An Integrated Approach for Treatment of Acute Type A Aortic Dissection
Abstract
1. Introduction
2. Methods
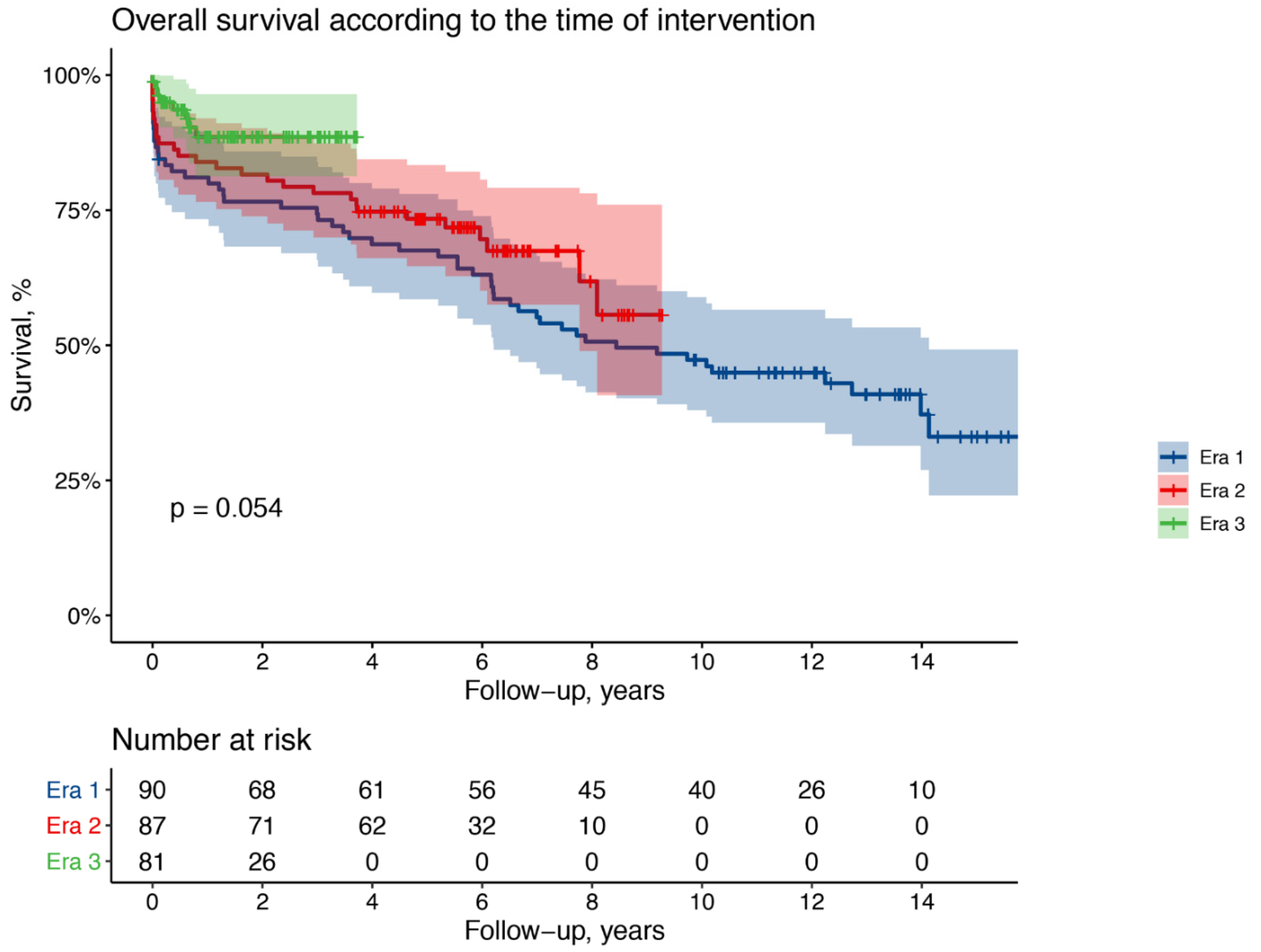
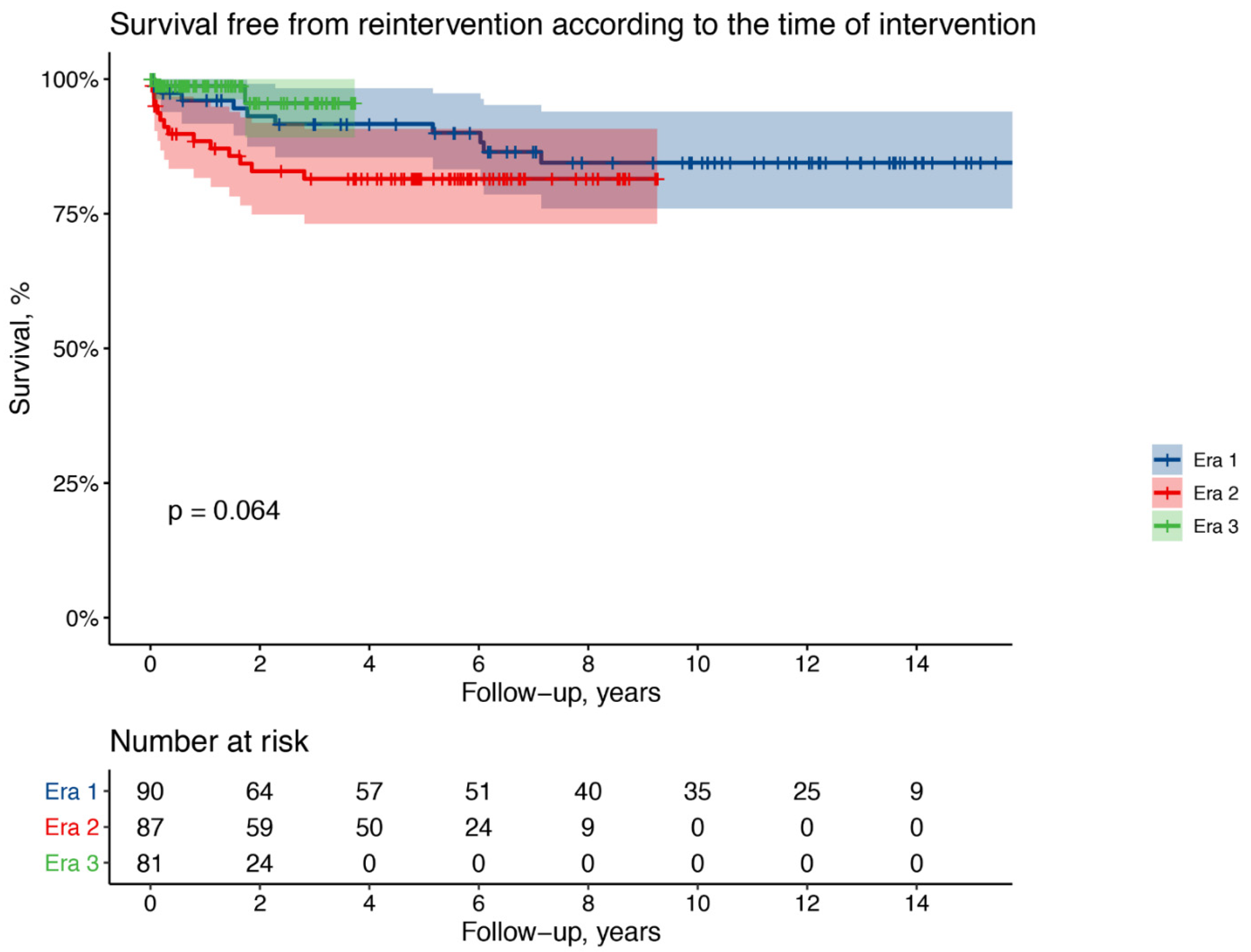
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Bakey, M.E.; Cooley, D.A.; Creech, O. Surgical considerations of dissecting aneurysm of the aorta. Ann. Surg. 1955, 142, 586–612. [Google Scholar] [CrossRef]
- De Bakey, M.E.; Henly, W.S.; Cooley, D.A.; Morris, G.C.; Crawford, E.S.; Beall, A.C. Surgical management of dissecting aneurysms of the aorta. J. Thorac. Cardiovasc. Surg. 1965, 49, 130–149. [Google Scholar] [CrossRef]
- Daily, P.O.; Trueblood, H.W.; Stinson, E.B.; Wuerflein, R.D.; Shumway, N.E. Management of acute aortic dissections. Ann. Thorac. Surg. 1970, 10, 237–247. [Google Scholar] [CrossRef]
- Attar, S.; Fardin, R.; Ayella, R.; McLaughlin, J.S. Medical vs surgical treatment of acute dissecting aneurysms. Arch. Surg. 1971, 103, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.; Miller, D.C. Evolution of surgical therapy for Stanford acute type A aortic dissection. Ann. Cardiothorac. Surg. 2016, 5, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.; Awais, M.; Woznicki, E. Presentation, diagnosis, and outcomes of acute aortic dissection: Seventeen-year trends from the international registry of acute aortic dissection. J. Vasc. Surg. 2016, 63, 552–553. [Google Scholar] [CrossRef]
- Lechiancole, A.; Vendramin, I.; Sponga, S.; Piani, D.; Benedetti, G.; Meneguzzi, M.; Ferrara, V.; Tullio, A.; Bortolotti, U.; Livi, U. Bentall procedure with the CarboSeal™ and CarboSeal Valsalva™ composite conduits: Long-term outcomes. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, I.; Lechiancole, A.; Piani, D.; Deroma, L.; Tullio, A.; Sponga, S.; Milano, A.D.; Onorati, F.; Bortolotti, U.; Livi, U. Type A acute aortic dissection with ≥40 mm aortic root. Results of conserva-tive and replacement strategies at long-term follow-up. Eur. J. Cardio-Thorac. Surg. 2021, 59, 1115–1122. [Google Scholar] [CrossRef]
- Harky, A.; Bashir, M.; Antoniou, A.; Bilal, H. The changing surgical approach to acute type A aortic dissection. J. Vis. Surg. 2018, 4, 151. [Google Scholar] [CrossRef]
- Westaby, S.; Saito, S.; Katsumata, T. Acute type A dissection: Conservative methods provide consistently low mortality. Ann. Thorac. Surg. 2002, 73, 707–713. [Google Scholar] [CrossRef]
- Thorburn, W.M. The myth of occam’s razor. Mind 1918, XXVII, 345–353. [Google Scholar] [CrossRef]
- Li, B.; Ma, W.G.; Liu, Y.M.; Sun, L.Z. Is extended arch replacement justified for acute type A aortic dissection? Interact. Cardiovasc. Thorac. Surg. 2015, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Moeller, E.; Nores, M.; Stamou, S.C. Repair of acute type-a aortic dissection in the present era: Outcomes and controversies. Aorta 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.R.; Miller, D.C. Aortic arch replacement for dissection. Oper. Tech. Thorac. Cardiovasc. Surg. 1999, 4, 33–57. [Google Scholar] [CrossRef][Green Version]
- Spielvogel, D.; Etz, C.D.; Silovitz, D.; Lansman, S.L.; Griepp, R.B. Aortic arch replacement with a trifurcated graft. Ann. Thorac. Surg. 2007, 83, S791–S795. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Vendramin, I.; Mazzaro, E.; Piccoli, G.; Bassi, F.; Gasparini, D.; Livi, U. Transluminal stenting in type A acute aortic dissection: Does the Djumbodis system has any impact on false lumen evolution? Ann. Thorac. Surg. 2010, 90, 1450–1456. [Google Scholar] [CrossRef]
- Vendramin, I.; Piani, D.; Lechiancole, A.; Sponga, S.; Sponza, M.; Puppato, M.; Bortolotti, U.; Livi, U. Late complications with the use of the Djumbodis in patients with type A acute aortic dissection. Interact. CardioVasc. Thorac. Surg. 2020, 31, 704–707. [Google Scholar] [CrossRef]
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using ‘elephant trunk’ prosthesis. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef]
- Roselli, E.E.; Isabella, M.A. Frozen Elephant Trunk Procedure. Oper. Tech. Thorac. Cardiovasc. Surg. 2013, 18, 87–100. [Google Scholar] [CrossRef][Green Version]
- Vendramin, I.; Piani, D.; Lechiancole, A.; de Manna, N.D.; Sponga, S.; Puppato, M.; Muser, D.; Bortolotti, U.; Livi, U. Do oral anticoagulants impact outcomes and false lumen patency after repair of acute type A aortic dissection? J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Giordano, R.; Di Tommaso, E.; Di Palo, G.; Iannelli, G. Treatment with transfemoral bare-metal stent of residual aortic arch dissection after surgical repair of acute type an aortic dissection. J. Thorac. Dis. 2018, 10, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, L.; Di Tommaso, E.; Giordano, R.; Pilato, E.; Iannelli, G. Off-Label treatment with transfemoral bare stents for isola-ted aortic arch dissection. Ann. Thorac. Surg. 2021, 111, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, I.; Lechiancole, A.; Frigatti, P.; Sponza, M.; Sponga, S.; Piani, D.; Bortolotti, U.; Livi, U. Aortic arch aneurysm and Kommerell’s diverticulum. Repair with a sin-gle-stage hybrid approach. J. Card. Surg. 2019, 34, 641–644. [Google Scholar] [CrossRef]
- Ma, W.-G.; Zhang, W.; Zhu, J.-M.; Ziganshin, B.A.; Zhi, A.-H.; Zheng, J.; Liu, Y.-M.; Elefteriades, J.A.; Sun, L.-Z. Long-term outcomes of frozen elephant trunk for type A aortic dissection in patients with Marfan syndrome. J. Thorac. Cardiovasc. Surg. 2017, 154, 1175–1189.e2. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Mohamed, H.; Vitulli, P.; Petrou, M. Axillary versus femoral arterial cannulation in type A acute aortic dissection: Evidence from a meta-analysis of comparative studies and adjusted risk estimates. Eur. J. Cardio-Thoracic Surg. 2015, 48, 953–959. [Google Scholar] [CrossRef]
| Era 1 (n = 90) | Era 2 (n = 87) | Era 3 (n = 81) | p Value | |
|---|---|---|---|---|
| Clinical profile | ||||
| Male sex, n (%) | 58 (64) | 67 (77) | 41 (51) | 0.02 |
| Age (years, mean ± sd) | 64 ± 12 | 63 ± 12 | 66 ± 13 | 0.13 |
| LVEF, % | 56 ± 8 | 51 ± 8 | 56 ± 9 | <0.01 |
| n. (%) | n. (%) | n. (%) | ||
| Chronic renal failure | 22 (24) | 17 (19) | 12 (15) | 0.28 |
| COPD | 11(12) | 5 (6) | 4 (5) | 0.14 |
| Previous cardiac surgery | 4 (4) | 6 (7) | 2 (2) | 0.39 |
| Bicuspid aortic valve | 6 (7) | 4 (5) | 2 (2) | 0.43 |
| CAD | 7 (8) | 8 (9) | 4 (5) | 0.56 |
| Previous stroke | 4 (4) | 2 (2) | 0 (0) | 0.15 |
| Chronic AF | 9 (10) | 7 (8) | 12 (15) | 0.35 |
| Presentation | ||||
| Tamponade/shock/hypotension | 31 (34) | 33 (38) | 18 (22) | 0.07 |
| Syncope | 15 (17) | 21 (22) | 8 (11) | 0.39 |
| Focal neurologic damage | 13 (14) | 16 (18) | 13 (16) | 0.77 |
| Coma | 0 (0) | 2 (2) | 2 (3) | 0.17 |
| Splanchnic malperfusion | 14 (16) | 10 (11) | 3 (4) | 0.01 |
| - Mesenteric | 3 (3) | 1 (1) | 0 (0) | |
| - Renal | 6 (7) | 6 (7) | 2 (2) | |
| - Lower limb | 5 (6) | 4 (5) | 2 (2) | |
| Risk factors | ||||
| Arterial hypertension | 64 (71) | 69 (79) | 63 (78) | 0.36 |
| Smoking | 23 (26) | 22 (25) | 29 (36) | 0.23 |
| Dyslipidemia | 8 (9) | 10 (11) | 18 (22) | 0.03 |
| Diabetes | 4 (4) | 3 (3) | 6 (7) | 0.47 |
| Era 1 (n = 90) | Era 2 (n = 87) | Era 3 (n = 81) | p Value | |
|---|---|---|---|---|
| Surgical procedures | n (%) | n (%) | n (%) | |
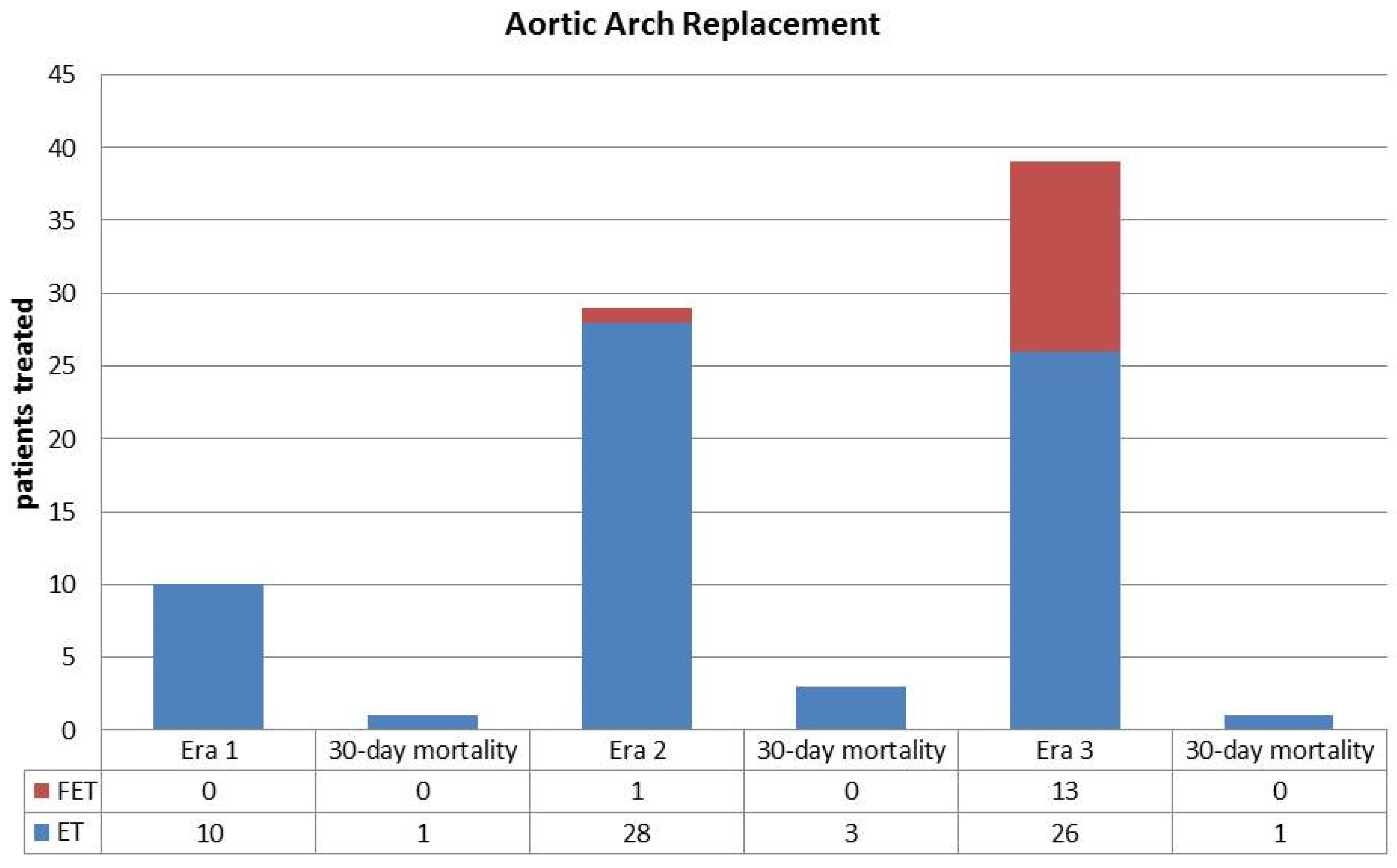
| Aortic Arch Replacement | 10 (11) | 29 (33) | 39 (48) | <0.01 |
| - Elephant Trunk | 10 (11) | 28 (32) | 26 (32) | <0.01 |
| - Frozen Elephant Trunk | 0 (0) | 1 (1) | 13 (16) | <0.01 |
| Aortic Root Replacement | 11(12) | 14 (15) | 7 (8) | 0.16 |
| - Bentall | 9 (10) | 12 (14) | 4 (5) | |
| - Reimplantation | 2 (2) | 2 (2) | 3 (4) | |
| - Remodeling | 0 (0) | 2 (2) | 0 (0) | |
| Aortic Valve Replacement | 8 (9) | 1 (1) | 3 (4) | 0.04 |
| - Mechanical | 5 (6) | 0 (0) | 0 (0) | |
| - Biological | 3 (3) | 1 (1) | 3 (4) | |
| Aortic Valve Repair | 5 (6) | 7 (8) | 3 (4) | 0.62 |
| Associated CABG | 3 (3) | 6 (7) | 6 (7) | 0.45 |
| Associated MV surgery | 1 (1) | 0 (0) | 0 (0) | 0.89 |
| Mean CPB time, minutes ± SD | 224 ± 67 | 231 ± 86 | 206 ± 73 | 0.14 |
| Mean ACC time, minutes ± SD | 118 ± 51 | 122 ± 58 | 119 ± 53 | 0.84 |
| Mean circulatory arrest, minutes ± SD | 50 ± 23 | 48 ± 22 | 39 ± 15 | <0.01 |
| Operative Setting | n (%) | n (%) | n (%) | |
| Axillary artery cannulation | 8 (9) | 75 (86) | 74 (91) | <0.01 |
| Femoral artery cannulation | 81 (91) | 12 (14) | 7 (9) | <0.01 |
| Antegrade cerebral perfusion | 25 (28) | 82 (94) | 81 (100 | <0.01 |
| Retrograde cerebral perfusion | 54 (60) | 5 (6) | 0 (0) | <0.01 |
| Era 1 (n = 90) | Era 2 (n = 87) | Era 3 (n = 81) | p Value | |
|---|---|---|---|---|
| 30-day mortality, n (%) | 12 (13) | 10 (11) | 3 (4) | 0.07 |
| - Hemorrhagic shock, n (%) | 5 (5) | 4 (5) | 1 (1) | |
| - MOF, n (%) | 2 (2) | 3 (3) | 1 (1) | |
| - Cardiogenic shock, n (%) | 4 (4) | 1 (1) | 1 (1) | |
| - Cerebrovascular accident, n (%) | 1 (1) | 2 (2) | - | |
| Chest re-exploration, n (%) | 17 (19) | 19 (22) | 5 (6) | 0.02 |
| Acute renal failure, n (%) | 38 (42) | 34 (39) | 41 (51) | 0.3 |
| Dialysis, n (%) | 15 (17) | 16 (18) | 12 (15) | 0.8 |
| High inotropic support, n (%) | 34 (38) | 30 (34) | 17 (21) | 0.04 |
| Coma, n (%) | 5 (6) | 3 (3) | 1 (1) | 0.12 |
| - Postoperative onset, n (%) | 5 (6) | 3 (3) | 1 (1) | 0.12 |
| Neurological focal deficit, n (%) | 12 (13) | 9 (10) | 8 (10) | 0.23 |
| - Postoperative onset, n (%) | 9 (10) | 5 (6) | 3 (4) | 0.18 |
| Paraplegia, n (%) | 1 (1) | 1 (1) | 2 (2) | 0.52 |
| - Postoperative onset, n (%) | 0 (0) | 0 (0) | 1 (1) | 0.64 |
| Transient ischemic attack, n (%) | 0 (0) | 3 (3) | 3 (4) | 0.11 |
| Median ICU stay, days (range) | 8 (1–26) | 9 (1–24) | 7 (1–17) | 0.25 |
| Median hospital stay, days (range) | 20 (5–65) | 22 (4–53) | 18 (4–49) | 0.17 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| RR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.01 (0.98–1.04) | 0.52 | ||
| Male gender | 1.42 (0.62–3.29) | 0.41 | ||
| Chronic renal failure | 1.01 (0.40–2.57) | 0.98 | ||
| Previous cardiac surgery | 1.78 (0.47–6.69) | 0.39 | ||
| Bicuspid aortic valve | 2.80 (0.97–8.05) | 0.06 | ||
| Coronary artery disease | 1.90 (0.27–13.30) | 0.52 | ||
| Previous stroke | 5.73 (2.34–14.01) | 0.01 | ||
| Chronic AF | 2.59 (1.13–5.95) | 0.02 | ||
| Arterial hypertension | 2.17 (1.05–4.54) | 0.03 | ||
| Smoke | 1.27 (0.53–3.06) | 0.59 | ||
| Diabetes | 2.57 (0.88–7.49) | 0.08 | ||
| Tamponade/shock | 3.82 (1.76–8.27) | 0.001 | 4.35 (1.88–10.06) | 0.001 |
| Syncope | 2.36 (0.58–9.67) | 0.23 | ||
| Neurological damage | 2.42 (1.12–5.24) | 0.03 | 2.41 (1.03–5.63) | 0.04 |
| Splanchnic malperfusion | 1.45 (0.53–3.93) | 0.47 | ||
| Aortic arch replacement | 1.52 (0.59–3.89) | 0.38 | ||
| AVR mechanical | 0.98 (0.25–3.88) | 0.98 | ||
| AVR biological | 3.09 (1.21–7.85) | 0.02 | ||
| Aortic valve repair | 0.67 (0.10–4.66) | 0.69 | ||
| CABG | 2.21 (0.74–6.55) | 0.15 | ||
| CPB time | 1.01 (1.00–1.01) | 0.001 | ||
| ACC time | 1.02 (1.01–1.03) | 0.002 | 1.02 (1.01–1.04) | <0.01 |
| Circulatory arrest | 1.01 (1.00–1.01) | 0.03 | ||
| Antegrade cerebral perfusion | 1.46 (0.57–3.74) | 0.43 | ||
| Retrograde cerebral perfusion | 0.46 (0.14–1.48) | 0.19 | ||
| Era 2 * | 0.86 (0.39–1.89) | 0.71 | ||
| Era 3 * | 0.28 (0.08–0.95) | 0.04 | ||
| Era 1 | Era 2 | Era 3 | |
|---|---|---|---|
| Late deaths, n. (%) | 41 (45) | 18 (21) | 5 (6) |
| Aortic related, n. (%) | 11 (27) | 6 (33) | 3 (60) |
| Cardiac related, n. (%) | 8 (19) | 2 (11) | - |
| Neoplasia, n. (%) | 5 (12) | 5 (28) | - |
| Infection, n. (%) | 10 (24) | 2 (11) | 2 (40) |
| Other, n. (%) | 7 (18) | 3 (17) | - |
| N. (%) | Era 1 10 (11) | Era 2 12 (14) | Era 3 2 (3) |
|---|---|---|---|
| TEVAR | 5(6) | 5 (6) | 0 (0) |
| Median time, years (range) | 2.2 (0.1–7.1) | 0.7 (0.1–1.4) | - |
| Arch replacement and ET | 1 (1) | 2 (2) | 1 (1) |
| Arch replacement and FET | 2 (2) | 0 (0) | 0 (0) |
| Bentall operation | 1 (1) | 1 (1) | 1 (1) |
| Aortic root remodeling | 0 (0) | 2 (2) | 0 (0) |
| Proximal pseudoaneurysm repair | 1 (1) | 1 (2) | 0 (0) |
| Median time, years (range) | 3.8 (0.6–6) | 1.1 (0.1–2.8) | 1 (0.1–1.7) |
| Perioperative Complications | ET | FET | p Value |
|---|---|---|---|
| Chest re-exploration, n (%) | 2 (8) | 0 (0) | 0.52 |
| Acute renal failure, n (%) | 18 (72) | 5 (38) | 0.07 |
| Dialysis, n (%) | 8 (30) | 0 (0) | 0.03 |
| High inotropic support, n (%) | 9 (34) | 2 (15) | 0.24 |
| Coma, n (%) | 1 (4) | 0 (0) | 0.67 |
| Neurological focal deficit, n (%) | 3 (12) | 1 (8) | 0.68 |
| Paraplegia, n (%) | 2 (8) | 0 (0) | 0.47 |
| Transient ischemic attack, n (%) | 5 (20) | 1 (8) | 0.64 |
| Median ICU stay, days (range) | 7 (1–28) | 5 (1–24) | 0.55 |
| Median hospital stay, days (range) | 21 (1–65) | 15 (1–53) | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendramin, I.; Lechiancole, A.; Piani, D.; Sponga, S.; Di Nora, C.; Muser, D.; Bortolotti, U.; Livi, U. An Integrated Approach for Treatment of Acute Type A Aortic Dissection. Medicina 2021, 57, 1155. https://doi.org/10.3390/medicina57111155
Vendramin I, Lechiancole A, Piani D, Sponga S, Di Nora C, Muser D, Bortolotti U, Livi U. An Integrated Approach for Treatment of Acute Type A Aortic Dissection. Medicina. 2021; 57(11):1155. https://doi.org/10.3390/medicina57111155
Chicago/Turabian StyleVendramin, Igor, Andrea Lechiancole, Daniela Piani, Sandro Sponga, Concetta Di Nora, Daniele Muser, Uberto Bortolotti, and Ugolino Livi. 2021. "An Integrated Approach for Treatment of Acute Type A Aortic Dissection" Medicina 57, no. 11: 1155. https://doi.org/10.3390/medicina57111155
APA StyleVendramin, I., Lechiancole, A., Piani, D., Sponga, S., Di Nora, C., Muser, D., Bortolotti, U., & Livi, U. (2021). An Integrated Approach for Treatment of Acute Type A Aortic Dissection. Medicina, 57(11), 1155. https://doi.org/10.3390/medicina57111155








