Chronic and Recurrent Herpes Zoster Ophthalmicus
Abstract
:1. Introduction
2. Methods
3. Results
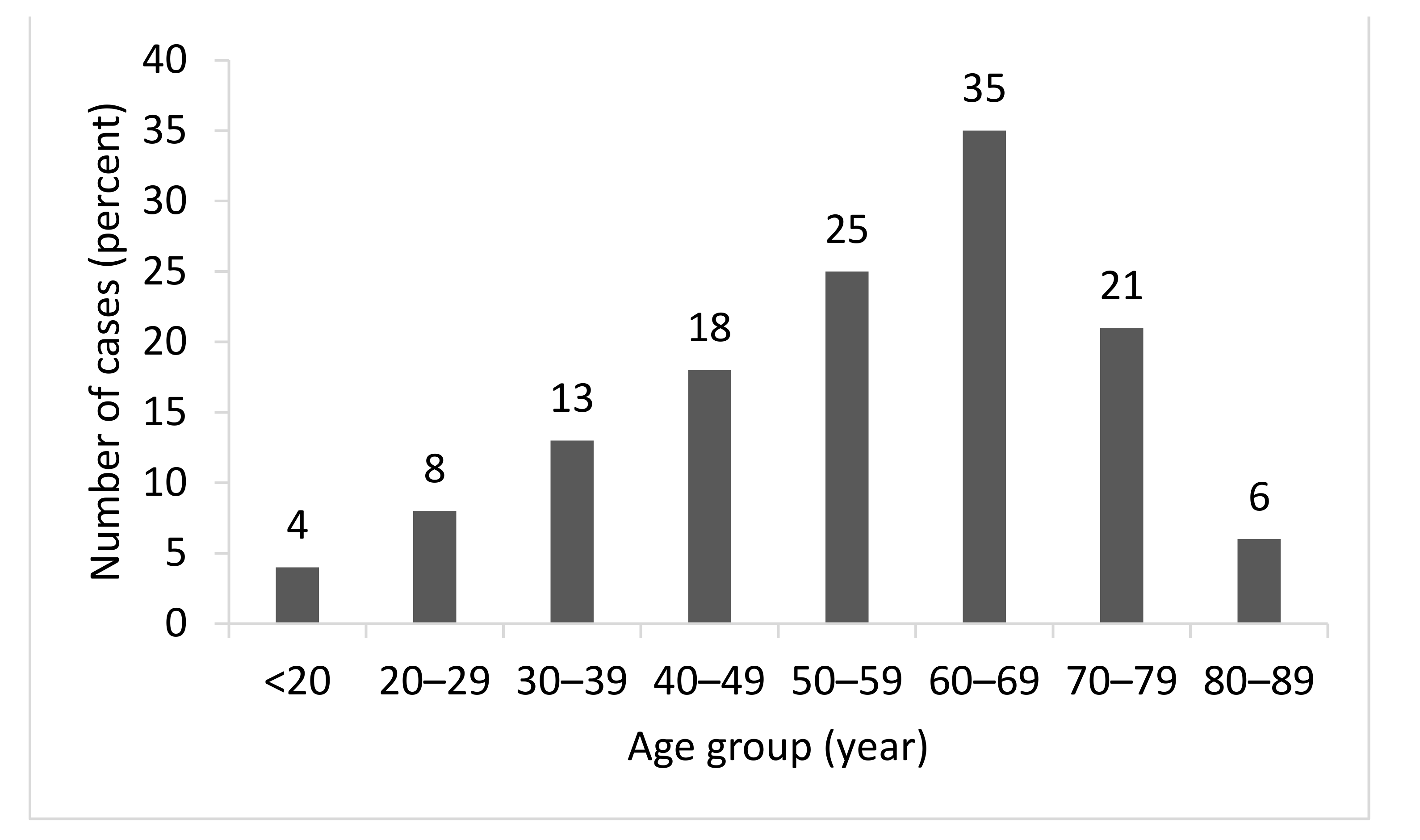
3.1. Baseline Characteristics
3.2. Clinical Manifestations
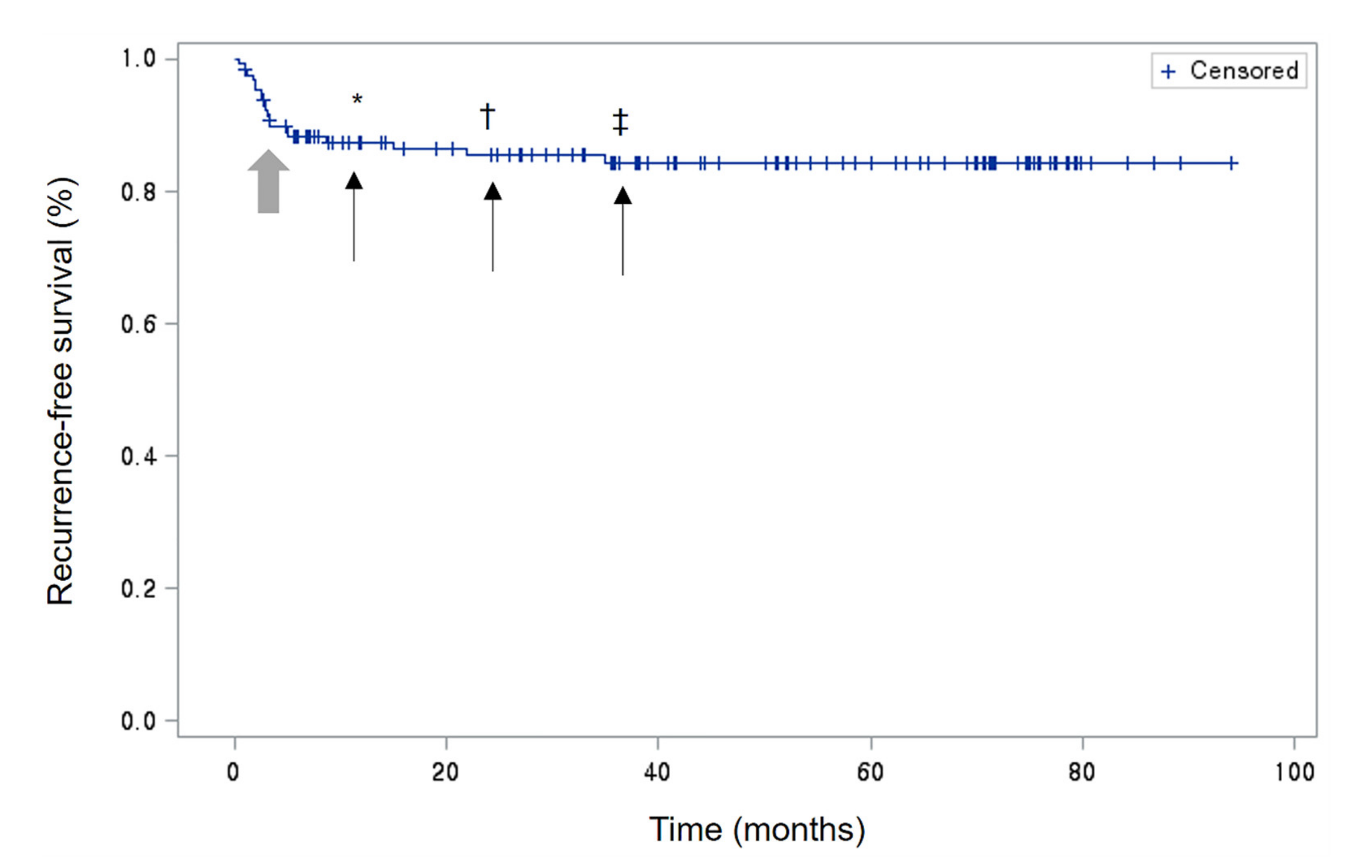
3.3. Clinical Course
3.3.1. Chronic Disease
3.3.2. Recurrent Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hope-Simpson, R.E. The nature of herpes zoster: A long-term study and a new hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.L.; Hall, A.J. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect. Dis. 2004, 4, 26–33. [Google Scholar] [CrossRef]
- Davies, E.C.; Pavan-Langston, D.; Chodosh, J. Herpes zoster ophthalmicus: Declining age at presentation. Br. J. Ophthalmol. 2016, 100, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Gilden, D. The global epidemiology of herpes zoster. Neurology 2013, 81, 928–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimland, D.; Moanna, A. Increasing incidence of herpes zoster among Veterans. Clin. Infect. Dis. 2010, 50, 1000–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drolet, M.; Brisson, M.; Schmader, K.E.; Levin, M.J.; Johnson, R.; Oxman, M.N.; Patrick, D.; Blanchette, C.; Mansi, J.A. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: A prospective study. Cmaj 2010, 182, 1731–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, R.S., III; Tindall, J.P. Geriatric herpes zoster. J. Am. Geriatr. Soc. 1971, 19, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ragozzino, M.W.; Melton, L.J., III; Kurland, L.T.; Chu, C.P.; Perry, H.O. Population-based study of herpes zoster and its sequelae. Medicine 1982, 61, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Liesegang, T.J. Varicella-zoster virus eye disease. Conea 1999, 18, 511–531. [Google Scholar] [CrossRef]
- Sandor, E.V.; Millman, A.; Croxson, T.S.; Mildvan, D. Herpes zoster ophthalmicus in patients at risk for the acquired immune deficiency syndrome (AIDS). Am. J. Ophthalmol. 1986, 101, 153–155. [Google Scholar] [CrossRef]
- Liesegang, T.J. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008, 115, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Wollan, P.C.; St Sauver, J.L.; Butterfield, L.C. Herpes zoster eye complications: Rates and trends. Mayo Clin. Proc. 2013, 88, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hales, C.M.; Harpaz, R.; Joesoef, M.R.; Bialek, S.R. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann. Intern. Med. 2013, 159, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.; Harpaz, R.; Molinari, N.A.; Jumaan, A.; Zhou, F. Herpes zoster incidence among insured persons in the United States, 1993–2006: Evaluation of impact of varicella vaccination. Clin. Infect. Dis. 2011, 52, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yih, W.K.; Brooks, D.R.; Lett, S.M.; Jumaan, A.O.; Zhang, Z.; Clements, K.M.; Seward, J.F. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 2005, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, R.S.; Cross, K.W.; Fleming, D.M. The incidence of shingles and its implications for vaccination policy. Vaccine 2003, 21, 2541–2547. [Google Scholar] [CrossRef]
- Tran, K.D.; Falcone, M.M.; Choi, D.S.; Goldhardt, R.; Karp, C.L.; Davis, J.L.; Galor, A. Epidemiology of Herpes Zoster Ophthalmicus: Recurrence and Chronicity. Ophthalmology 2016, 123, 1469–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yawn, B.P.; Wollan, P.C.; Kurland, M.J.; St Sauver, J.L.; Saddier, P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin. Proc. 2011, 86, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miserocchi, E.; Fogliato, G.; Bianchi, I.; Bandello, F.; Modorati, G. Clinical features of ocular herpetic infection in an italian referral center. Cornea 2014, 33, 565–570. [Google Scholar] [CrossRef] [PubMed]
| Demographic | No. (%) Mean ± SD or Range |
|---|---|
| Sex | |
| Male | 66 (50.77) |
| Female | 64 (49.23) |
| Age at presentation (years) | |
| Mean | 55.18 ± 17.66 |
| Range | 11–89 |
| Immune status | |
| Immunocompetent | 116 (89.23) |
| Immunocompromised | 14 (10.77) |
| Treatment duration (days) | |
| Mean | 82.22 ± 199.56 |
| Range | 3–1575 |
| Chronic HZO | 31 (23.85) |
| Acute HZO | 99 (76.15) |
| Recurrent HZO | 19 (14.62) |
| No recurrent HZO | 111 (85.38) |
| Clinical Manifestation | Frequency (%) |
|---|---|
| No eye involvement, only rash | 26 (20) |
| Conjunctivitis | 49 (37.69) |
| Epithelial keratitis | 60 (46.15) |
| Stromal keratitis | 10 (7.69) |
| Endothelial keratitis | 15 (11.54) |
| Uveitis | 8 (6.15) |
| Increased IOP | 10 (7.7) |
| Cranial nerve palsy | 2 (1.5) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p Value 1 | OR (95% CI) | p Value 1 |
| Age (years) | 0.985 (0.963–1.018) | 0.1911 | 0.963 (0.927–1.001) | 0.0530 |
| Sex | 1.900 (0.834–4.331) | 0.1268 | 1.248 (0.344–4.533) | 0.7124 |
| Immune status | 0.500 (0.106–2.367) | 0.3823 | ||
| Conjunctivitis | 2.133 (0.941–4.839) | 0.0698 | 8.698 (1.619–46.736) | 0.0154 |
| Epithelial keratitis | 2.682 (1.160–6.200) | 0.0211 | 41.495 (1.82–20.29) | 0.0005 |
| Stromal keratitis | 40.070 (4.826–332.720) | 0.0006 | 46.605 (2.597–836.357) | 0.0005 |
| Endotheliitis | 13.062 (3.773–45.221) | <0.0001 | 3.171 (0.401–25.079) | 0.0693 |
| Uveitis | 11.640 (2.214–61.193) | 0.0037 | 6.519 (0.324–131.281) | 0.3798 |
| Increased IOP | 16.869 (3.356–84.800) | 0.0006 | 4.815 (0.437–53.019) | 0.1940 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p Value 1 | HR (95% CI) | p Value 1 |
| Age (years) | 1.000 (0.974–1.025) | 0.9723 | 1.027 (0.980–1.067) | 0.1661 |
| Sex | 3.161(1.138–8.777) | 0.0272 | 2.606 (0.690–9.837) | 0.1576 |
| Immune status | 1.052 (0.243–4.556) | 0.9455 | ||
| Conjunctivitis | 2.321 (0.933–5.771) | 0.0700 | 2.671 (0.654–10.910) | 0.1713 |
| Epithelial keratitis | 2.876 (1.092–7.571) | 0.0324 | 5.550 (1.207–29.993) | 0.0465 |
| Stromal keratitis | 14.924 (5.460–40.795) | <0.0001 | 18.811 (2.929–120.824) | 0.0020 |
| Endotheliitis | 5.638 (2.214–14.353) | 0.0003 | 0.221 (0.039–1.246) | 0.0871 |
| Uveitis | 5.441 (1782–16.612) | 0.0029 | 1.484 (0.218–10.094) | 0.6863 |
| Increased IOP | 14.925 (5.818–38.286) | <0.0001 | 7.306 (1.606–33.242) | 0.0101 |
| Primary Tx duration | 1.003 (1.002–1.004) | <0.0001 | 0.999 (0.997–1.001) | 0.4745 |
| Chronic course | 32.146 (3.473–297.543) | 0.0022 | 34.405 (3.647–324.611) | 0.0020 |
| Patient Number | Sex | Age (Years) | Immune Status | First Ocular Manifestation | Recurred Ocular Manifestation |
|---|---|---|---|---|---|
| 1 | F | 40 | competent | S,U | S |
| 2 | M | 35 | competent | S,I | S |
| 3 | F | 22 | competent | E | S |
| 4 | F | 58 | competent | C,E,I | C |
| 5 | M | 76 | competent | C,E,S,e | E,S,e |
| 6 | F | 57 | competent | E | S |
| 7 | F | 54 | competent | C,e | E |
| 8 | M | 63 | competent | E,S,I | S, I |
| 9 | F | 61 | competent | C,S,e,U | S,e |
| 10 | F | 59 | competent | C,E | S |
| 11 | F | 62 | compromised | C,E | S,e |
| 12 | F | 71 | competent | C,E,e | S |
| 13 | F | 23 | competent | E,S | S |
| 14 | F | 44 | compromised | C,E,S | E,S |
| 15 | M | 53 | competent | C | C |
| 16 | F | 71 | competent | E,e,U | E |
| 17 | F | 62 | competent | C,E | E |
| 18 | F | 67 | competent | C,E | E |
| 19 | M | 70 | competent | E | E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Han, J.; Yang, C.M.; Choi, C.Y.; Khoramnia, R.; Chung, T.-Y.; Lim, D.H. Chronic and Recurrent Herpes Zoster Ophthalmicus. Medicina 2021, 57, 999. https://doi.org/10.3390/medicina57100999
Lee SM, Han J, Yang CM, Choi CY, Khoramnia R, Chung T-Y, Lim DH. Chronic and Recurrent Herpes Zoster Ophthalmicus. Medicina. 2021; 57(10):999. https://doi.org/10.3390/medicina57100999
Chicago/Turabian StyleLee, Soo Min, Jisang Han, Chan Min Yang, Chul Young Choi, Ramin Khoramnia, Tae-Young Chung, and Dong Hui Lim. 2021. "Chronic and Recurrent Herpes Zoster Ophthalmicus" Medicina 57, no. 10: 999. https://doi.org/10.3390/medicina57100999
APA StyleLee, S. M., Han, J., Yang, C. M., Choi, C. Y., Khoramnia, R., Chung, T.-Y., & Lim, D. H. (2021). Chronic and Recurrent Herpes Zoster Ophthalmicus. Medicina, 57(10), 999. https://doi.org/10.3390/medicina57100999







