The Key Role of Ambulatory Blood Pressure Monitoring in the Detection of Masked Hypertension and Other Phenomena in Frail Geriatric Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawes, C.M.; Hoorn, S.V.; Rodgers, A. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Daly, D.D.; DePalma, S.; Minissian, M.B.; Orringer, C.E.; Smith, S.C. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll Cardiol. 2017, 70, 1785–1822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.J. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: New challenges of the old problem. Arch. Intern. Med. 2004, 164, 2126–2134. [Google Scholar] [CrossRef]
- Gijón-Conde, T.; Graciani, A.; López-García, E.; García-Esquinas, E.; Laclaustra, M.; Ruilope, L.M.; Rodríguez-Artalejo, F.; Banegas, J.R. Frailty, Disability, and Ambulatory Blood Pressure in Older Adults. J. Am. Med. Dir. Assoc. 2018, 19, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla García, J.D.; Jaén Águila, F.; Fernández Torres, C.; Gil Extremera, B.; Jiménez Alonso, J. Ambulatory blood pressure monitoring in the elderly. Int. J. Hypertens. 2012, 2012, 548286. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991, 265, 3255–3264. [Google Scholar] [CrossRef]
- Gasowski, J.; on behalf of the Systolic Hypertension in Europe Investigators; Staessen, J.; Celis, H.; Fagard, R.; Thijs, L.; Birkenhäger, W.; Bulpitt, C.; Fletcher, A.; Arabidze, G.; et al. Systolic Hypertension in Europe (Syst-Eur) trial phase 2: Objectives, protocol, and initial progress. Systolic Hypertension in Europe Investigators. J. Hum. Hypertens. 1999, 13, 135–145. [Google Scholar] [CrossRef][Green Version]
- Beckett, N.S.; Peters, R.; Fletcher, A.E.; Staessen, J.A.; Liu, L.; Dumitrascu, D.; Stoyanovsky, V.; Antikainen, R.L.; Nikitin, Y.; Anderson, C.; et al. Treatment of hypertension in patients 80 years of age or older. N. Engl. J. Med. 2008, 358, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Bulpitt, C.; Fletcher, A.; Beckett, N.; Coope, J.; Gil-Extremera, B.; Forette, F.; Nachev, C.; Potter, J.; Sever, P.; Staessen, J.A.; et al. Hypertension in the Very Elderly Trial (HYVET): Protocol for the main trial. Drugs Aging 2001, 18, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D.; Supiano, M.A.; Applegate, W.B.; Berlowitz, D.; Campbell, R.C.; Chertow, G.M.; Fine, L.J.; Haley, W.E.; Hawfield, A.T.; Ix, J.H.; et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA 2016, 315, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Labat, C.; Rossignol, P.; Fay, R.; Rolland, Y.; Valbusa, F.; Salvi, P.; Zamboni, M.; Manckoundia, P.; Hanon, O.; et al. Treatment With Multiple Blood Pressure Medications, Achieved Blood Pressure, and Mortality in Older Nursing Home Residents: The PARTAGE Study. JAMA Intern. Med. 2015, 175, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Horio, T.; Matayoshi, T.; Kamide, K. Masked hypertension: Subtypes and target organ damage. Clin. Exp. Hypertens. 2008, 30, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; O’Brien, E.; Staessen, J.A. Masked hypertension: Understanding its complexity. Eur. Heart J. 2017, 38, 1112–1118. [Google Scholar] [CrossRef]
- Yano, Y.; Bakris, G.L. Recognition and management of masked hypertension: A review and novel approach. J. Am. Soc. Hypertens. 2013, 7, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Pierdomenico, A.M.; Coccina, F.; Porreca, E. Prognosis of Masked and White Coat Uncontrolled Hypertension Detected by Ambulatory Blood Pressure Monitoring in Elderly Treated Hypertensive Patients. Am. J. Hypertens. 2017, 30, 1106–1111. [Google Scholar] [CrossRef]
- Babu, M.; Drawz, P. Masked Hypertension in CKD: Increased Prevalence and Risk for Cardiovascular and Renal Events. Curr. Cardiol. Rep. 2019, 21, 58. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Cacciolati, C.; Hanon, O.; Alpérovitch, A.; Dufouil, C.; Tzourio, C. Masked hypertension in the elderly: Cross-sectional analysis of a population-based sample. Am. J. Hypertens. 2011, 24, 674–680. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kikuya, M.; Metoki, H.; Asayama, K.; Obara, T.; Hashimoto, J.; Totsune, K.; Hoshi, H.; Satoh, H.; Imai, Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J. Am. Coll Cardiol. 2005, 46, 508–515. [Google Scholar] [CrossRef]
- Spannella, F.; Filipponi, A.; Giulietti, F.; Balietti, P.; Bernardi, B.; Rosettani, G.; Sarzani, R. Prognostic role of masked and white-coat hypertension: 10-Year mortality in treated elderly hypertensives. J. Hum. Hypertens. 2019, 33, 741–747. [Google Scholar] [CrossRef]
- Bobrie, G.; Chatellier, G.; Genes, N.; Clerson, P.; Vaur, L.; Vaisse, B.; Menard, J.; Mallion, J.-M. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA 2004, 291, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Thijs, L.; Hansen, T.W.; Li, Y.; Boggia, J.; Kikuya, M.; Björklund-Bodegård, K.; Ohkubo, T.; Jeppesen, J.; Torp-Pedersen, C.; et al. Significance of white-coat hypertension in older persons with isolated systolic hypertension: A meta-analysis using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes population. Hypertension 2012, 59, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Thijs, L.; Li, Y.; Hansen, T.; Boggia, J.; Liu, Y.; Asayama, K.; Björklund-Bodegård, K.; Ohkubo, T.; Jeppesen, J.; et al. Masked hypertension in diabetes mellitus: Treatment implications for clinical practice. Hypertension 2013, 61, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; O’Brien, E.; Thijs, L.; Asayama, K.; Staessen, J.A. Masked hypertension: A phenomenon of measurement. Hypertension 2015, 65, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sinha, A.D.; Light, R.P. Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Lüders, S.; Middeke, M. Antihypertensiva immer abends—Bloß nicht oder sinnvoll? (Antihypertensives always evenings-absolutely not or sensible?). Internist 2020, 61, 980–988. [Google Scholar] [CrossRef]
- Parati, G.; Ochoa, J.E.; Bilo, G.; Agarwal, R.; Covic, A.; Dekker, F.; Fliser, D.; Heine, G.H.; Jager, K.J.; Gargani, L.; et al. Hypertension in Chronic Kidney Disease Part 2: Role of Ambulatory and Home Blood Pressure Monitoring for Assessing Alterations in Blood Pressure Variability and Blood Pressure Profiles. Hypertension 2016, 67, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Inokuchi, T.; Hoshide, S.; Kanemaru, Y.; Shimada, K.; Kario, K. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am. J. Hypertens. 2011, 24, 285–291. [Google Scholar] [CrossRef]
- Kario, K.; Schwartz, J.E.; Pickering, T.G. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension 1999, 34, 685–691. [Google Scholar] [CrossRef][Green Version]
- Guo, H.; Tabara, Y.; Igase, M.; Yamamoto, M.; Ochi, N.; Kido, T.; Uetani, E.; Taguchi, K.; Miki, T.; Kohara, K. Abnormal nocturnal blood pressure profile is associated with mild cognitive impairment in the elderly: The J-SHIPP study. Hypertens. Res. 2010, 33, 32–36. [Google Scholar] [CrossRef]
- Goldstein, I.B.; Bartzokis, G.; Guthrie, D.; Shapiro, D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology 2002, 59, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; De Pascalis, V.; Favieri, F.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance: A Systematic Review. J. Clin. Med. 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Jacobs, M.J.; Wong, N.D.; L’Italien, G.J.; Lapuerta, P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001, 37, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Young, J.H.; Klag, M.J.; Muntner, P.; Whyte, J.L.; Pahor, M.; Coresh, J. Blood pressure and decline in kidney function: Findings from the Systolic Hypertension in the Elderly Program (SHEP). J. Am. Soc. Nephrol. 2002, 13, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Uallachain, G.N.; Murphy, G.; Avalos, G. The RAMBLER study: The role of ambulatory blood pressure measurement in routine clinical practice: A cross-sectional study. Iran. Med. J. 2006, 99, 276–279. [Google Scholar]
| Basic Clinical Parameters Sample (n = 118) Mean ± SD | ||
|---|---|---|
| Gender | Male (n = 42) | Female (n = 76) |
| Patients | 42 | 76 |
| Age | 81.6 ± 4.4 | 87.1 ± 6.2 |
| Weight (kg) | 74.2 ± 17.2 | 70.2 ± 15.8 |
| Height (cm) | 178.9 ± 9.1 | 161.1 ± 9.1 |
| Body mass index | 24.3 ± 5.4 | 22.1 ± 4.4 |
| Frailty Index (points) | 0.6 ± 0.2 | 0.5 ± 0.2 |
| BP Measurement | Time Period | Systolic MBP (mmHg) | Diastolic MBP (mmHg) |
|---|---|---|---|
| (n = 118) Mean ± SD | |||
| Office BP | |||
| 126.1 ± 8.1 | 74.9 ± 6.9 | ||
| ABPM | 24 h | 124.3 ± 29.8 | 72.8 ± 13.9 |
| Daytime | 125.9 ± 30.2 | 74.6 ± 12.9 | |
| Nighttime | 124.1 ± 32.1 | 70.9 ± 15.1 | |
| N | % | |
|---|---|---|
| (n = 118) | ||
| Correct office BP compensation (target values) | 118 | 100 |
| Correct ABPM compensation (target values): | 33 | 28 |
| 24 h systolic + diastolic BP | 33 | 28 |
| 24 h systolic BP | 33 | 28 |
| 24 h diastolic BP | 60 | 51 |
| Daytime systolic + diastolic BP | 33 | 28 |
| Daytime systolic BP | 33 | 28 |
| Daytime diastolic BP | 49 | 58 |
| Night systolic + diastolic BP | 8 | 7 |
| Night systolic BP | 15 | 13 |
| Night diastolic BP | 35 | 30 |
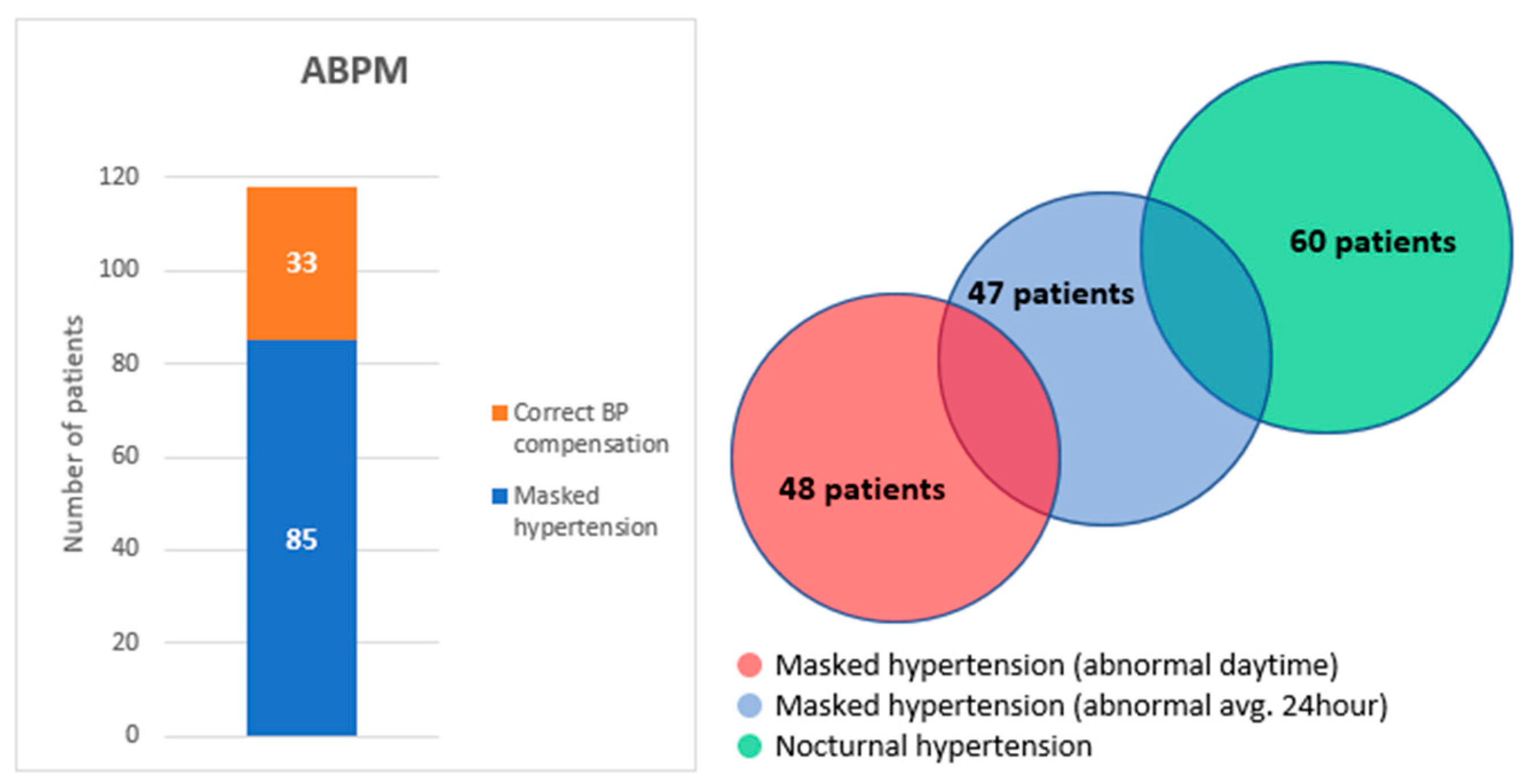
| Masked hypertension: | 85 | 72 |
| Masked hypertension (abnormal avg. 24 h) | 47 | 40 |
| Masked hypertension (abnormal daytime) | 48 | 41 |
| Nocturnal hypertension | 60 | 51 |
| N | % | |
|---|---|---|
| ACE inhibitors | 67 | 57 |
| Angiotensin receptor blockers (ARBs) | 39 | 33 |
| Calcium channel blockers (CCBs) | 62 | 53 |
| Diuretics | 43 | 36 |
| Beta blockers | 38 | 32 |
| Centrally acting antihypertensives | 19 | 16 |
| Monotherapy | 27 | 23 |
| Dual therapy | 32 | 27 |
| Triple therapy | 59 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koudelka, M.; Sovová, E. The Key Role of Ambulatory Blood Pressure Monitoring in the Detection of Masked Hypertension and Other Phenomena in Frail Geriatric Patients. Medicina 2021, 57, 1221. https://doi.org/10.3390/medicina57111221
Koudelka M, Sovová E. The Key Role of Ambulatory Blood Pressure Monitoring in the Detection of Masked Hypertension and Other Phenomena in Frail Geriatric Patients. Medicina. 2021; 57(11):1221. https://doi.org/10.3390/medicina57111221
Chicago/Turabian StyleKoudelka, Marek, and Eliška Sovová. 2021. "The Key Role of Ambulatory Blood Pressure Monitoring in the Detection of Masked Hypertension and Other Phenomena in Frail Geriatric Patients" Medicina 57, no. 11: 1221. https://doi.org/10.3390/medicina57111221
APA StyleKoudelka, M., & Sovová, E. (2021). The Key Role of Ambulatory Blood Pressure Monitoring in the Detection of Masked Hypertension and Other Phenomena in Frail Geriatric Patients. Medicina, 57(11), 1221. https://doi.org/10.3390/medicina57111221





