The Pharmacokinetic Profile and Bioavailability of Enteral N-Acetylcysteine in Intensive Care Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. NAC Formulations
2.3. Study Procedures
2.4. Analysis of the Blood Samples
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Characteristics of the Lung Group
3.3. Characteristics of the Brain Group
3.4. Characteristics of the Gut Group
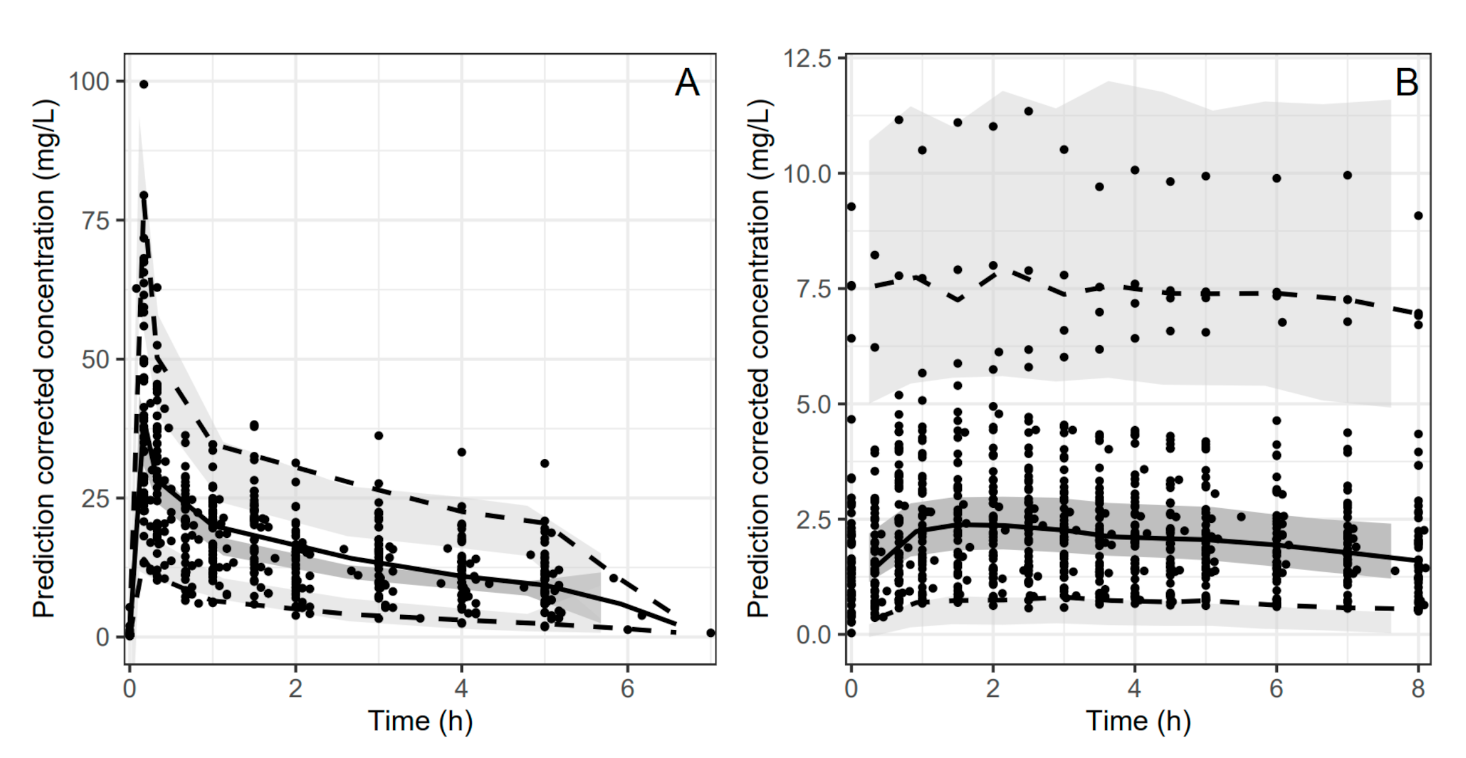
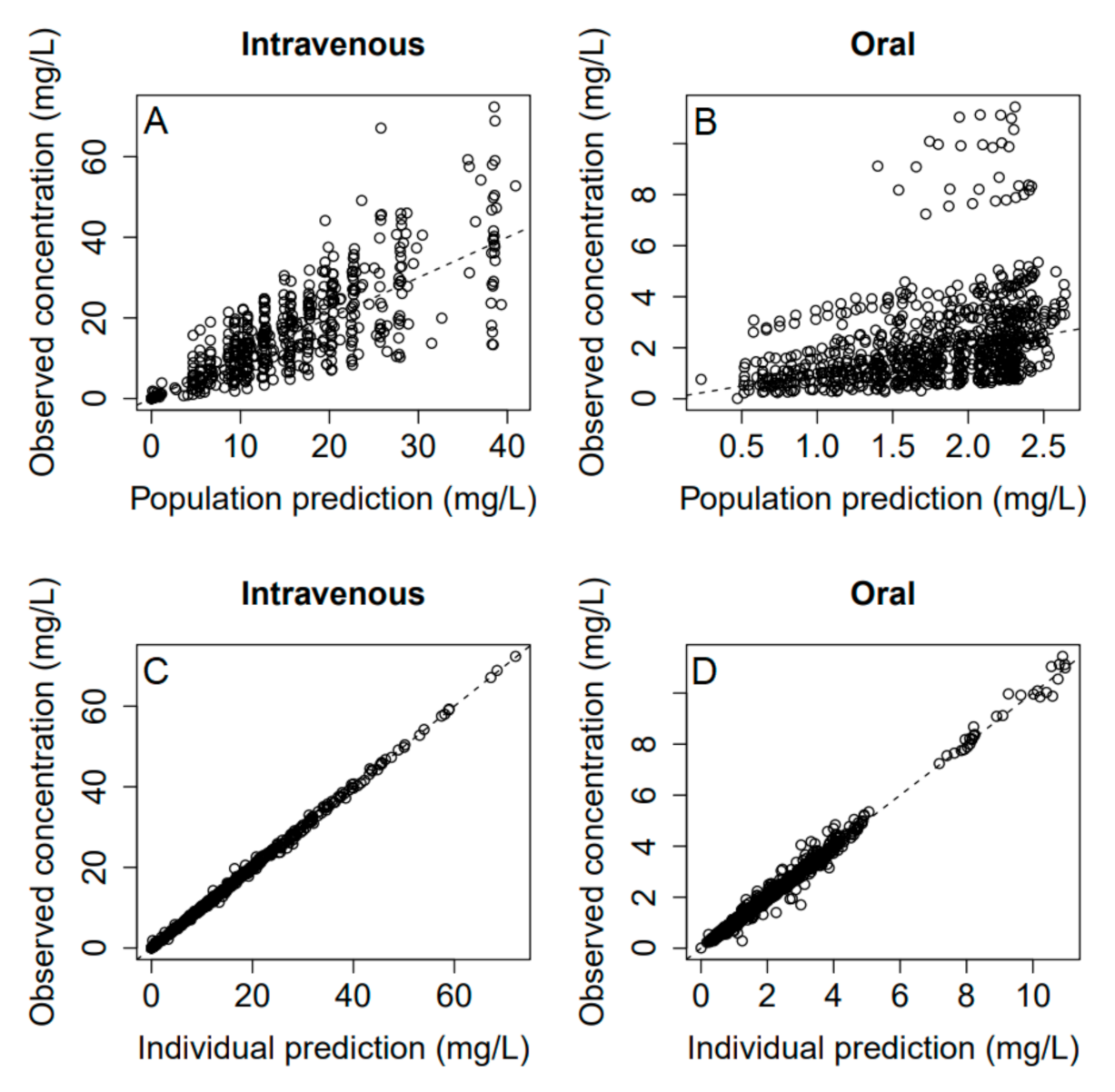
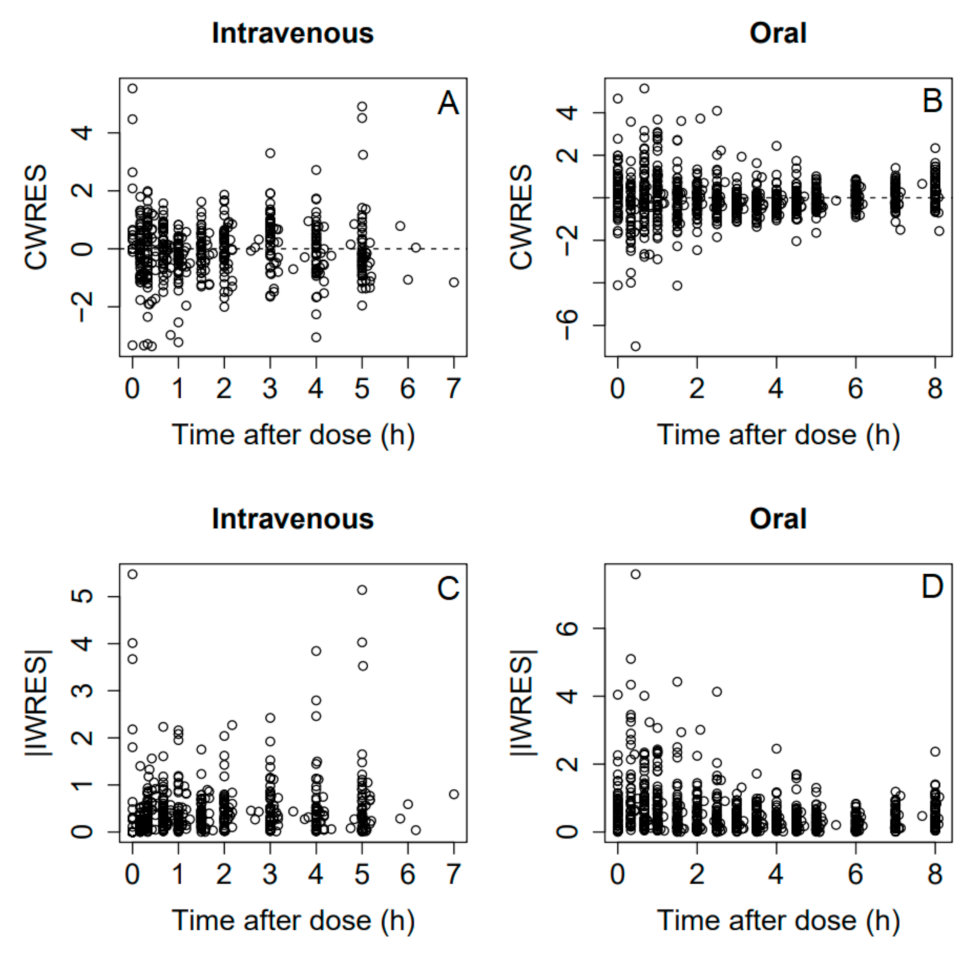
3.5. Pharmacokinetics and Bioavailability Evaluation
4. Discussion
4.1. Group Differences in PK
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheffner, A.L. Mucolytic-nu-acylated Sulfhydryl Compositions and Process for Treating Animal Mucus. U.S. Patent 3,091,569, 28 May 1963. [Google Scholar]
- Freye, R.; Berk, M. (Eds.) The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine, 1st ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar] [CrossRef]
- Grassi, C.; Morandini, G.C.; Frigerio, G. Clinical evaluation of systemic acetylcysteine by different routes of administration. Curr. Ther. Res. Clin. Exp. 1973, 15, 165–179. [Google Scholar]
- Grandjean, E.M.; Berthet, P.; Ruffmann, R.; Leuenberger, P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: A meta-analysis of published double-blind, placebo-controlled clinical trials. Clin. Ther. 2000, 22, 209–221. [Google Scholar] [CrossRef]
- Stey, C.; Steurer, J.; Bachmann, S.; Medici, T.C.; Tramer, M.R. The effect of oral N-acetylcysteine in chronic bronchitis: A quantitative systematic review. Eur. Respir. J. 2000, 16, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.F.; Harris, R.; Stout-Delgado, H.W. Targeted antioxidants as therapeutics for treatment of pneumonia in the elderly. Transl. Res. 2020, 220, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Poe, F.L.; Corn, J. N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2. Med. Hypotheses 2020, 143, 109862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ju, Y.; Ma, Y.; Wang, T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia A randomized controlled trial. Medicine 2018, 97, e13087. [Google Scholar] [CrossRef]
- Sharafkhah, M.; Abdolrazaghnejad, A.; Zarinfar, N.; Mohammadbeigi, A.; Massoudifar, A.; Abaszadeh, S. Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: A randomized, double blind, placebo-controlled clinical trial. Med. Gas Res. 2018, 8, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Masoompour, S.M.; Anushiravani, A.; Norouz, A.T. Evaluation of the Effect of Nebulized N-Acetylcysteine on Respiratory Secretions in Mechanically Ventilated Patients: Randomized Clinical Trial. Iran. J. Med. Sci. 2015, 40, 309–315. [Google Scholar]
- Rank, N.; Michel, C.; Haertel, C.; Lenhart, A.; Welte, M.; Meier-Hellmann, A.; Spies, C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit. Care Med. 2000, 28, 3799–3807. [Google Scholar] [CrossRef]
- Millea, P.J. N-Acetylcysteine: Multiple Clinical Applications. Am. Fam. Physician 2009, 80, 265–269. [Google Scholar]
- Liu, Y.M.; Liu, Y.; Lu, C.A.; Jia, J.Y.; Liu, G.Y.; Weng, L.P.; Wang, J.Y.; Li, G.X.; Wang, W.; Li, S.J.; et al. Relative Bioavailability of Generic and Branded Acetylcysteine Effervescent Tablets: A Single-Dose, Open-Label, Randomized-Sequence, Two-Period Crossover Study in Fasting Healthy Chinese Male Volunteers. Clin. Ther. 2010, 32, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, I.E.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Salamon, S.; Kramar, B.; Marolt, T.P.; Poljsak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClure, E.A.; Gipson, C.D.; Malcolm, R.J.; Kalivas, P.W.; Gray, K.M. Potential Role of N-Acetylcysteine in the Management of Substance Use Disorders. CNS Drugs 2014, 28, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodenstein, D.; De Coster, A.; Gazzaniga, A. Pharmacokinetics of oral acetylcysteine: Absorption, binding and metabolism in patients with respiratory disorders. Clin. Pharmacokinet. 1978, 3, 247–254. [Google Scholar] [CrossRef]
- Boman, G.; Backer, U.; Larsson, S.; Melander, B.; Wahlander, L. Oral acetylcysteine reduces exacerbation rate in chronic-bronchitis-report of a trial organized by the swedish society for pulmonary-diseases. Eur. J. Respir. Dis. 1983, 64, 405–415. [Google Scholar] [PubMed]
- Borgstrom, L.; Kagedal, B.; Paulsen, O. Pharmacokinetics of n-acetylcysteine in man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and bioavailability of reduced and oxidized n-acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Holdiness, M.R. Clinical pharmacokinetics of n-acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Di Stefano, A.F.D.; Radicioni, M. Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study. Adv. Ther. 2021, 38, 468–478. [Google Scholar] [CrossRef]
- Boucher, B.A.; Wood, G.C.; Swanson, J.M. Pharmacokinetic changes in critical illness. Crit. Care Clin. 2006, 22, 255–271. [Google Scholar] [CrossRef]
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to Drug Pharmacokinetics in the Critically Ill Patient. Chest 2012, 141, 1327–1336. [Google Scholar] [CrossRef]
- Roberts, D.J.; Hall, R.I. Drug absorption, distribution, metabolism and excretion considerations in critically ill adults. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1067–1084. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Tansley, G.; Hall, R. Pharmacokinetic considerations for drugs administered in the critically ill. Br. J. Hosp. Med. 2015, 76, 89–94. [Google Scholar] [CrossRef]
- Btaiche, I.F.; Chan, L.N.; Pleva, M.; Kraft, M.D. Critical Illness, Gastrointestinal Complications, and Medication Therapy during Enteral Feeding in Critically Ill Adult Patients. Nutr. Clin. Pract. 2010, 25, 32–49. [Google Scholar] [CrossRef]
- Cook, A.M.; Peppard, A.; Magnuson, B. Nutrition Considerations in Traumatic Brain Injury. Nutr. Clin. Pract. 2008, 23, 608–620. [Google Scholar] [CrossRef]
- Jones, A.L.; Jarvie, D.R.; Simpson, D.; Hayes, P.C.; Prescott, L.F. Pharmacokinetics of N-acetylcysteine are altered in patients with chronic liver disease. Aliment. Pharmacol. Ther. 1997, 11, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Nolin, T.D.; Ouseph, R.; Himmefarb, J.; McMenamin, M.E.; Ward, R.A. Multiple-Dose Pharmacokinetics and Pharmacodynamics of N-Acetylcysteine in Patients with End-Stage Renal Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1588–1594. [Google Scholar] [CrossRef]
- Hernandez, S.H.; Howland, M.; Schiano, T.D.; Hoffman, R.S. The pharmacokinetics and extracorporeal removal of N-acetylcysteine during renal replacement therapies. Clin. Toxicol. 2015, 53, 941–949. [Google Scholar] [CrossRef]
- UpToDate. Acetylcysteine: Drug Information. Available online: https://www.uptodate.com/contents/acetylcysteine-drug-information?search=acetylcysteine&source=panel_search_result&selectedTitle=1~128&usage_type=panel&kp_tab=drug_general&display_rank=1#F130222 (accessed on 11 January 2020).
- Lexicomp. Drug Interactions. Available online: https://www.uptodate.com/drug-interactions/?search=acetylcysteine&topicId=9317&source=responsive_topic#di-analyze (accessed on 11 January 2020).
- Paulsen, O.; Borgstrom, L.; Kagedal, B.; Walder, M. No effect of oral n-acetylcysteine on the bioavailability of erythromycin and bacampicillin. Eur. Respir. J. 1988, 1, 171–175. [Google Scholar] [PubMed]
- Summary of Product Characteristics. ACC 100 mg/mL Solution for Injection. Available online: https://www.ravimiregister.ee/Data/SPC/SPC_1035379.pdf (accessed on 11 January 2020).
- Summary of Product Characteristics. ACC 200 mg Powder for Oral Solution. Available online: https://www.ravimiregister.ee/Data/SPC/SPC_1039238.pdf (accessed on 11 January 2020).
- Bald, E.; Glowacki, R. 2-Chloro-1-methylquinolinium tetrafluoroborate as an effective and thiol specific uv-tagging reagent for liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1323–1339. [Google Scholar] [CrossRef]
- Glowacki, R.; Bald, E. Determination of N-Acetylcysteine and Main Endogenous Thiols in Human Plasma by HPLC with Ultraviolet Detection in the Form of Their S-Quinolinium Derivatives. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2530–2544. [Google Scholar] [CrossRef]
- McCaughan, B.; Kay, G.; Di Salvo, A.; Cox, P.J.; Cairns, D. Synthesis, Characterization, Photo-physical Evaluation and Crystal Structure of 2-Methoxy-1-Methylquinolinium Tetrafluoroborate, a Potentially Selective Agent for the Quantitative Measurement of Biologically Relevant Thiols. J. Chem. Crystallogr. 2010, 40, 417–422. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Liu, X.X. Handling Missing Dosing History in Population Pharmacokinetic Modeling: An Extension to MDM Method. CPT Pharmacomet. Syst Pharmacol. 2019, 8, 11. [Google Scholar] [CrossRef]
- Rabbie, S.C.; Martin, P.D.; Flanagan, T.; Basit, A.W.; Standing, J.F. Estimating the variability in fraction absorbed as a paradigm for informing formulation development in early clinical drug development. Eur. J. Pharm. Sci. 2016, 89, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Reintam, A.; Parm, P.; Kitus, R.; Starkopf, J.; Kern, H. Gastrointestinal Failure score in critically ill patients: A prospective observational study. Crit. Care 2008, 12, R90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padar, M.; Starkopf, J.; Uusvel, G.; Blaser, A.R. Gastrointestinal failure affects outcome of intensive care. J. Crit. Care 2019, 52, 103–108. [Google Scholar] [CrossRef]
- Oliva, A.; Bianchi, A.; Russo, A.; Ceccarelli, G.; Cancelli, F.; Aloj, F.; Fegatelli, D.A.; Mastroianni, C.M.; Venditti, M. Effect of N-Acetylcysteine Administration on 30-Day Mortality in Critically Ill Patients with Septic Shock Caused by Carbapenem-Resistant Klebsiella pneumoniae and Acinetobacter baumannii: A Retrospective Case-Control Study. Antibiotics 2021, 10, 271. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Median (Interquartile Range) | ||
|---|---|---|---|
| Lung Group | Brain Group | Gut Group | |
| Number of patients | 18 | 19 | 17 |
| Sex (male) | 13 | 15 | 11 |
| Age (years) | 65 (59–71) | 61 (50–69) | 71 (66–78) |
| Height (cm) | 175 (167–180) | 175 (170–180) | 169 (167–175) |
| Weight (kg) | 78 (71–96) | 80 (73–85) | 76 (66–90) |
| BMI (kg/m2) | 25 (23–29) | 26 (24–28) | 26 (24–29) |
| APACHE II score on admission day | 22 (19–26) | 22 (20–25) | 19 (12–23) |
| Median (Interquartile Range) | ||||||
|---|---|---|---|---|---|---|
| Lung Group | Brain Group | Gut Group | ||||
| First Day | Second Day | First Day | Second Day | First Day | Second Day | |
| FLUID BALANCE per 24 h | ||||||
| IV infusion (mL) A | 1641 (1116–2941) | 1464 (689–2603) | 1749 (1415–2935) | 1214 (787–2174) | 3023 (2248–3495) | 2599 (1680–3470) |
| PO liquids (mL) B | 1013 (348–1341) | 1061 (731–1286) | 840 (425–1195) | 890 (390–1275) | 0 (0–400) | 60 (0–450) |
| Urine (mL) | 2075 (1734–2893) | 1863 (1553–2123) | 2445 (2105–2860) | 1550 (1100–2050) | 1800 (1095–2850) | 1850 (1570–2200) |
| NGFT liquids (mL) | 25 (0–218) | 100 (0–296) | 40 (0–263) | 0 (0–25) | 50 (10–350) | 0 (0–750) |
| Fluid balance (mL) | 1044 (93–1452) | 388 (119–1350) | 630 ((–609)–1533) | 550 ((–40)–924) | 1188 (337–2285) | 1183 (275–1982) |
| LAB TESTS | ||||||
| HGB (g/L) | 106 (93–124) | 102 (92–125) | 106 (98–117) | 106 (97–118) | 103 (92–111) | 92 (88–97) |
| HCT (%) | 32 (27–41) | 32 (28–39) | 31 (30–34) | 31 (29–36) | 31 (27–33) | 28 (27–29) |
| CRP (mg/L) C | 150 (76–168) | 132 (91–231) | 90 (51–125) | 125 (86–179) | 185 (157–321) | 240 (131–313) |
| Creatinine (µmol/mL) | 81 (44–116) | 76 (43–97) | 63 (51–97) | 53 (49–78) | 71 (43–132) | 75 (49–124) |
| eGFR (mL/min/1.73 m2) D | 87 (60–116) | 91 (82–120) | 103 (67–122) | 108 (86–136) | 81 (45–110) | 77 (56–104) |
| Urea (mmol/L) E | 9 (4–11) | 9 (4–10) | 5 (3–7) | 5 (3–7) | 9 (6–12) | 8 (6–11) |
| Bilirubin (µmol/L) | 12 (7–24) | 12 (7–26) | 8 (6–16) | 8 (6–16) | 11 (9–20) | 12 (10–20) |
| AST (U/L) | 45 (33–74) | 45 (33–67) | 33 (22–85) | 32 (22–65) | 30 (19–58) | 23 (19–58) |
| ALT (U/L) | 34 (19–66) | 36 (19–64) | 31 (13–50) | 37 (13–52) | 15 (11–45) | 14 (10–26) |
| Protein (U/L) F | 61 (59–67) | 62 (61–64) | 61 (58–63) | 61 (58–63) | 55 (52–60) | 55 (54–60) |
| Albumin (g/L) G | 27 (25–30) | 29 (27–32) | 34 (32–35) | 34 (32–35) | 26 (25–29) | 27 (25–32) |
| Lactate (mmol/L) H | 1.5 (1.1–1.8) | 1.4 (1.2–1.8) | 1.1 (0.8–1.5) | 1.1 (0.8–1.2) | 1.3 (1.1–1.6) | 1.1 (1.0–1.9) |
| SOFA score | 9 (7–11) | 8 (6–12) | 8 (6–10) | 7 (4–8) | 7 (5–11) | 6 (4–11) |
| GIF-score I | 1 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 1 (1–1) | 1 (1–2) |
| PK Characteristic | Median (Interquartile Range) | ||
|---|---|---|---|
| Lung Group | Brain Group | Gut Group | |
| Cmax IV (mg/L) | 37.7 (28.4–50.4) | 23.6 (17.2–42.0) | 40.0 (33.9–53.9) |
| Cmax PO (mg/L) | 2.7 (1.5–4.3) | 1.9 (1.5–2.8) | 2.8 (2.3–4.3) |
| tmax PO (min) | 120 (50–150) | 90 (60–120) | 60 (60–120) |
| AUC0–5 IV (mg/L×h) | 79 (64–97) | 53 (32–59) | 98 (54–110) |
| AUC0–8 PO (mg/L×h) | 18 (10–24) | 9 (6–14) | 17 (12–26) |
| Parameter | Estimate (Standard Error) | IIV a (Standard Error) | Shrinkage (%) |
|---|---|---|---|
| V (L) = V0·Θ1 brain group | |||
| V0 | 11.80 (1.44) | 56.3 (5.5) | 1.8 |
| Θ1 | 1.59 (0.29) | - | - |
| CL (L/h) = CL0·Θ2 brain group | |||
| CL0 | 3.57 (0.20) | 56.8 (11.5) | 0.1 |
| Θ2 | 1.99 (0.28) | - | - |
| KA (1/h) = KA0·Θ3 brain group | |||
| KA0 | 0.33 (0.09) | 110 (14.5) | 6.0 |
| Θ3 | 2.63 (0.98) | - | - |
| F b | −2.03 (0.07) | 46.1 (7.0) | 10.1 |
| V2 (L) = V20·Θ4 brain group | |||
| V20 | 12.0 (2.0) | 66.9 (21.1) | 5.3 |
| Θ4 | 2.07 (0.47) | - | - |
| Q2 (L/h) = Q20·Θ5 brain group | |||
| Q20 | 4.60 (0.73) | 63.2 (13.1) | 18.4 |
| Θ5 | 2.16 (0.49) | - | - |
| V3 (L) | 8.62 (0.98) | 52.1 (7.7) | 10.9 |
| Q3 (L/h) | 43.8 (6.46) | 55.2 (10.3) | 25.5 |
| Residual variability | |||
| Intravenous, proportional (%) | 3.4 (1.3) | 21.6 | |
| Oral, proportional (%) | 8.2 (1.8) | 9.6 | |
| Intravenous, additive (mg/L) | 0.3 (0.1) | 21.6 | |
| Oral, additive (mg/L) | 0.07 (0.03) | 9.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teder, K.; Maddison, L.; Soeorg, H.; Meos, A.; Karjagin, J. The Pharmacokinetic Profile and Bioavailability of Enteral N-Acetylcysteine in Intensive Care Unit. Medicina 2021, 57, 1218. https://doi.org/10.3390/medicina57111218
Teder K, Maddison L, Soeorg H, Meos A, Karjagin J. The Pharmacokinetic Profile and Bioavailability of Enteral N-Acetylcysteine in Intensive Care Unit. Medicina. 2021; 57(11):1218. https://doi.org/10.3390/medicina57111218
Chicago/Turabian StyleTeder, Kersti, Liivi Maddison, Hiie Soeorg, Andres Meos, and Juri Karjagin. 2021. "The Pharmacokinetic Profile and Bioavailability of Enteral N-Acetylcysteine in Intensive Care Unit" Medicina 57, no. 11: 1218. https://doi.org/10.3390/medicina57111218
APA StyleTeder, K., Maddison, L., Soeorg, H., Meos, A., & Karjagin, J. (2021). The Pharmacokinetic Profile and Bioavailability of Enteral N-Acetylcysteine in Intensive Care Unit. Medicina, 57(11), 1218. https://doi.org/10.3390/medicina57111218





