Current Status and Trends of Minimally Invasive Gastrectomy in Korea
Abstract
1. Introduction
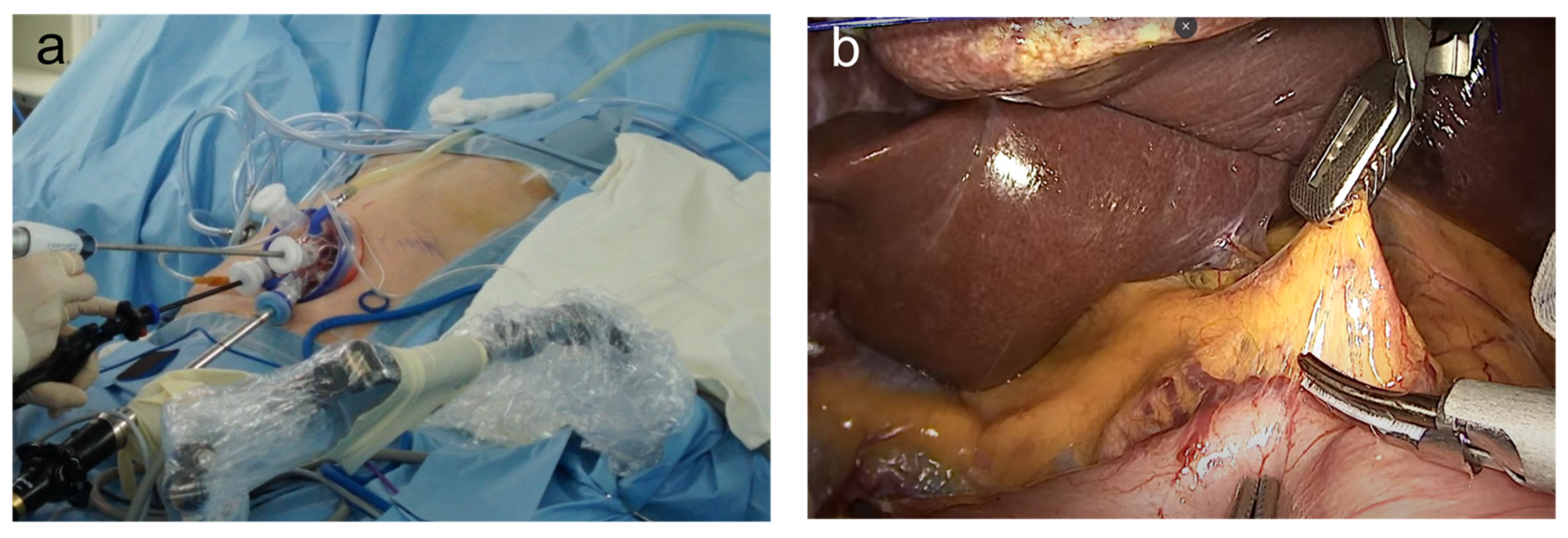
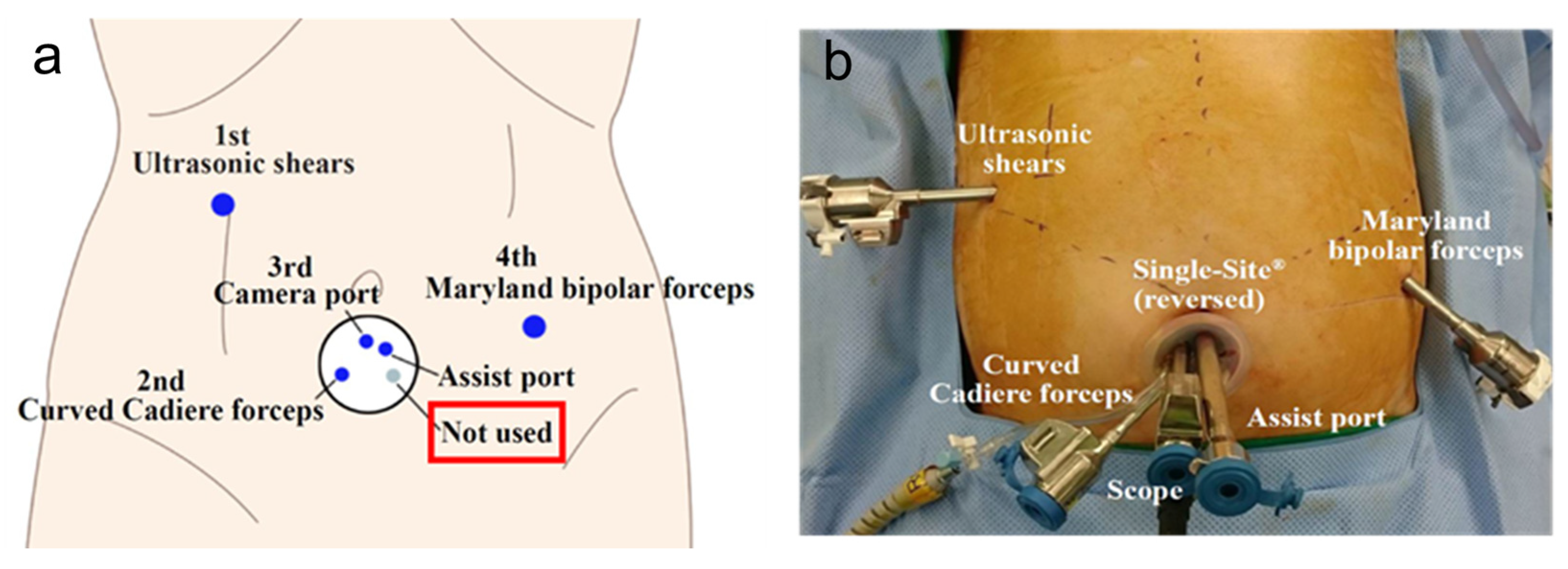
2. Reduced-Port and Single-Incision Laparoscopic Gastrectomy
2.1. Concept
2.2. Operative Procedures
2.3. Technical Feasibility and Surgical Outcomes
2.4. Oncologic Validity
2.5. Controversial Issues and Future Perspectives
3. Robotic Gastrectomy
3.1. Concept
3.2. Technical Feasibility and Surgical Outcomes
3.3. Operative Time and Learning Curve
3.4. Oncologic Outcomes
3.5. Future Perspectives
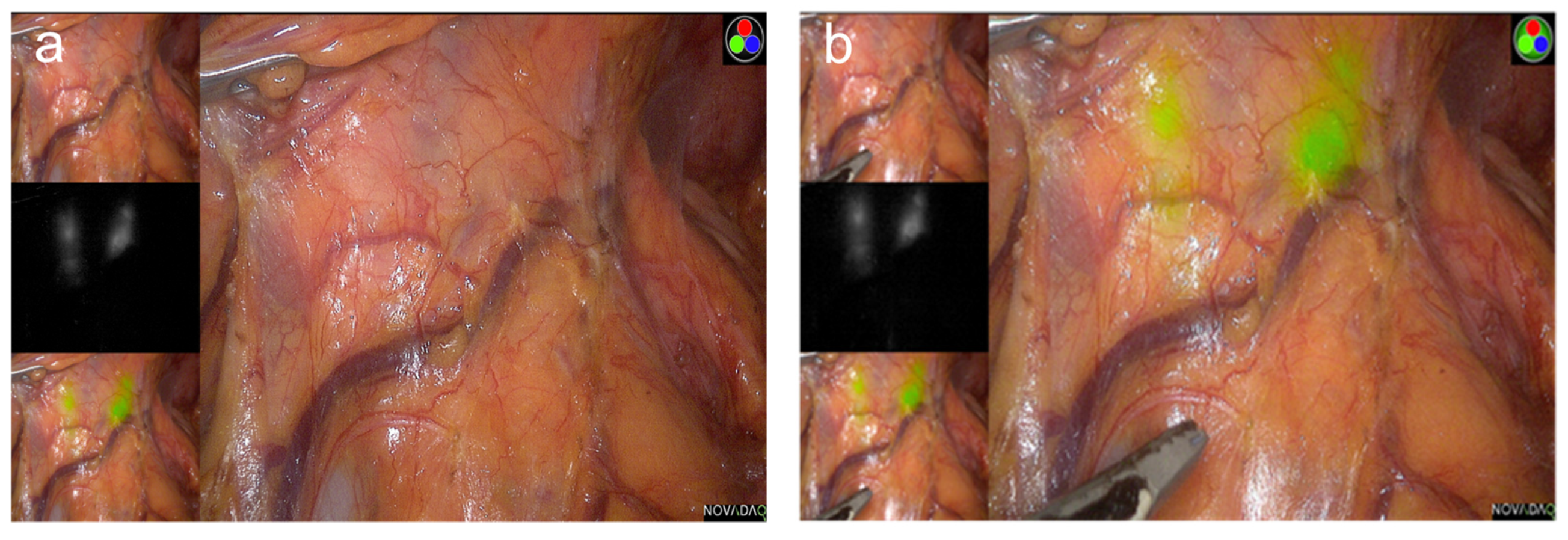
4. Fluorescence Image-Guided Gastrectomy
4.1. Concept
4.2. Oncologic Outcomes
4.3. Sentinel-Node Navigation Surgery
4.4. Technical Advantages
4.5. Controversial Issues and Future Perspectives
5. Oncometabolic Surgery
5.1. Concept
5.2. Patient Selection
5.3. Efficacy
5.4. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J. Gastric Cancer 2016, 16, 131–140. [Google Scholar] [CrossRef]
- Kitano, S.; Iso, Y.; Moriyama, M.; Sugimachi, K. Laparoscopy-assisted Billroth I gastrectomy. Surg. Laparosc. Endosc. 1994, 4, 146–148. [Google Scholar]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Song, K.Y.; Ryu, S.Y. Long-term results of laparoscopic gastrectomy for gastric cancer: A large-scale case-control and case-matched Korean multicenter study. J. Clin. Oncol. 2014, 32, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Effect of laparoscopic distal gastrectomy vs. open distal gastrectomy on long-term survival among patients with stage I gastric cancer: The KLASS-01 randomized clinical trial. JAMA Oncol. 2019, 5, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Hyung, W.J.; Cho, G.S.; Kim, M.C.; Han, S.U.; Kim, W.; Ryu, S.W.; Lee, H.J.; Song, K.Y. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: An interim report—A phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann. Surg. 2010, 251, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: Short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann. Surg. 2016, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Baik, Y.H.; Yun, Y.H.; Nam, B.H.; Kim, D.H.; Choi, I.J.; Bae, J.M. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: Results of a prospective randomized clinical trial. Ann. Surg. 2008, 248, 721–727. [Google Scholar] [CrossRef]
- Lee, H.J.; Hyung, W.J.; Yang, H.K.; Han, S.U.; Park, Y.K.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann. Surg. 2019, 270, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Hyung, W.J.; Yang, H.K.; Park, Y.K.; Lee, H.J.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; Hur, H.; et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: The KLASS-02-RCT randomized clinical trial. J. Clin. Oncol. 2020, 38, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Chang, M.S.; Han, D.S. Comparing the surgical outcomes of dual-port laparoscopic distal gastrectomy and three-port laparoscopic distal gastrectomy for gastric cancer. Ann. Surg. Treat. Res. 2021, 100, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Son, S.Y.; Jung, D.H.; Park, D.J.; Kim, H.H. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: Comparative study with multi-port laparoscopic distal gastrectomy. J. Am. Coll. Surg. 2014, 219, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Son, S.Y.; Jung, D.H.; Park, Y.S.; Shin, D.J.; Park, D.J.; Kim, H.H. Solo intracorporeal esophagojejunostomy reconstruction using a laparoscopic scope holder in single-port laparoscopic total gastrectomy for early gastric cancer. J. Gastric Cancer 2015, 15, 132–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahn, S.H.; Son, S.Y.; Lee, C.M.; Jung, D.H.; Park, J.; Kim, H.H. Intracorporeal uncut Roux-en-Y gastrojejunostomy reconstruction in pure single-incision laparoscopic distal gastrectomy for early gastric cancer: Unaided stapling closure. J. Am. Coll. Surg. 2014, 218, e17–e21. [Google Scholar] [CrossRef] [PubMed]
- Jeong, O.; Park, Y.K.; Ryu, S.Y. Early experience of duet laparoscopic distal gastrectomy (duet-LDG) using three abdominal ports for gastric carcinoma: Surgical technique and comparison with conventional laparoscopic distal gastrectomy. Surg. Endosc. 2016, 30, 3559–3566. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, S.M.; Ha, M.H.; Seo, J.E.; Choi, M.G.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, J.H. Comparison of reduced port totally laparoscopic-assisted total gastrectomy (duet TLTG) and conventional laparoscopic-assisted total gastrectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, e132–e136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Ha, M.H.; Seo, J.E.; Kim, J.E.; Choi, M.G.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, J.H. Comparison of reduced port totally laparoscopic distal gastrectomy (duet TLDG) and conventional laparoscopic-assisted distal gastrectomy. Ann. Surg. Oncol. 2015, 22, 2567–2572. [Google Scholar] [CrossRef]
- Lee, H.H.; Song, K.Y.; Lee, J.S.; Park, S.M.; Kim, J.J. Delta-shaped anastomosis, a good substitute for conventional Billroth I technique with comparable long-term functional outcome in totally laparoscopic distal gastrectomy. Surg. Endosc. 2015, 29, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Lee, E.J.; Kim, G.Y. Evaluation of reduced port laparoscopic distal gastrectomy performed by a novice surgeon. J. Gastric Cancer 2021, 21, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Lee, H.H. Is the 5-ports approach necessary in laparoscopic gastrectomy? Feasibility of reduced-port totally laparoscopic gastrectomy for the treatment of gastric cancer: A prospective cohort study. Int. J. Surg. 2016, 29, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Song, K.Y.; Jung, Y.J.; Kim, J.H.; Park, C.H.; Lee, H.H. Right-side approach-duet totally laparoscopic distal gastrectomy (R-Duet TLDG) using a three-port to treat gastric cancer. J. Gastrointest. Surg. 2018, 22, 578–586. [Google Scholar] [CrossRef]
- Hyun, M.H.; Lee, C.H.; Kwon, Y.J.; Cho, S.I.; Jang, Y.J.; Kim, D.H.; Kim, J.H.; Park, S.H.; Mok, Y.J.; Park, S.S. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: Comparisons of surgery, complications, and surgical stress. Ann. Surg. Oncol. 2013, 20, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Han, S.U.; Yang, H.K.; Kim, Y.W.; Lee, H.J.; Ryu, K.W.; Park, J.M.; An, J.Y.; Kim, M.C.; Park, S.; et al. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann. Surg. 2016, 263, 103–109. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, M.S.; Song, K.J.; Woo, Y.; Hyung, W.J. Rapid and safe learning of robotic gastrectomy for gastric cancer: Multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur. J. Surg. Oncol. 2014, 40, 1346–1354. [Google Scholar] [CrossRef]
- Son, T.; Lee, J.H.; Kim, Y.M.; Kim, H.I.; Noh, S.H.; Hyung, W.J. Robotic spleen-preserving total gastrectomy for gastric cancer: Comparison with conventional laparoscopic procedure. Surg. Endosc. 2014, 28, 2606–2615. [Google Scholar] [CrossRef]
- Woo, Y.; Hyung, W.J.; Pak, K.H.; Inaba, K.; Obama, K.; Choi, S.H.; Noh, S.H. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch. Surg. 2011, 146, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Son, S.Y.; Cui, L.H.; Shin, H.J.; Hur, H.; Han, S.U. Real-time vessel navigation using indocyanine green fluorescence during robotic or laparoscopic gastrectomy for gastric cancer. J. Gastric Cancer 2017, 17, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kong, S.H.; Park, J.H.; Son, Y.G.; Huh, Y.J.; Suh, Y.S.; Lee, H.J.; Yang, H.K. Assessment of the completeness of lymph node dissection using near-infrared imaging with indocyanine green in laparoscopic gastrectomy for gastric cancer. J. Gastric Cancer 2018, 18, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.G.; Son, T.; Kim, H.I.; Hyung, W.J. Fluorescent lymphography-guided lymphadenectomy during robotic radical gastrectomy for gastric cancer. JAMA Surg. 2019, 154, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, Y.W.; Ryu, K.W.; Nam, B.H.; Lee, Y.J.; Jeong, S.H.; Park, J.H.; Hur, H.; Han, S.U.; Min, J.S.; et al. Assessment of laparoscopic stomach preserving surgery with sentinel basin dissection versus standard gastrectomy with lymphadenectomy in early gastric cancer-A multicenter randomized phase III clinical trial (SENORITA trial) protocol. BMC Cancer 2016, 16, 340. [Google Scholar] [CrossRef]
- Park, S.H.; Berlth, F.; Choi, J.H.; Park, J.H.; Suh, Y.S.; Kong, S.H.; Park, D.J.; Lee, H.J.; Yang, H.K. Near-infrared fluorescence-guided surgery using indocyanine green facilitates secure infrapyloric lymph node dissection during laparoscopic distal gastrectomy. Surg. Today 2020, 50, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, C.M.; Park, S.; Jung, D.H.; Jang, Y.J.; Kim, J.H.; Park, S.H.; Mok, Y.J. Long-term follow-up for type 2 diabetes mellitus after gastrectomy in non-morbidly obese patients with gastric cancer: The legitimacy of onco-metabolic surgery. J. Gastric Cancer 2017, 17, 283–294. [Google Scholar] [CrossRef]
- Omori, T.; Oyama, T.; Akamatsu, H.; Tori, M.; Ueshima, S.; Nishida, T. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg. Endosc. 2011, 25, 2400–2404. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.; Nicholson, J. Single incision laparoscopic surgery in general surgery: A review. Ann. R. Coll. Surg. Engl. 2011, 93, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Geng, J. Comparative analysis of clavien-dindo grade and risk factors of complications after dual-port laparoscopic distal gastrectomy and hand-assisted laparoscopic gastrectomy. J. Oncol. 2021, 2021, 4747843. [Google Scholar] [CrossRef]
- Kawamura, H.; Tanioka, T.; Shibuya, K.; Tahara, M.; Takahashi, M. Comparison of the invasiveness between reduced-port laparoscopy-assisted distal gastrectomy and conventional laparoscopy-assisted distal gastrectomy. Intern. Surg. 2013, 98, 247–253. [Google Scholar] [CrossRef]
- Kashiwagi, H.; Kumagai, K.; Monma, E.; Nozue, M. Dual-port distal gastrectomy for the early gastric cancer. Surg. Endosc. 2015, 29, 1321–1326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omori, T.; Fujiwara, Y.; Moon, J.; Sugimura, K.; Miyata, H.; Masuzawa, T.; Kishi, K.; Miyoshi, N.; Tomokuni, A.; Akita, H.; et al. Comparison of single-incision and conventional multi-port laparoscopic distal gastrectomy with D2 lymph node dissection for gastric cancer: A propensity score-matched analysis. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 817–824. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Yamamoto, K.; Hara, H.; Shinno, N.; Yamamoto, M.; Sugimura, K.; Wada, H.; Takahashi, H.; Yasui, M.; Miyata, H.; et al. A randomized controlled trial of single-port versus multi-port laparoscopic distal gastrectomy for gastric cancer. Surg. Endosc. 2021, 35, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Zuiki, T.; Hosoya, Y.; Kaneda, Y.; Kurashina, K.; Saito, S.; Ui, T.; Haruta, H.; Hyodo, M.; Sata, N.; Lefor, A.T.; et al. Stenosis after use of the double-stapling technique for reconstruction after laparoscopy-assisted total gastrectomy. Surg. Endosc. 2013, 27, 3683–3689. [Google Scholar] [CrossRef]
- Kosuga, T.; Hiki, N.; Nunobe, S.; Ohashi, M.; Kubota, T.; Kamiya, S.; Sano, T.; Yamaguchi, T. Does the single-stapling technique for circular-stapled esophagojejunostomy reduce anastomotic complications after laparoscopic total gastrectomy? Ann. Surg. Oncol. 2015, 22, 3606–3612. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.S.; Park, J.H.; Kim, T.H.; Huh, Y.J.; Son, Y.G.; Yang, J.Y.; Kong, S.H.; Lee, H.J.; Yang, H.K. Unaided stapling technique for pure single-incision distal gastrectomy in early gastric cancer: Unaided delta-shaped anastomosis and uncut roux-en-Y anastomosis. J. Gastric Cancer 2015, 15, 105–112. [Google Scholar] [CrossRef]
- Lee, C.M.; Park, D.W.; Jung, D.H.; Jang, Y.J.; Kim, J.H.; Park, S.; Park, S.H. Single-port laparoscopic proximal gastrectomy with double tract reconstruction for early gastric cancer: Report of a case. J. Gastric Cancer 2016, 16, 200–206. [Google Scholar] [CrossRef][Green Version]
- Kang, S.H.; Won, Y.; Lee, K.; Youn, S.I.; Min, S.H.; Park, Y.S.; Ahn, S.H.; Kim, H.H. Three-dimensional (3D) visualization provides better outcome than two-dimensional (2D) visualization in single-port laparoscopic distal gastrectomy: A propensity-matched analysis. Langenbeck’s Arch. Surg. 2021, 406, 473–478. [Google Scholar] [CrossRef]
- Kim, A.; Lee, C.M.; Park, S. Is it beneficial to utilize an articulating instrument in single-port laparoscopic gastrectomy? J. Gastric Cancer 2021, 21, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Jeong, O.; Seo, H.S.; Choi, M.G.; Ryu, S.Y.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, J.H. Long-term oncological outcomes of reduced three-port laparoscopic gastrectomy for early-stage gastric carcinoma: A retrospective large-scale multi-institutional study. J. Gastric Cancer 2021, 21, 93–102. [Google Scholar] [CrossRef]
- Omori, T.; Fujiwara, Y.; Yamamoto, K.; Yanagimoto, Y.; Sugimura, K.; Masuzawa, T.; Kishi, K.; Takahashi, H.; Yasui, M.; Miyata, H.; et al. The safety and feasibility of single-port laparoscopic gastrectomy for advanced gastric cancer. J. Gastrointest. Surg. 2019, 23, 1329–1339. [Google Scholar] [CrossRef]
- Kodera, Y. Reduced port surgery for gastric cancer: Another giant leap for mankind? Gastric Cancer 2013, 16, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Getzen, T.E.; McKee, H.J.; Laud, P. Economic and demographic trends signal an impending physician shortage. Health Aff. 2002, 21, 140–154. [Google Scholar] [CrossRef]
- Leibrandt, T.J.; Pezzi, C.M.; Fassler, S.A.; Reilly, E.F.; Morris, J.B. Has the 80-hour work week had an impact on voluntary attrition in general surgery residency programs? J. Am. Coll. Surg. 2006, 202, 340–344. [Google Scholar] [CrossRef]
- Chen, K.; Xu, X.W.; Zhang, R.C.; Pan, Y.; Wu, D.; Mou, Y.P. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J. Gastroenterol. 2013, 19, 5365–5376. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z.; Liu, S.; Hu, L.; He, Y.; Fang, Y.; Wan, X. Short-term outcomes and prognosis of laparoscopy-assisted total gastrectomy in elderly patients with stomach cancer. Surg. Endosc. 2020, 34, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Suh, Y.S.; Kim, T.H.; Choi, Y.H.; Choi, J.H.; Kong, S.H.; Park, D.J.; Lee, H.J.; Yang, H.K. Postoperative morbidity and quality of life between totally laparoscopic total gastrectomy and laparoscopy-assisted total gastrectomy: A propensity-score matched analysis. BMC Cancer 2021, 21, 1016. [Google Scholar] [CrossRef]
- Desai, P.M. Pain management and pulmonary dysfunction. Crit. Care Clin. 1999, 15, 151–166. [Google Scholar] [CrossRef]
- Ford, G.T.; Rosenal, T.W.; Clergue, F.; Whitelaw, W.A. Respiratory physiology in upper abdominal surgery. Clin. Chest Med. 1993, 14, 237–252. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, H.J.; Park, J.H.; Kim, T.H.; Son, Y.G.; Huh, Y.J.; Choi, J.H.; Kim, S.H.; Park, J.H.; Suh, Y.S.; et al. Clinical significance of intra-operative gastroscopy for tumor localization in totally laparoscopic partial gastrectomy. J. Gastrointest. Surg. 2021, 25, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Sugimachi, K. Robot-assisted gastric surgery. Surg. Clin. N. Am. 2003, 83, 1429–1444. [Google Scholar] [CrossRef]
- Bobo, Z.; Xin, W.; Jiang, L.; Quan, W.; Liang, B.; Xiangbing, D.; Ziqiang, W. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: Meta-analysis and trial sequential analysis of prospective observational studies. Surg. Endosc. 2019, 33, 1033–1048. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Zhang, B.; Maher, H.; Wang, X.F.; Cai, X.J. Robotic versus laparoscopic gastrectomy for gastric cancer: A systematic review and updated meta-analysis. BMC Surg. 2017, 17, 93. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, H.I.; Han, S.U.; Yang, H.K.; Kim, Y.W.; Lee, H.J.; An, J.Y.; Kim, M.C.; Park, S.; Song, K.Y.; et al. Who may benefit from robotic gastrectomy? A subgroup analysis of multicenter prospective comparative study data on robotic versus laparoscopic gastrectomy. Eur. J. Surg. Oncol. 2016, 42, 1944–1949. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.M.; Woo, Y.; Obama, K.; Noh, S.H.; Hyung, W.J. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: Comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg. Endosc. 2015, 29, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- Arita, T.; Ichikawa, D.; Konishi, H.; Komatsu, S.; Shiozaki, A.; Hiramoto, H.; Hamada, J.; Shoda, K.; Kawaguchi, T.; Hirajima, S.; et al. Increase in peritoneal recurrence induced by intraoperative hemorrhage in gastrectomy. Ann. Surg. Oncol. 2015, 22, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.K.; Kubota, H.; Tachibana, M.; Kotoh, T.; Tabara, H.; Watanabe, R.; Kohno, H.; Nagasue, N. Long-term survival of transmural advanced gastric carcinoma following curative resection: Multivariate analysis of prognostic factors. World J. Surg. 2000, 24, 588–593. [Google Scholar] [CrossRef]
- Kamei, T.; Kitayama, J.; Yamashita, H.; Nagawa, H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J. Surg. 2009, 33, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Uyama, I.; Suda, K.; Nakauchi, M.; Kinoshita, T.; Noshiro, H.; Takiguchi, S.; Ehara, K.; Obama, K.; Kuwabara, S.; Okabe, H.; et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: A multi-institutional prospective single-arm study. Gastric Cancer 2019, 22, 377–385. [Google Scholar] [CrossRef]
- Marano, A.; Choi, Y.Y.; Hyung, W.J.; Kim, Y.M.; Kim, J.; Noh, S.H. Robotic versus laparoscopic versus open gastrectomy: A meta-analysis. J. Gastric Cancer 2013, 13, 136–148. [Google Scholar] [CrossRef]
- Yang, S.Y.; Roh, K.H.; Kim, Y.N.; Cho, M.; Lim, S.H.; Son, T.; Hyung, W.J.; Kim, H.I. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann. Surg. Oncol. 2017, 24, 1770–1777. [Google Scholar] [CrossRef]
- Giulianotti, P.C.; Coratti, A.; Angelini, M.; Sbrana, F.; Cecconi, S.; Balestracci, T.; Caravaglios, G. Robotics in general surgery: Personal experience in a large community hospital. Arch. Surg. 2003, 138, 777–784. [Google Scholar] [CrossRef]
- Eom, B.W.; Yoon, H.M.; Ryu, K.W.; Lee, J.H.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Choi, I.J.; Lee, J.S.; Kook, M.C.; et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur. J. Surg. Oncol. 2012, 38, 57–63. [Google Scholar] [CrossRef]
- Tokunaga, M.; Sugisawa, N.; Kondo, J.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Early phase II study of robot-assisted distal gastrectomy with nodal dissection for clinical stage IA gastric cancer. Gastric Cancer 2014, 17, 542–547. [Google Scholar] [CrossRef]
- An, J.Y.; Kim, S.M.; Ahn, S.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Successful robotic gastrectomy does not require extensive laparoscopic experience. J. Gastric Cancer 2018, 18, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Lan, Y.T.; Fang, W.L.; Chen, J.H.; Lo, S.S.; Hsieh, M.C.; Li, A.F.; Chiou, S.H.; Wu, C.W. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J. Gastrointest. Surg. 2012, 16, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, C.; Makino, H.; Yamamoto, N.; Sato, T.; Oshima, T.; Nagano, Y.; Fujii, S.; Akiyama, H.; Otsuka, Y.; Ono, H.A.; et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg. Laparosc. Endosc. Percutaneous Tech. 2008, 18, 236–241. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, M.C.; Park, M.S.; Hyung, W.J. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg. Endosc. 2012, 26, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, W.J.; Hyung, W.J.; Kim, H.I.; Han, S.U.; Kim, Y.W.; Ryu, K.W.; Park, S. Comprehensive learning curve of robotic surgery: Discovery from a multicenter prospective trial of robotic gastrectomy. Ann. Surg. 2021, 273, 949–956. [Google Scholar] [CrossRef]
- Kong, S.H.; Lee, H.J.; Ahn, H.S.; Kim, J.W.; Kim, W.H.; Lee, K.U.; Yang, H.K. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: The reappraisal of positive lymph node ratio as a proper N-staging. Ann. Surg. 2012, 255, 50–58. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Kim, Y.W.; Reim, D.; Park, J.Y.; Eom, B.W.; Kook, M.C.; Ryu, K.W.; Yoon, H.M. Role of robot-assisted distal gastrectomy compared to laparoscopy-assisted distal gastrectomy in suprapancreatic nodal dissection for gastric cancer. Surg. Endosc. 2016, 30, 1547–1552. [Google Scholar] [CrossRef]
- Obama, K.; Kim, Y.M.; Kang, D.R.; Son, T.; Kim, H.I.; Noh, S.H.; Hyung, W.J. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer 2018, 21, 285–295. [Google Scholar] [CrossRef]
- Roh, C.K.; Lee, S.; Son, S.Y.; Hur, H.; Han, S.U. Textbook outcome and survival of robotic versus laparoscopic total gastrectomy for gastric cancer: A propensity score matched cohort study. Sci. Rep. 2021, 11, 15394. [Google Scholar] [CrossRef]
- Choi, S.; Song, J.H.; Lee, S.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Trends in clinical outcomes and long-term survival after robotic gastrectomy for gastric cancer: A single high-volume center experience of consecutive 2000 patients. Gastric Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, M.; Suda, K.; Susumu, S.; Kadoya, S.; Inaba, K.; Ishida, Y.; Uyama, I. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: A single institutional retrospective cohort study. Surg. Endosc. 2016, 30, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Li, B.; Bai, B.; Liu, Y.; Lian, B.; Zhao, Q. Robotic versus laparoscopic gastrectomy with D2 lymph node dissection for advanced gastric cancer: A propensity score-matched analysis. Cancer Manag. Res. 2018, 10, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xi, H.; Qiao, Z.; Li, J.; Zhang, K.; Xie, T.; Shen, W.; Cui, J.; Wei, B.; Chen, L. Comparison of robotic- and laparoscopic-assisted gastrectomy in advanced gastric cancer: Updated short- and long-term results. Surg. Endosc. 2019, 33, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.K.; Kim, Y.N.; Jang, D.S.; Kim, Y.M.; Son, T.; Hyung, W.J.; Kim, H.I. Safety and feasibility of reduced-port robotic distal gastrectomy for gastric cancer: A phase I/II clinical trial. Surg. Endosc. 2017, 31, 4002–4009. [Google Scholar] [CrossRef]
- Seo, W.J.; Son, T.; Roh, C.K.; Cho, M.; Kim, H.I.; Hyung, W.J. Reduced-port totally robotic distal subtotal gastrectomy with lymph node dissection for gastric cancer: A modified technique using Single-Site(®) and two additional ports. Surg. Endosc. 2018, 32, 3713–3719. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Son, T.; Kim, J.; Seo, W.J.; Rho, C.K.; Cho, M.; Kim, H.I.; Hyung, W.J. Intracorporeal delta-shaped gastroduodenostomy in reduced-port robotic distal subtotal gastrectomy: Technical aspects and short-term outcomes. Surg. Endosc. 2018, 32, 4344–4350. [Google Scholar] [CrossRef]
- Smith, D.D.; Schwarz, R.R.; Schwarz, R.E. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J. Clin. Oncol. 2005, 23, 7114–7124. [Google Scholar] [CrossRef]
- Katai, H.; Yoshimura, K.; Fukagawa, T.; Sano, T.; Sasako, M. Risk factors for pancreas-related abscess after total gastrectomy. Gastric Cancer 2005, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Tokunaga, M.; Bando, E.; Tanizawa, Y.; Kawamura, T.; Terashima, M. Evaluation of postoperative pancreatic fistula after total gastrectomy with D2 lymphadenectomy by ISGPF classification. J. Gastrointest. Surg. 2011, 15, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Sasako, M.; Katai, H.; Maruyama, K. Amylase concentration of drainage fluid after total gastrectomy. Br. J. Surg. 1997, 84, 1310–1312. [Google Scholar] [PubMed]
- Park, J.Y.; Kook, M.C.; Eom, B.W.; Yoon, H.M.; Kim, S.J.; Rho, J.Y.; Kim, S.K.; Kim, Y.I.; Cho, S.J.; Lee, J.Y.; et al. Practical intraoperative pathologic evaluation of sentinel lymph nodes during sentinel node navigation surgery in gastric cancer patients-Proposal of the pathologic protocol for the upcoming SENORITA trial. Surg. Oncol. 2016, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Son, T.; Kim, H.I.; Noh, S.H.; Hyung, W.J. Robotic D2 lymph node dissection during distal subtotal gastrectomy for gastric cancer: Toward procedural standardization. Ann. Surg. Oncol. 2016, 23, 2409–2410. [Google Scholar] [CrossRef]
- Park, S.H.; Suh, Y.S.; Park, J.H.; Kim, T.H.; Son, Y.G.; Huh, Y.J.; Kim, Y.; Lee, H.B.; Kong, S.H.; Lee, H.J.; et al. Near-infrared image-guided laparoscopic omental flap for breast cancer. Asian J. Endosc. Surg. 2020, 13, 250–255. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Oh, S.J.; Kang, W.H.; Hyung, W.J.; Choi, S.H.; Noh, S.H. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: Lessons learned from an initial 100 consecutive procedures. Ann. Surg. 2009, 249, 927–932. [Google Scholar] [CrossRef]
- Verbeek, F.P.; Schaafsma, B.E.; Tummers, Q.R.; van der Vorst, J.R.; van der Made, W.J.; Baeten, C.I.; Bonsing, B.A.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L.; et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg. Endosc. 2014, 28, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Xie, J.W.; Zhong, Q.; Wang, J.B.; Lin, J.X.; Lu, J.; Cao, L.L.; Lin, M.; Tu, R.H.; Huang, Z.N.; et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: A randomized clinical trial. JAMA Surg. 2020, 155, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Park, Y.S.; Son, S.Y.; Lee, J.H.; Lee, H.S.; Park, Y.S.; Lee, K.H.; Kim, Y.H.; Park, K.U.; Lee, W.W.; et al. Long-term oncologic outcomes of laparoscopic sentinel node navigation surgery in early gastric cancer: A single-center, single-arm, phase II trial. Ann. Surg. Oncol. 2018, 25, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.W.; Kim, Y.W.; Min, J.S.; Yoon, H.M.; An, J.Y.; Eom, B.W.; Hur, H.; Lee, Y.-J.; Cho, G.S.; Park, Y.; et al. Results of interim analysis of the multicenter randomized phase III SENORITA trial of laparoscopic sentinel node oriented, stomach-preserving surgery versus laparoscopic standard gastrectomy with lymph node dissection in early gastric cancer. J. Clin. Oncol. 2017, 35, 4028. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, T.; Chung, Y.E.; Cho, M.; Kim, Y.M.; Kwon, I.G.; Kim, H.I.; Hyung, W.J. Real-time identification of aberrant left hepatic arterial territories using near-infrared fluorescence with indocyanine green during gastrectomy for gastric cancer. Surg. Endosc. 2021, 35, 2389–2397. [Google Scholar] [CrossRef]
- Sparreboom, C.L.; Wu, Z.Q.; Ji, J.F.; Lange, J.F. Integrated approach to colorectal anastomotic leakage: Communication, infection, and healing disturbances. World J. Gastroenterol. 2016, 22, 7226–7235. [Google Scholar] [CrossRef] [PubMed]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; Harmsen, H.J.; Olinga, P.; Havenga, K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: A pilot study. Surg. Endosc. 2016, 30, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.J.; Lee, H.J.; Kim, T.H.; Choi, Y.S.; Park, J.H.; Son, Y.G.; Suh, Y.S.; Kong, S.H.; Yang, H.K. Efficacy of assessing intraoperative bowel perfusion with near-infrared camera in laparoscopic gastric cancer surgery. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 476–483. [Google Scholar] [CrossRef]
- Yoshida, M.; Kubota, K.; Kuroda, J.; Ohta, K.; Nakamura, T.; Saito, J.; Kobayashi, M.; Sato, T.; Beck, Y.; Kitagawa, Y.; et al. Indocyanine green injection for detecting sentinel nodes using color fluorescence camera in the laparoscopy-assisted gastrectomy. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. S3), 29–33. [Google Scholar] [CrossRef]
- Miyashiro, I.; Kishi, K.; Yano, M.; Tanaka, K.; Motoori, M.; Ohue, M.; Ohigashi, H.; Takenaka, A.; Tomita, Y.; Ishikawa, O. Laparoscopic detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging. Surg. Endosc. 2011, 25, 1672–1676. [Google Scholar] [CrossRef]
- Kong, S.H.; Noh, Y.W.; Suh, Y.S.; Park, H.S.; Lee, H.J.; Kang, K.W.; Kim, H.C.; Lim, Y.T.; Yang, H.K. Evaluation of the novel near-infrared fluorescence tracers pullulan polymer nanogel and indocyanine green/γ-glutamic acid complex for sentinel lymph node navigation surgery in large animal models. Gastric Cancer 2015, 18, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Song, K.Y.; Park, C.H.; Jeon, H.M. Training of surgical endoscopists in Korea: Assessment of the learning curve using a cumulative sum model. J. Surg. Educ. 2012, 69, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Gagner, M. Potential of surgery for curing type 2 diabetes mellitus. Ann. Surg. 2002, 236, 554–559. [Google Scholar] [CrossRef]
- Jiménez, A.; Casamitjana, R.; Flores, L.; Viaplana, J.; Corcelles, R.; Lacy, A.; Vidal, J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann. Surg. 2012, 256, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef]
- Ho, T.W.; Wu, J.M.; Yang, C.Y.; Lai, H.S.; Lai, F.; Tien, Y.W. Total gastrectomy improves glucose metabolism on gastric cancer patients: A nationwide population-based study. Surg. Obes. Relat. Dis. 2016, 12, 635–641. [Google Scholar] [CrossRef]
- Kang, K.C.; Shin, S.H.; Lee, Y.J.; Heo, Y.S. Influence of gastrectomy for stomach cancer on type 2 diabetes mellitus for patients with a body mass index less than 30 kg/m(2). J. Korean Surg. Soc. 2012, 82, 347–355. [Google Scholar] [CrossRef][Green Version]
- Kwon, Y.; Jung Kim, H.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. A systematic review and meta-analysis of the effect of Billroth reconstruction on type 2 diabetes: A new perspective on old surgical methods. Surg. Obes. Relat. Dis. 2015, 11, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.W.; Li, J.L.; Wu, Y.; Xia, G.K.; Schwarz, R.E.; He, Y.L.; Zhang, C.H. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: A retrospective cohort study. Dig. Dis. Sci. 2014, 59, 1017–1024. [Google Scholar] [CrossRef]
- Lee, W.J.; Hur, K.Y.; Lakadawala, M.; Kasama, K.; Wong, S.K.; Chen, S.C.; Lee, Y.C.; Ser, K.H. Predicting success of metabolic surgery: Age, body mass index, C-peptide, and duration score. Surg. Obes. Relat. Dis. 2013, 9, 379–384. [Google Scholar] [CrossRef]
- Lee, W.; Ahn, S.H.; Lee, J.H.; Park, D.J.; Lee, H.J.; Kim, H.H.; Yang, H.K. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes. Surg. 2012, 22, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, K.Y.; Lee, S.C.; Yang, D.H.; Kim, B.C. The effect of long Roux-en-Y gastrojejunostomy in gastric cancer patients with type 2 diabetes and body mass index <35 kg/m2: Preliminary results. Ann. Surg. Treat. Res. 2015, 88, 215–221. [Google Scholar] [CrossRef][Green Version]
- Kwon, Y.; Abdemur, A.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. The foregut theory as a possible mechanism of action for the remission of type 2 diabetes in low body mass index patients undergoing subtotal gastrectomy for gastric cancer. Surg. Obes. Relat. Dis. 2014, 10, 235–242. [Google Scholar] [CrossRef]
- Pak, J.; Kwon, Y.; Lo Menzo, E.; Park, S.; Szomstein, S.; Rosenthal, R.J. Impact of gastrointestinal bypass on nonmorbidly obese type 2 diabetes mellitus patients after gastrectomy. Surg. Obes. Relat. Dis. 2015, 11, 1266–1272. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, D.H.; Park, B.J.; Kim, S.; Park, S.; Rosenthal, R.J. Impact of preoperative visceral fat proportion on type 2 diabetes in patients with low body mass index after gastrectomy. Surg. Obes. Relat. Dis. 2017, 13, 1361–1368. [Google Scholar] [CrossRef]
- Korean Gastric Cancer Association. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S89–S97. [Google Scholar] [CrossRef]
- An, J.Y.; Kim, Y.M.; Yun, M.A.; Jeon, B.H.; Noh, S.H. Improvement of type 2 diabetes mellitus after gastric cancer surgery: Short-term outcome analysis after gastrectomy. World J. Gastroenterol. 2013, 19, 9410–9417. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, J.W.; Ahn, C.W.; Choi, S.H. Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: A prospective pilot study. J. Korean Surg. Soc. 2013, 84, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Huh, Y.J.; Park, S.; Park, Y.S.; Park, D.J.; Kwon, J.W.; Lee, J.H.; Heo, Y.S.; Choi, S.H. Multicenter results of long-limb bypass reconstruction after gastrectomy in patients with gastric cancer and type II diabetes. Asian J. Surg. 2020, 43, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Park, D.J.; Kim, K.H.; Park, D.J.; Lee, Y.; Park, K.B.; Min, S.H.; Ahn, S.H.; Kim, H.H. Nutritional safety of oncometabolic surgery for early gastric cancer patients: A prospective single-arm pilot study using a historical control group for comparison. Surg. Endosc. 2020, 34, 275–283. [Google Scholar] [CrossRef]
- Renehan, A.G.; Yeh, H.C.; Johnson, J.A.; Wild, S.H.; Gale, E.A.; Møller, H. Diabetes and cancer: Evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia 2012, 55, 1619–1632. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Kim, J.-M.; Park, S.-S. Current Status and Trends of Minimally Invasive Gastrectomy in Korea. Medicina 2021, 57, 1195. https://doi.org/10.3390/medicina57111195
Park S-H, Kim J-M, Park S-S. Current Status and Trends of Minimally Invasive Gastrectomy in Korea. Medicina. 2021; 57(11):1195. https://doi.org/10.3390/medicina57111195
Chicago/Turabian StylePark, Shin-Hoo, Jong-Min Kim, and Sung-Soo Park. 2021. "Current Status and Trends of Minimally Invasive Gastrectomy in Korea" Medicina 57, no. 11: 1195. https://doi.org/10.3390/medicina57111195
APA StylePark, S.-H., Kim, J.-M., & Park, S.-S. (2021). Current Status and Trends of Minimally Invasive Gastrectomy in Korea. Medicina, 57(11), 1195. https://doi.org/10.3390/medicina57111195





