The Role of Bradykinin Receptors in the Etiopathogenesis of Chronic Spontaneous Urticaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Participants and Blood Collection
2.3. Cell Staining and Flow Cytometry
2.4. Statistical Analyses
3. Results
3.1. Comparison of Lymphocytes and Monocytes Subpopulations Distribution
3.2. Expression of Bradykinin Receptors
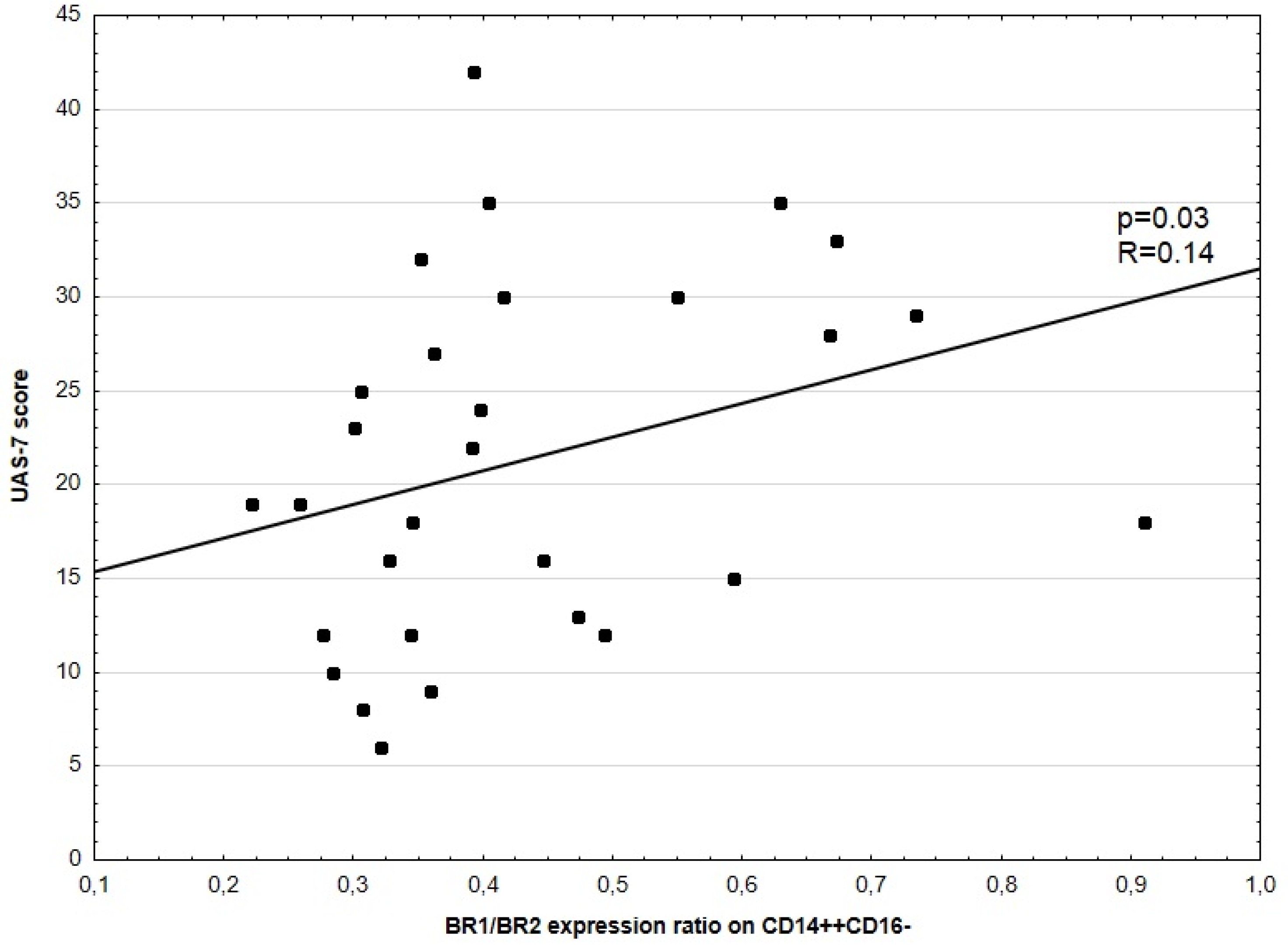
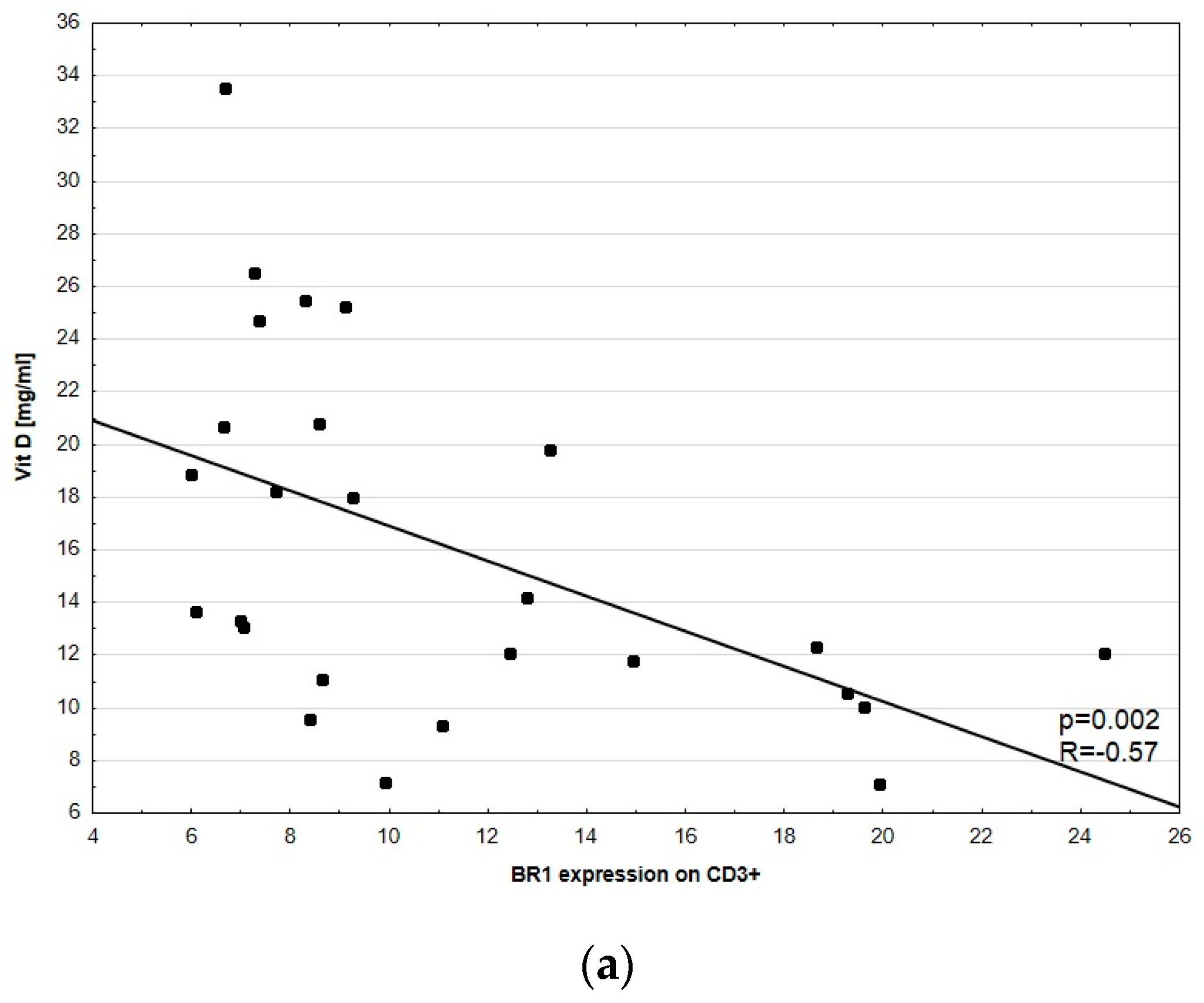
3.3. Disease Activity Correlation with BR1 and BR2
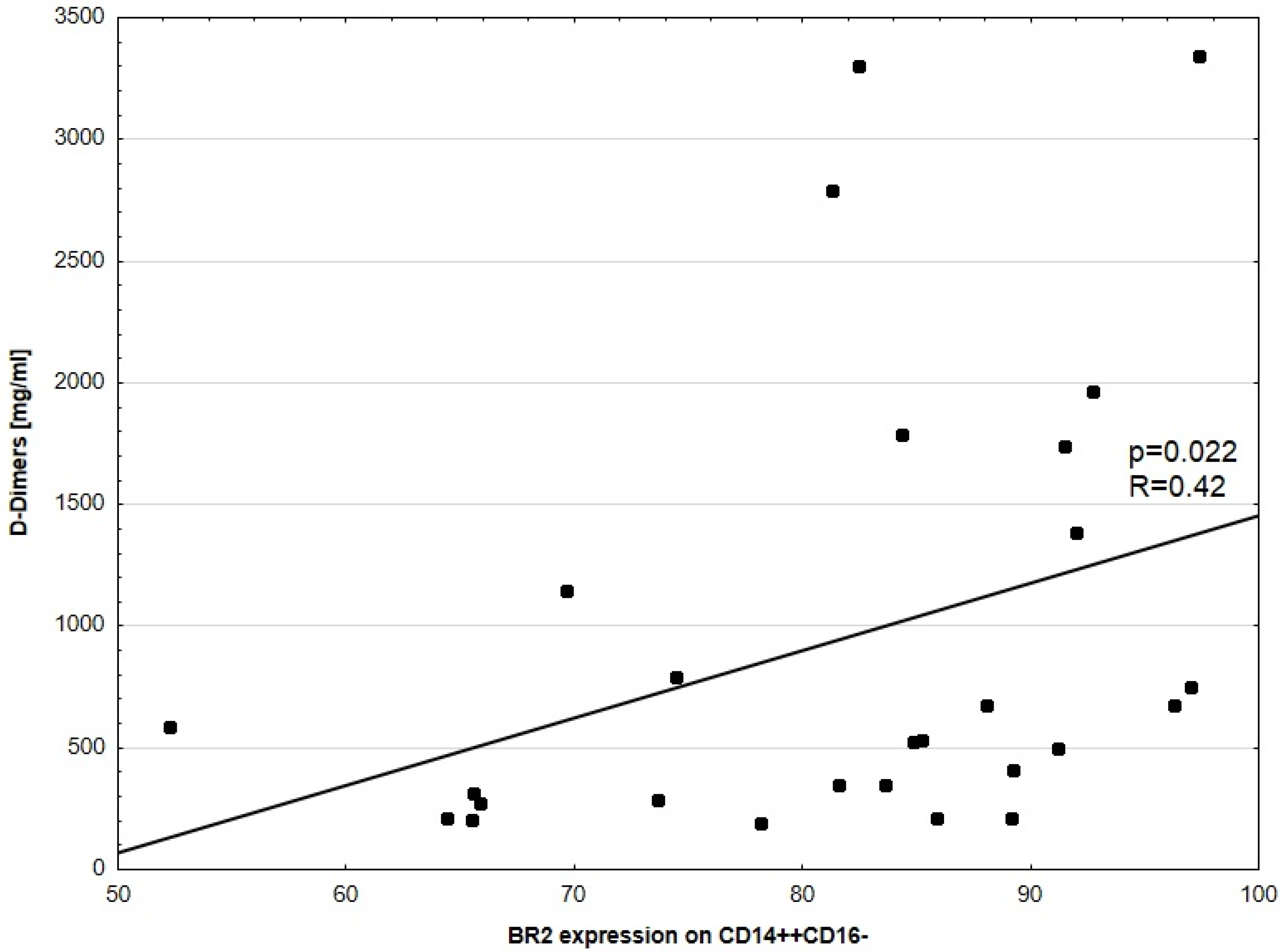
3.4. D-Dimers Concentration and BR Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuberbier, T.; Aberer, W.; Asero, R.; Latiff, A.H.A.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Bedrikow, R.B.; et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, I.; Pratesi, F.; Ribatti, D.; Migliorini, P. Mediators of Inflammation and Angiogenesis in Chronic Spontaneous Urticaria: Are They Potential Biomarkers of the Disease? Mediat. Inflamm. 2017, 2017, 4123694. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.S.; Jeon, J.; Kim, J.H.; Oh, C.H. Severity of acute and chronic urticaria correlates with D-dimer level, but not C-reactive protein or total IgE. Clin. Exp. Dermatol. 2014, 39, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B.; Ying, S.; Ardelean, E.; Mlynek, A.; Kita, H.; Clark, P.; Maurer, M. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial wheals with low-level persistence in uninvolved skin. Br. J. Derm. 2014, 171, 505–511. [Google Scholar] [CrossRef]
- Caproni, M.; Giomi, B.; Volpi, W.; Melani, L.; Schincaglia, E.; Macchia, D.; Manfredi, M.; D’Agata, A.; Fabbri, P. Chronic idiopathic urticaria: Infiltrating cells and related cytokines in autologous serum-induced wheals. Clin. Immunol. 2005, 114, 284–292. [Google Scholar] [CrossRef]
- Elias, J.; Boss, E.; Kaplan, A.P. Studies of the cellular infiltrate of chronic idiopathic urticaria: Prominence of T lymphocytes, monocytes, and mast cells. J. Allergy Clin. Immunol. 1986, 78, 914–918. [Google Scholar] [CrossRef]
- Bhoola, K.D.; Figueroa, C.D.; Worthy, K. Bioregulation of kinins: Kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992, 44, 1–80. [Google Scholar]
- Regoli, D.; Barabé, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980, 32, 1–46. [Google Scholar]
- Calixto, J.B.; Cabrini, D.A.; Ferreira, J.; Campos, M. Kinins in pain and inflammation. Pain 2000, 87, 1–5. [Google Scholar] [CrossRef]
- Leeb-Lundberg, L.M.F.; Marceau, F.; Müller-Esterl, W.; Pettibone, D.J.; Zuraw, B. International Union of Pharmacology. XLV. Classification of the Kinin Receptor Family: From Molecular Mechanisms to Pathophysiological Consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef]
- Triwongwaranat, D.; Kulthanan, K.; Chularojanamontri, L.; Pinkaew, S. Correlation between plasma D-dimer levels and the severity of patients with chronic urticaria. Asia Pac. Allergy 2013, 3, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Asero, R. D-dimer: A biomarker for antihistamine-resistant chronic urticaria. J. Allergy Clin. Immunol. 2013, 132, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Rosén, K.; Hsieh, H.-J.; Saini, S.; Grattan, C.; Gimenéz-Arnau, A.; Agarwal, S.; Doyle, R.; Canvin, J.; Kaplan, A.; et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N. Engl. J. Med. 2013, 368, 924–935. [Google Scholar] [CrossRef]
- Maurer, M.; Weller, K.; Bindslev-Jensen, C.; Giménez-Arnau, A.; Bousquet, P.J.; Bousquet, J.; Canonica, G.W.; Church, M.K.; Godse, K.V.; Grattan, C.E.H.; et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report1. Allergy 2010, 66, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Staubach, P.; Raap, U.; Richter-Huhn, G.; Bauer, A.; Ruëff, F.; Jakob, T.; Yazdi, A.S.; Mahler, V.; Wagner, N.; et al. H1-antihistamine-refractory chronic spontaneous urticaria: It’s worse than we thought—First results of the multicenter real-life AWARE study. Clin. Exp. Allergy 2017, 47, 684–692. [Google Scholar] [CrossRef]
- Hofman, Z.L.; Elzen, M.T.V.D.; Kuijpers, J.; De Maat, S.; Hack, C.E.; Knulst, A.C.; Röckmann, H.; Maas, C. Evidence for bradykinin release in chronic spontaneous urticaria. Clin. Exp. Allergy 2020, 50, 343–351. [Google Scholar] [CrossRef]
- Kaplan, A.P.; Joseph, K. The bradykinin-forming cascade and its role in hereditary angioedema. Ann. Allergy Asthma Immunol. 2010, 104, 193–204. [Google Scholar] [CrossRef]
- Lai, J.; Luo, M.C.; Chen, Q.; Porreca, F. Pronociceptive actions of dynorphin via bradykinin receptors. Neurosci. Lett. 2008, 437, 175–179. [Google Scholar] [CrossRef]
- Cruden, N.L.; Newby, D.E. Therapeutic potential of icatibant (HOE-140, JE-049). Expert Opin. Pharmacother. 2008, 9, 2383–2390. [Google Scholar] [CrossRef]
- Abraham, W.M.; Scuri, M.; Farmer, S.G. Peptide and non-peptide bradykinin receptor antagonists: Role in allergic airway disease. Eur. J. Pharmacol. 2006, 533, 215–221. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/firazyr-epar-product-information_en.pdf (accessed on 1 October 2021).
- Zanichelli, A.; Magerl, M.; Longhurst, H.; Fabien, V.; Maurer, M. Hereditary angioedema with C1 inhibitor deficiency: Delay in diagnosis in Europe. Allergy Asthma Clin. Immunol. 2013, 9, 29. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Cremonesi, P.; Hoffmann, T.K.; Hollingsworth, J. Angioedema in the emergency department: A practical guide to differential diagnosis and management. Int. J. Emerg. Med. 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, P.W.; Hack, C.E.; Eerenberg, A.J.; Struyvenberg, A.; van der Zwan, J.K. Activation of the contact system in insect-sting anaphylaxis: Association with the development of angioedema and shock. Blood 1993, 82, 1732–1739. [Google Scholar] [CrossRef]
- Brown, S.G.A.; Stone, S.F.; Fatovich, D.M.; Burrows, S.A.; Holdgate, A.; Celenza, A.; Coulson, A.; Hartnett, L.; Nagree, Y.; Cotterell, C.; et al. Anaphylaxis: Clinical patterns, mediator release, and severity. J. Allergy Clin. Immunol. 2013, 132, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Delong, L.K.; Culler, S.; Saini, S.S.; Beck, L.A.; Chen, S.C. Annual Direct and Indirect Health Care Costs of Chronic Idiopathic Urticaria. Arch. Dermatol. 2008, 144, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Magerl, M.; Metz, M.; Siebenhaar, F.; Weller, K.; Krause, K. Practical algorithm for diagnosing patients with recurrent wheals or angioedema. Allergy 2013, 68, 816–819. [Google Scholar] [CrossRef]
- Barlow, R.J.; Ross, E.L.; Macdonald, D.M.; Black, A.K.; Greaves, M.W. Mast cells and T lymphocytes in chronic urticaria. Clin. Exp. Allergy 1995, 25, 317–322. [Google Scholar] [CrossRef]
- ObtułOwicz, A.; PirOwska, M.; Pastuszczak, M.; Wojas-Pelc, A. Coagulation/fibrynolysis and inflammation markers in patients with chronic spontaneous urticaria (CSU). Prz. Lek. 2017, 74, 565–569. [Google Scholar]
- Niewiarowska-Sendo, A.; Łabędź-Masłowska, A.; Kozik, A.; Guevara-Lora, I. Bradykinin B2 receptor and dopamine D2 receptor cooperatively contribute to the regulation of neutrophil adhesion to endothelial cells. Acta Biochim. Pol. 2018, 65, 367–375. [Google Scholar] [CrossRef]
- Terzuoli, E.; Meini, S.; Cucchi, P.; Catalani, C.; Cialdai, C.; Maggi, C.A.; Donnini, S. Antagonism of bradykinin B2 receptor prevents inflammatory responses in human endothelial cells by quenching the NF-kB pathway activation. PLoS ONE 2014, 9, e84358. [Google Scholar] [CrossRef]
- Guevara-Lora, I.; Labedz, A.; Skrzeczynska-Moncznik, J.; Kozik, A. Bradykinin and des-Arg10-kallidin enhance the adhesion of polymorphonuclear leukocyes to extracellular matrix proteins and endothelial cells. Cell Adhes. Commun. 2011, 18, 67–71. [Google Scholar] [CrossRef]
- Figueroa, C.D.; Matus, C.E.; Pavicic, F.; Sarmiento, J.; Hidalgo, M.A.; Burgos, R.A.; Gonzalez, C.B.; Bhoola, K.D.; Ehrenfeld, I. Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate. Immun. 2015, 21, 289–304. [Google Scholar] [CrossRef]
- Condotta, S.A.; Richer, M.J. The immune battlefield: The impact of inflammatory cytokines on CD8+ T-cell immunity. PLoS Pathog. 2017, 13, e1006618. [Google Scholar] [CrossRef]
- Haring, J.S.; Badovinac, V.; Harty, J.T. Inflaming the CD8+ T Cell Response. Immunology 2006, 25, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Dyga, W.; Obtułowicz, A.; Bogdali, A. Expression of bradykinin recpetors on PBMC collected from patients with HAE and spontaneous urticaria. In Proceedings of the Bradykinin Symposium, Berlin, Germany, 5–6 September 2018; p. 16. [Google Scholar]
- Obtułowicz, K. Bradykinin-mediated angioedema. Pol. Arch. Intern. Med. 2016, 126, 76–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nussberger, J.; Cugno, M.; Cicardi, M. Bradykinin-mediatedangioedema. N. Engl. J. Med. 2002, 3, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Cunill, A.S.; Björkqvist, J.; Senter, R.; Guilarte, M.; Cardona, V.; Labrador-Horrillo, M.; Nickel, K.F.; Butler, L.; Luengo, O.; Kumar, P.; et al. Plasma contact system activation drives anaphylaxis in severe mast cell–mediated allergic reactions. J. Allergy Clin. Immunol. 2015, 135, 1031–1043.e6. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.M.; Sugimoto, S.L.; Curd, J.G.; Christiansen, S.C.; Zuraw, B.L. High-molecular-weight kininogen is cleaved in active erythema multiforme. J. Allergy Clin. Immunol. 1989, 83, 802–810. [Google Scholar] [CrossRef]
- Hofman, Z.; de Maat, S.; Hack, C.E.; Maas, C. Bradykinin: Inflammatory Product of the Coagulation System. Clin. Rev. Allergy Immunol. 2016, 51, 152–161. [Google Scholar] [CrossRef]
- Maurer, M.; Raap, U.; Staubach, P.; Richter-Huhn, G.; Bauer, A.; Oppel, E.M.; Hillen, U.; Baeumer, D.; Reinhardt, M.; Chapman-Rothe, N. Antihistamine-resistant chronic spontaneous urticaria: 1-year data from the AWARE study. Clin. Exp. Allergy 2019, 49, 655–662. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kwon, S.-H.; Yun, J.-H.; Jeong, H.-S.; Kim, H.-R.; Lee, E.H.; Ye, S.-K.; Cho, C.-H. STAT3 activation in endothelial cells is important for tumor metastasis via increased cell adhesion molecule expression. Oncogene 2017, 36, 5445–5459. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Jiang, W.; Wang, H.; Li, H.; Tang, B.; Liu, B.; Jiang, H.; Sun, X. The IL-6/STAT3 pathway regulates adhesion molecules and cytoskeleton of endothelial cells in thromboangiitis obliterans. Cell. Signal. 2018, 44, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.S.; Kaplan, A.P. Chronic Spontaneous Urticaria: The Devil’s Itch. J. Allergy Clin. Immunol. Pract. 2018, 6, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Jung, K.E.; Koo, D.W.; Lee, J.S. Vitamin D as a Marker for Disease Severity in Chronic Urticaria and Its Possible Role in Pathogenesis. Ann. Dermatol. 2015, 27, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Tavakol, M.; Hirbod-Mobarakeh, A.; Gharagozlou, M.; Aghamohammadi, A.; Tavakol, Z.; Momenzadeh, K.; Nabavi, M.; Dabbaghzade, A.; Mosallanejad, A.; et al. Vitamin D deficiency in chronic idiopathic urticaria. Iran. J. Allergy Asthma Immunol. 2015, 14, 222–227. [Google Scholar]
- Quirk, S.K.; Rainwater, E.; Shure, A.K.; Agrawal, D.K. Vitamin D in Atopic Dermatitis, Chronic Urticaria and Allergic Contact Dermatitis. Expert Rev. Clin. Immunol. 2016, 12, 839–847. [Google Scholar] [CrossRef]
- Tuchinda, P.; Kulthanan, K.; Chularojanamontri, L.; Arunkajohnsak, S.; Sriussadaporn, S. Relationship between vitamin D and chronic spontaneous urticaria: A systematic review. Clin. Transl. Allergy 2018, 8, 51. [Google Scholar] [CrossRef]


| Number of CSU Patients | 30 |
| Sex Male/Female n (%) | 6 (20%)/24 (80%) |
| Mean age (range) | 46.13 (+/−15.06) |
| Disease Severity by UAS-7 | |
| Symptom-free n (%) | 0 (0%) |
| Mild n (%) | 9 (30%) |
| Moderate to severe n (%) | 21 (70%) |
| Subpopulation of Cells | Healthy Control Group Median (Q1–Q3) (%) | CSU Group Median (Q1–Q3) (%) | p-Value |
|---|---|---|---|
| T lymphocytes CD3+ | 61.4 (56.1–66.7) | 65.9 (52.9–71.3) | <0.25 |
| Th CD4+ | 58.3 (54.7–62.6) | 69.3 (64.5–75.0) | <0.0001 |
| Tc CD8+ | 33.3 (30.9–37.3) | 24.6 (20.2–29.3) | <0.0001 |
| Monocytes HLA-DR+CD14+ | 32.1 (21.5–38.8) | 32.4 (21.6–46.0) | <0.73 |
| Monocytesintermediatesubset CD14++CD16+ | 7.8 (4.8–13.0) | 5.16 (3.5–8.9) | <0.09 |
| Monocytes Classicsubset CD14++CD16− | 86.0 (78.6–90.0) | 89.6 (86.6–92.9) | <0.06 |
| Healthy Control Group (% of Cells) | CSU Patients (% of Cells) | p-Value | |
|---|---|---|---|
| BR1 level on CD3+ (range) | 3.8 (3.08–5.20) | 9.12 (7.17–16.78) | <0.001 |
| BR1 level on CD4+ (range) | 2.90 (2.31–4.28) | 6.96 (5.78–10.57) | <0.001 |
| BR1 level on CD8+ (range) | 6.05 (4.51–8.95) | 12.84 (9.77–22.64) | <0.001 |
| BR2 level on CD3+ (range) | 7.72 (4.44–18.06) | 9.02 (3.97–14.23) | <0.773 |
| BR2 level on CD4+ (range) | 7.63 (4.36–14.93) | 9.72 (3.66–21.06) | <0.824 |
| BR2 level on CD8+ (range) | 12.21 (4.58–22.94) | 12.99 (9.96–22.68) | <0.957 |
| BR1 CD3+ p-Value | BR1 CD4+ p-Value | BR1 CD8+ p-Value | BR2 CD3+ p-Value | BR 2 CD4+ p-Value | BR 2 CD8+ p-Value | |
|---|---|---|---|---|---|---|
| CRP | 0.675 | 0.952 | 0.915 | 0.742 | 0.319 | 0.884 |
| Fibrinogen | 0.638 | 0.253 | 0.783 | 0.680 | 0.818 | 0.787 |
| IgE level | 0.701 | 0.147 | 0.808 | 0.830 | 0.660 | 0.889 |
| Thrombocytes | 0.081 | 0.312 | 0.074 | 0.795 | 0.799 | 0.914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obtulowicz, A.; Dubiela, P.; Dyga, W.; Migacz-Gruszka, K.; Mikolajczyk, T.; Wojas-Pelc, A.; Obtulowicz, K. The Role of Bradykinin Receptors in the Etiopathogenesis of Chronic Spontaneous Urticaria. Medicina 2021, 57, 1133. https://doi.org/10.3390/medicina57101133
Obtulowicz A, Dubiela P, Dyga W, Migacz-Gruszka K, Mikolajczyk T, Wojas-Pelc A, Obtulowicz K. The Role of Bradykinin Receptors in the Etiopathogenesis of Chronic Spontaneous Urticaria. Medicina. 2021; 57(10):1133. https://doi.org/10.3390/medicina57101133
Chicago/Turabian StyleObtulowicz, Aleksander, Pawel Dubiela, Wojciech Dyga, Kamila Migacz-Gruszka, Tomasz Mikolajczyk, Anna Wojas-Pelc, and Krystyna Obtulowicz. 2021. "The Role of Bradykinin Receptors in the Etiopathogenesis of Chronic Spontaneous Urticaria" Medicina 57, no. 10: 1133. https://doi.org/10.3390/medicina57101133
APA StyleObtulowicz, A., Dubiela, P., Dyga, W., Migacz-Gruszka, K., Mikolajczyk, T., Wojas-Pelc, A., & Obtulowicz, K. (2021). The Role of Bradykinin Receptors in the Etiopathogenesis of Chronic Spontaneous Urticaria. Medicina, 57(10), 1133. https://doi.org/10.3390/medicina57101133






