Real-World Patient Characteristics and Treatment Patterns of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer: A Multicenter Retrospective Chart Review Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data
2.3. Statistical Analysis
3. Results
3.1. Patients
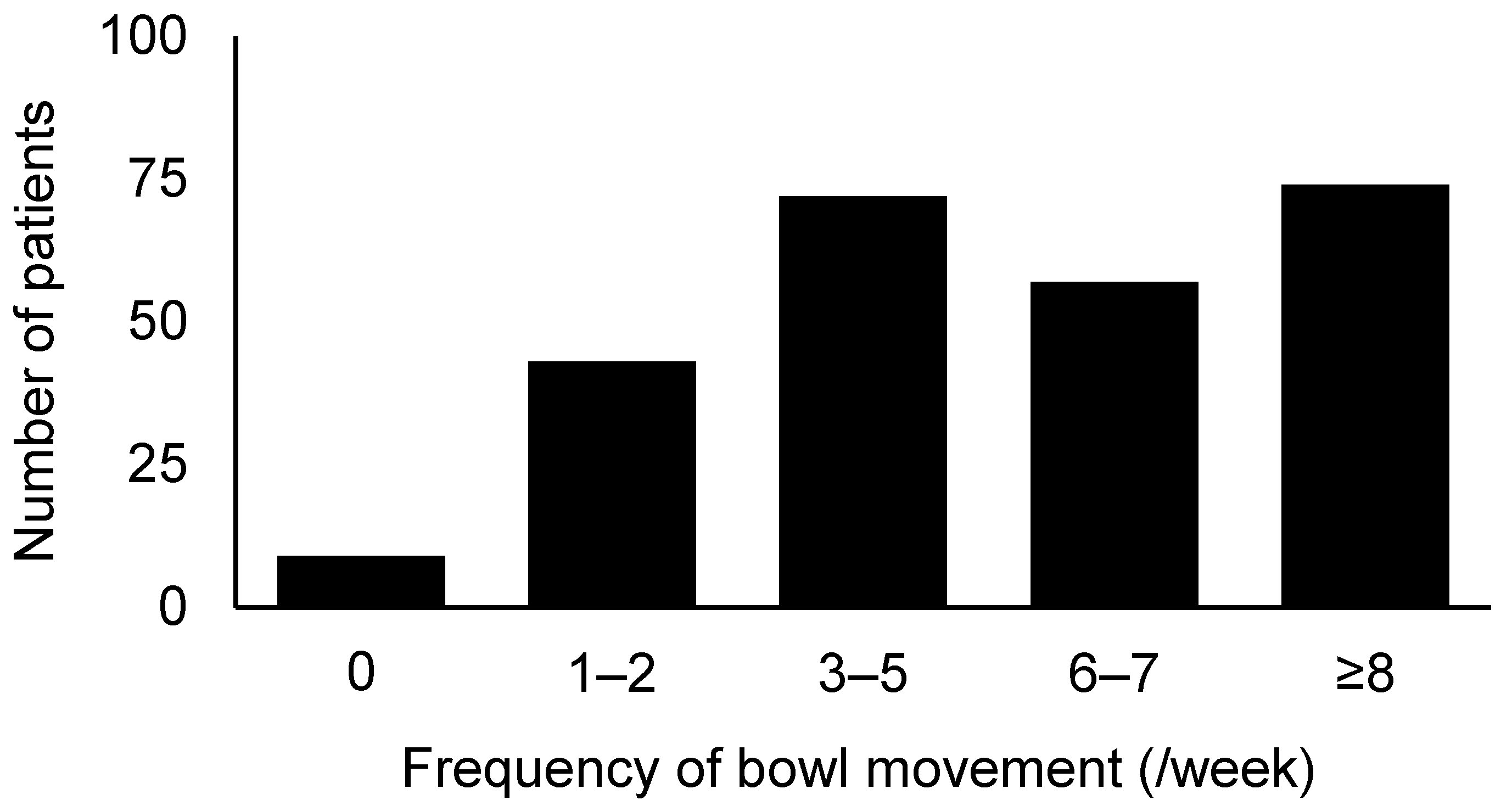
3.2. Efficacy and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokoro, A.; Imai, H.; Fumita, S.; Harada, T.; Noriyuki, T.; Gamoh, M.; Akashi, Y.; Sato, H.; Kizawa, Y. Incidence of opioid-induced constipation in Japanese patients with cancer pain: A prospective observational cohort study. Cancer Med. 2019, 8, 4883–4891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, H.A. Naldemedine: A Review in Opioid-Induced Constipation. Drugs 2019, 79, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Esmadi, M.; Ahmad, D.; Hewlett, A. Efficacy of naldemedine for the treatment of opioid-induced constipation: A meta-analysis. J. Gastrointest. Liver Dis. 2019, 28, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.; Wild, J.; Reddy, J.; Yamada, T.; Arjona Ferreira, J.C. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol. Hepatol. 2017, 2, 555–564. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized Phase III and Extension Studies of Naldemedine in Patients with Opioid-Induced Constipation and Cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized phase III and extension studies: Efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann. Oncol. 2018, 29, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Oda, K.; Tauchi, K.; Nakata, K.; Shinozaki, K.; Yokota, T.; Suzuki, Y.; Narabayashi, M.; Boku, N. Phase IIb, Randomized, Double-Blind, Placebo-Controlled Study of Naldemedine for the Treatment of Opioid-Induced Constipa-tion in Patients with Cancer. J. Clin. Oncol. 2017, 35, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Nalamachu, S.; Morlion, B.; Reddy, J.; Baba, Y.; Yamada, T.; Arjona Ferreira, J.C. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic noncancer pain: A randomized, double-blind, placebo-controlled phase 3 study. Pain 2018, 159, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Yamada, T.; Arjona Ferreira, J.C. A Phase 2b, Randomized, Double-Blind Placebo-Controlled Study to Evaluate the Efficacy and Safety of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Noncancer Pain. Pain Med. 2017, 18, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Adult Cancer Pain Version 2.2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf (accessed on 2 September 2021).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, J.; Webster, L.; Yamada, T.; Hale, M. Safety and Efficacy of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain Receiving Opioid Therapy: A Subgroup Analysis of Patients >/= 65 Years of Age. Drugs Aging 2020, 37, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashizume, J.; Ryu, E.; Nose, S.; Miyanaga, K.; Kishikawa, R.; Nakamura, T.; Muro, T.; Kodama, Y.; Yamashita, H.; Ishii, K.; et al. Predictors for Diarrhea After Administration of Naldemedine: Analysis Focusing on the Administration Period of Opioid Analgesics Before the Start of Naldemedine. Palliat. Care Res. 2020, 15, 101–109. [Google Scholar] [CrossRef]
- Takagi, Y.; Osawa, G.; Kato, Y.; Ikezawa, E.; Kobayashi, C.; Aruga, E. Prevention and management of diarrhea associated with naldemedine among patients receiving opioids: A retrospective cohort study. BMC Gastroenterol. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Ikemura, K.; Mizutani, E.; Iwamoto, T.; Okuda, M. Opioid therapy duration before naldemedine treatment is a significant independent risk of diarrhea: A retrospective cohort study. J. Pharm. Health Care Sci. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.; Kessoku, T.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; Kato, T.; Honda, Y.; Ogawa, Y.; Imajo, K.; Higurashi, T.; et al. Comparing the effectiveness of magnesium oxide and naldemedine in preventing opioid-induced constipation: A proof of concept, single institutional, two arm, open-label, phase II, randomized controlled trial: The MAGNET study. Trials 2020, 21, 453. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Komiya, S.; Uzawa, N.; Inoue, K.; Itoh, T.; Aoki, S.; Shibasaki, M.; Suzuki, T. Involvement of supraspinal and peripheral naloxonazine-insensitive opioid receptor sites in the expression of mu-opioid receptor agonist-induced physical dependence. Eur. J. Pharmacol. 2013, 715, 238–245. [Google Scholar] [CrossRef] [PubMed]


| Parameter | n (%) |
|---|---|
| Male | 179 (60.5%) |
| Age | |
| ≤64 | 77 (26.0%) |
| 65–74 | 101 (34.1%) |
| ≥75 | 118 (39.9%) |
| ECOG PS | |
| 0 | 24 (8.1%) |
| 1 | 55 (18.6%) |
| 2 | 88 (29.7%) |
| 3 | 92 (31.1%) |
| 4 | 37 (12.5%) |
| Primary tumor | |
| Thoracic | 82 (27.7%) |
| Pancreatic | 41 (13.9%) |
| Colorectal | 30 (10.1%) |
| Gastrointestinal | 22 (7.4%) |
| Breast | 17 (5.7%) |
| Blood | 14 (4.7%) |
| Prostate | 11 (3.7%) |
| Gynecologic | 11 (3.7%) |
| Head and neck | 10 (3.4%) |
| Others | 58 (19.6%) |
| Treatment * | |
| Anticancer medications | 80 (27.0%) |
| Radiation | 33 (11.1%) |
| Surgery | 6 (2.0%) |
| Chemoradiation | 4 (1.4%) |
| Past treatment | |
| Surgery (abdominal) | 102 (34.5%) |
| Radiation (abdominal/lumbar) | 35 (11.8%) |
| Comorbidities related to cancer | |
| Peritonitis | 33 (11.1%) |
| Brain tumor/metastasis | 25 (8.4%) |
| Meningitis | 3 (1.0%) |
| Gastrointestinal obstruction | 1 (0.3%) |
| Diabetes mellitus | |
| Yes | 35 (11.7%) |
| No | 261 (88.3%) |
| Parameter | n (%) |
|---|---|
| Daily dose of opioids * | |
| ≤19 mg | 106 (36.9%) |
| 20–49 mg | 112 (39.0%) |
| 50–99 mg | 35 (12.2%) |
| ≥100 mg | 34 (11.8%) |
| Regular use of opioids * | |
| Oxycodone | 165 (57.5%) |
| Morphine | 49 (17.1%) |
| Fentanyl | 42 (14.6%) |
| Hydromorphone | 21 (7.3%) |
| Others | 10 (3.5%) |
| Days from opioid initiation to starting naldemedine † | |
| ≤3 days | 68 (23.0%) |
| 4–7 days | 55 (18.6%) |
| 8–14 days | 53 (17.9%) |
| 15–29 days | 46 (15.5%) |
| 30–99 days | 43 (14.5%) |
| ≥100 days | 30 (10.1%) |
| Coadministration of laxatives ‡ | |
| Magnesium oxide | 190 (81.5%) |
| Sennoside | 81 (34.8%) |
| Bisacodyl | 31 (13.3%) |
| Lubiprostone | 26 (11.2%) |
| Sodium picosulfate hydrate | 23 (9.9%) |
| Others | 20 (8.6%) |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Diarrhea | 61 (20.6%) | 20 (6.8%) | 6 (2%) | 0 (0.0%) |
| Abdominal pain | 7 (2.4%) | 4 (1.4%) | 1 (0.3%) | ― |
| Nausea | 17 (5.7%) | 8 (2.7%) | 3 (1%) | ― |
| Anorexia | 30 (10.1%) | 9 (3%) | 4 (1.4%) | 0 (0.0%) |
| Vomiting | 3 (1%) | 3 (1%) | 2 (0.7%) | 0 (0.0%) |
| Fatigue | 19 (6.4%) | 4 (1.4%) | 3 (1%) | ― |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiruta, E.; Fujita, Y.; Imai, H.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; Matsuura, M.; et al. Real-World Patient Characteristics and Treatment Patterns of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer: A Multicenter Retrospective Chart Review Study. Medicina 2021, 57, 1233. https://doi.org/10.3390/medicina57111233
Hiruta E, Fujita Y, Imai H, Masuno T, Yamazaki S, Tanaka H, Kamiya T, Ito M, Takei S, Matsuura M, et al. Real-World Patient Characteristics and Treatment Patterns of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer: A Multicenter Retrospective Chart Review Study. Medicina. 2021; 57(11):1233. https://doi.org/10.3390/medicina57111233
Chicago/Turabian StyleHiruta, Eriko, Yukiyoshi Fujita, Hisao Imai, Takashi Masuno, Shigeki Yamazaki, Hajime Tanaka, Teruhiko Kamiya, Masako Ito, Satoshi Takei, Masato Matsuura, and et al. 2021. "Real-World Patient Characteristics and Treatment Patterns of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer: A Multicenter Retrospective Chart Review Study" Medicina 57, no. 11: 1233. https://doi.org/10.3390/medicina57111233
APA StyleHiruta, E., Fujita, Y., Imai, H., Masuno, T., Yamazaki, S., Tanaka, H., Kamiya, T., Ito, M., Takei, S., Matsuura, M., Nishiba, H., Mogi, J., Kotake, M., Koizuka, S., & Minato, K. (2021). Real-World Patient Characteristics and Treatment Patterns of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Cancer: A Multicenter Retrospective Chart Review Study. Medicina, 57(11), 1233. https://doi.org/10.3390/medicina57111233






