Google Scholar]

| Before Transplantation | 1 Month after Transplantation | 15 Months after Transplantation | |
|---|---|---|---|
| Creatinine (µmol/L) | ) | ||
| Urea (mmol/L) | 7.9 | 7.1 | |
| Potassium (mmol/L) | 4.53 | 4.06 | |
| Urine pH | 8.0 | 7.5 | 6.5 |
| Urine-specific gravity | 1012 | 1011 | 1006 |
| Blood in urine | - | - | 0.3 |
| Leukocytes in urine | - | ||
| Erythrocytes in urine (microscopic urinalysis) | - | - | - |
| Leukocytes in urine (microscopic urinalysis) | ) | - |
| Vascular Reconstruction Techniques of Severed Polar Artery | Indication | Method | Advantages | Disadvantages |
|---|---|---|---|---|
| Ligation | Upper polar artery which supplies < 25% is severed | Ligation | Does not affect the function of the graft | Cannot be applied for the lower polar artery |
| Direct repair | Distal end of the polar artery is preserved | Connection of two interrupted segments of the polar artery | Does not require additional vessels | Possible only if the aortic patch with polar artery bifurcation is retained |
| End-to-side anastomosis | Distal end of the polar artery is severed | End-to-side anastomosis with the main renal artery, external iliac artery, or internal iliac artery | The diameters of vessels can be different | If back-table preparation is not used, warm ischemia time is prolonged Higher risk of stenosis and thrombosis |
| End-to-end anastomosis | Distal end of the polar artery is severed | End-to-end anastomosis with hypogastric artery | Can be anastomosed after the main renal artery is reperfused Reduces ischemia time | The hypogastric artery is not always available due to atherosclerosis The size or diameter can be insufficient |
| Side-to-side conjoined anastomosis | Two equal-sized arteries | Common ostium is made with another renal artery | Reduction in warm ischemia time due to single artery anastomosis | Arteries need to be comparable in size |
| Vascular interposition graft | Insufficient artery length | Vascular interposition graft is made from donor or recipient’s vessel (saphenous vein, internal iliac artery, inferior epigastric artery, or gonadal vein) or PTFE; it is anastomosed to the severed polar artery and a larger vessel | No additional intraoperative or donor-site complications Decreases warm ischemia time due to back—table preparation | Saphenous vein graft has a higher risk of occlusion, aneurysms, and ruptures, and access to the vein requires an additional incision PTFE has the highest rate of thrombogenicity and infectability |
| Authors | Year | Age | Sex | Diagnosis | Donor’s Kidney | Number of Allograft Renal Arteries | Source of Gonadal Vein | Interposition GraftAnastomosed to | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|---|
| Chatzizacharias NA | 2010 | 28 | M | IgA nephropathy | Left | 2 | Donor | External iliac artery | NA |
| Veeramani M | 2010 | 49 | M | ADPKD, ESRD | Left | 2 | Donor | External iliac artery | 2 years |
| Uysal E | 2017 | 27 | M | ESRD | Right | 1 | Recipient | Internal iliac artery | 8 months |
| Tomizawa M | 2020 | 34 | M | ESRD | Right | 3 | Donor | Graft made from the internal iliac artery | 3 years |
| Present case | 2020 | 34 | F | Alport syndrome, MPGN, ESRD | Right | 2 | Donor | One of four renal arteries from a common trunk | 15 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikauskaitė, S.; Počepavičiūtė, K.; Velička, L.; Jankauskas, A.; Trumbeckas, D.; Šuopytė, E. Reconstruction of a Lower Polar Artery for Kidney Transplantation Using Donor Ovarian Vein: Case Report with Literature Review. Medicina 2021, 57, 1248. https://doi.org/10.3390/medicina57111248
Bikauskaitė S, Počepavičiūtė K, Velička L, Jankauskas A, Trumbeckas D, Šuopytė E. Reconstruction of a Lower Polar Artery for Kidney Transplantation Using Donor Ovarian Vein: Case Report with Literature Review. Medicina. 2021; 57(11):1248. https://doi.org/10.3390/medicina57111248
Chicago/Turabian StyleBikauskaitė, Saulė, Kamilė Počepavičiūtė, Linas Velička, Antanas Jankauskas, Darius Trumbeckas, and Erika Šuopytė. 2021. "Reconstruction of a Lower Polar Artery for Kidney Transplantation Using Donor Ovarian Vein: Case Report with Literature Review" Medicina 57, no. 11: 1248. https://doi.org/10.3390/medicina57111248
APA StyleBikauskaitė, S., Počepavičiūtė, K., Velička, L., Jankauskas, A., Trumbeckas, D., & Šuopytė, E. (2021). Reconstruction of a Lower Polar Artery for Kidney Transplantation Using Donor Ovarian Vein: Case Report with Literature Review. Medicina, 57(11), 1248. https://doi.org/10.3390/medicina57111248





